Distinct Climate Effects on Dahurian Larch Growth at an Asian Temperate-Boreal Forest Ecotone and Nearby Boreal Sites
Abstract
:1. Introduction
2. Materials and Methods
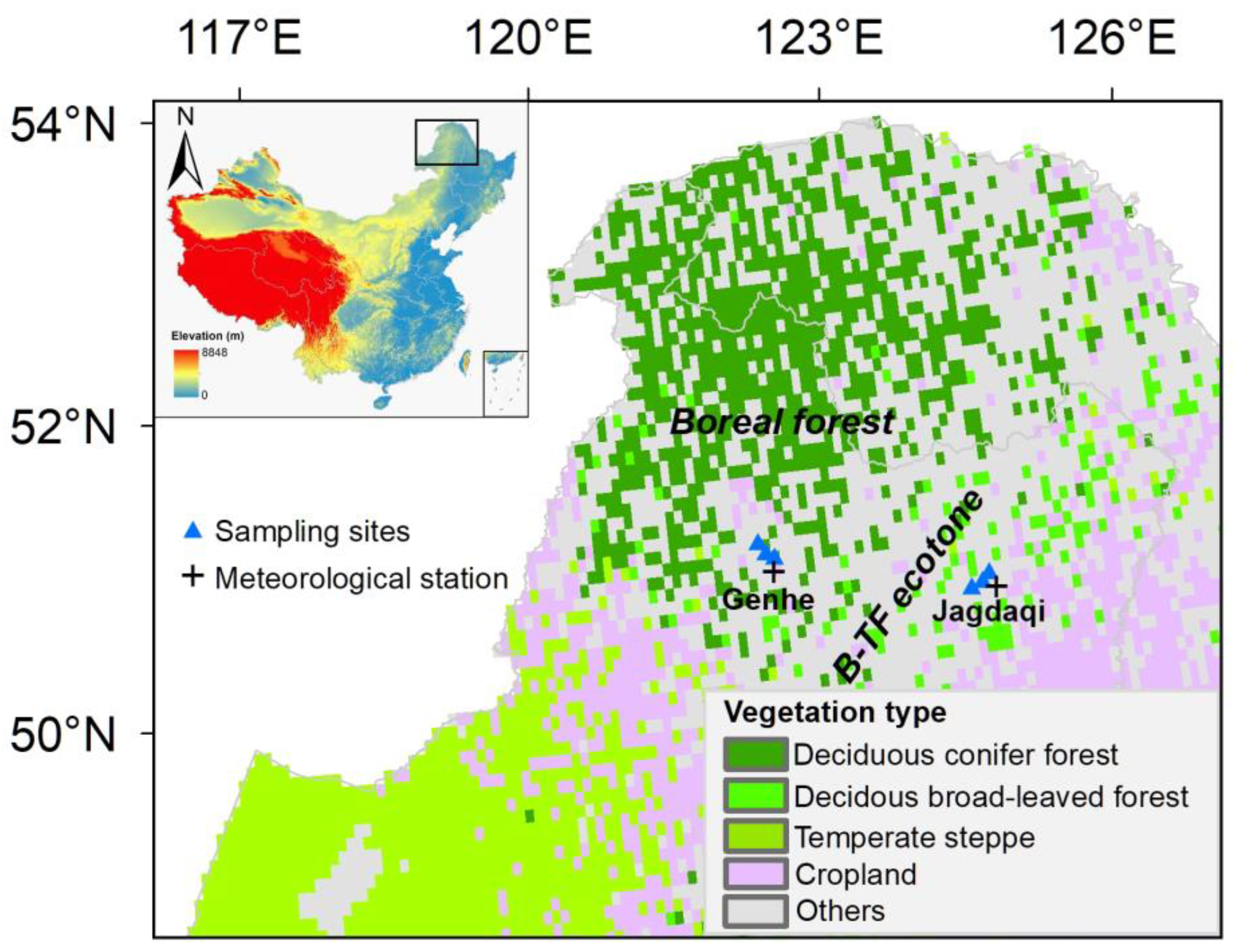
2.1. Study Areas
2.2. Field Sampling
2.3. Laboratory Measurements
2.4. Data on Phenology and Climate Variables
2.5. Calculation of Annual Basal Area Increment
2.6. Statistical Analysis
3. Results
3.1. Climate Trends and Soil Nutrients in the Two Bioregions
3.2. Changes of Annual Basal Area Increment with Tree Age
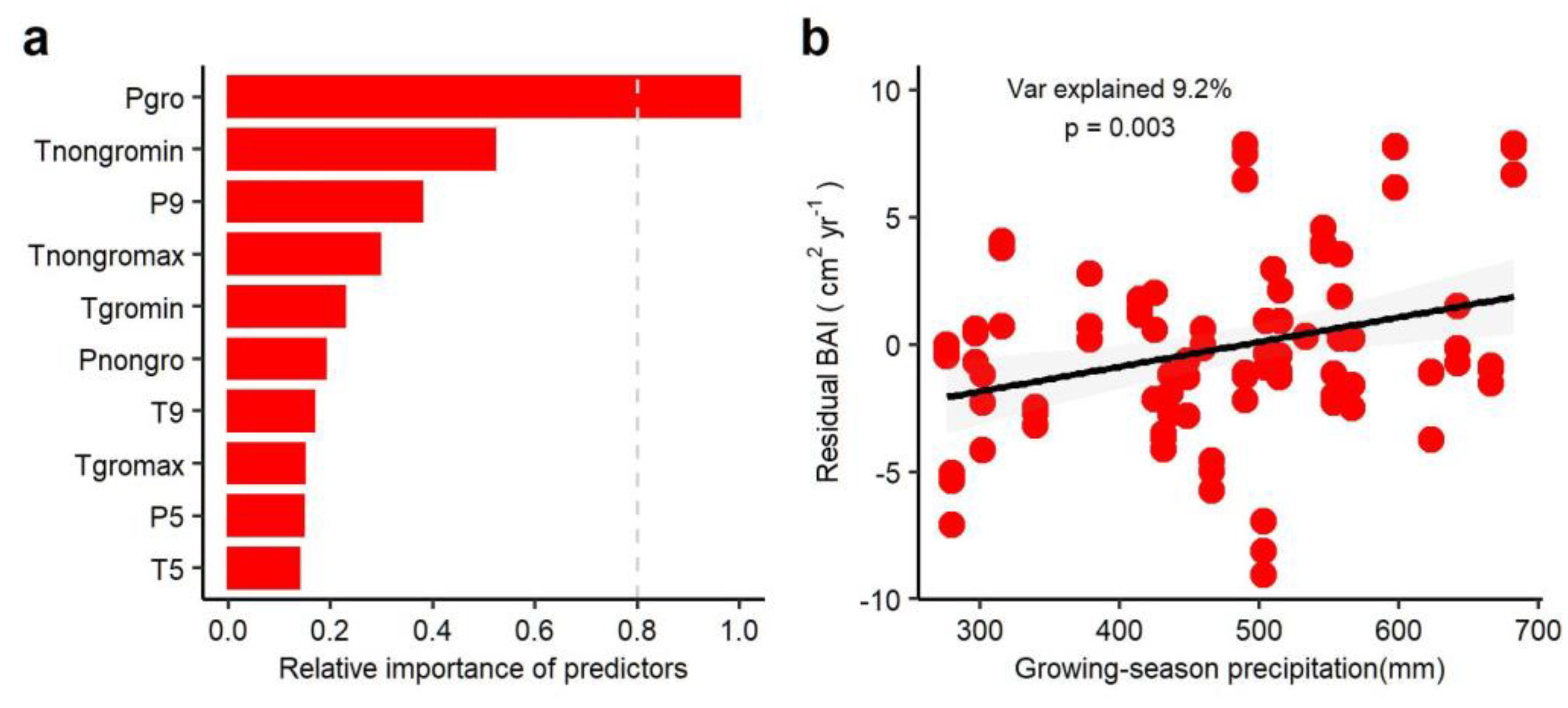
3.3. Climate Regulation of Tree Growth in Past Three Decades
4. Discussion
4.1. Responses of Tree Growth to Inter-Annual Variation in Temperature
4.2. Responses of Tree Growth to Inter-Annual Variations in Precipitation
4.3. Uncertainties and Future Research Needs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapin, F.; Callaghan, T.; Bergeron, Y.; Fukuda, M.; Johnstone, J.; Juday, G.; Zimov, S. Global change and the boreal forest: Thresholds, shifting states or gradual change? AMBIO J. Hum. Environ. 2004, 33, 361–365. [Google Scholar] [CrossRef]
- Soja, A.; Tchebakova, N.; French, N.; Flannign, M.; Shugart, H.; Stocks, B.; Sukhinin, A.; Parfenova, E.; Chapin, F.; Stackhouse, P. Climate-induced boreal forest change: Predictions versus current observations. Glob. Planet. Change 2007, 56, 274–296. [Google Scholar] [CrossRef] [Green Version]
- Beck, P.; Juday, G.; Alix, C.; Barber, V.; Winslow, S.; Sousa, E.; Goetz, S. Changes in forest productivity across Alaska consistent with biome shift. Ecol. Lett. 2011, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Bunn, A.; Berner, L. Latitudinal gradient in tree growth response to climate warming in the Siberian taiga. Glob. Chang. Biol. 2011, 17, 1935–1945. [Google Scholar] [CrossRef]
- Sherriff, R.; Miller, A.; Muth, K.; Schriver, M.; Batzel, R. Spruce growth responses to warming vary by ecoregion and ecosystem type near the forest-tundra boundary in south-west Alaska. J. Biogeogr. 2017, 44, 1457–1468. [Google Scholar] [CrossRef]
- Zhu, Z.; Piao, S.; Myneni, R.; Huang, M.; Zeng, Z.; Canadell, J.; Ciais, P.; Sitch, S.; Friedlingstein, P.; Arneth, A. Greening of the Earth and its drivers. Nat. Clim. Chang. 2016, 6, 791–795. [Google Scholar] [CrossRef]
- D’Orangeville, L.; Houle, D.; Duchesne, L.; Phillips, R.P.; Bergeron, Y.; Kneeshaw, D. Beneficial effects of climate warming on boreal tree growth may be transitory. Nat. Commun. 2018, 9, 3213. [Google Scholar] [CrossRef] [Green Version]
- Frelich, L.; Montgomery, R.; Reich, P. Seven ways a warming climate can kill the southern boreal forest. Forests 2021, 12, 560. [Google Scholar] [CrossRef]
- Fisichelli, N.; Frelich, L.; Reich, P. Temperate tree expansion into adjacent boreal forest patches facilitated by warmer temperatures. Ecography 2014, 37, 152–161. [Google Scholar] [CrossRef]
- Evans, P.; Brown, C. The boreal–temperate forest ecotone response to climate change. Environ. Rev. 2017, 25, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, M.; Murthy, R.; Griffin, K. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ. 2002, 25, 1729–1737. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Chen, J.; Piao, S.; Ciais, P.; Luo, Y.; & Wan, S. Terrestrial carbon cycle affected by non-uniform climate warming. Nat. Geosci. 2014, 7, 173–180. [Google Scholar] [CrossRef]
- Tan, J.; Piao, S.; Chen, A.; Zeng, Z.; Ciais, P.; Janssens, I.; Vicca, S. Seasonally different response of photosynthetic activity to daytime and night-time warming in the Northern Hemisphere. Glob. Chang. Biol. 2015, 21, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Walker, X.; Johnstone, J. Widespread negative correlations between black spruce growth and temperature across topographic moisture gradients in the boreal forest. Environ. Res. Lett. 2014, 9, 064016. [Google Scholar] [CrossRef]
- Kumarathunge, D.; Drake, J.; Tjoelker, M.; López, R.; Pfautsch, S.; Vårhammar, A.; Medlyn, B. The temperature optima for tree seedling photosynthesis and growth depend on water inputs. Glob. Chang. Biol. 2020, 26, 2544–2560. [Google Scholar] [CrossRef]
- Turnbull, M.; Tissue, D.; Murthy, R.; Wang, X.; Sparrow, A.; Griffin, K.L. Nocturnal warming increases photosynthesis at elevated CO2 partial pressure in Populus deltoides. N. Phytol. 2004, 161, 819–826. [Google Scholar] [CrossRef]
- Jarvis, P.; Linder, S. Constraints to growth of boreal forests. Nature 2004, 405, 904–905. [Google Scholar] [CrossRef]
- Reich, P.; Sendall, K.; Stefanski, A.; Rich, R.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2000, 562, 263–267. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Han, S.; Chen, Z.; Setälä, H.; Yu, J.; Zheng, X.; Guo, Y.; Gu, Y. Radial growth response of Larix gmelinii to climate along a latitudinal gradient in the Greater Khingan Mountains, Northeastern China. Forests 2016, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Jiang, Y.; Dong, M.; Du, E.; Zhou, Z.; Zhao, S.; Xu, H. Diverse responses of radial growth to climate across the southern part of the Asian boreal forests in northeast China. For. Ecol. Manag. 2020, 458, 117759. [Google Scholar] [CrossRef]
- Hynes, A.; Hamann, A. Moisture deficits limit growth of white spruce in the west-central boreal forest of North America. For. Ecol. Manag. 2020, 461, 117944. [Google Scholar] [CrossRef]
- Walker, X.; Mack, M.; Johnstone, J. Stable carbon isotope analysis reveals widespread drought stress in boreal black spruce forests. Glob. Chang. Biol. 2015, 21, 3102–3113. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.; Hollinger, D.; Bohrer, G.; Dragoni, D.; Munger, J.; Schmid, H.; Richardson, A. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Blume-Werry, G.; Kreyling, J.; Laudon, H.; Milbau, A. Short-term climate change manipulation effects do not scale up to long-term legacies: Effects of an absent snow cover on boreal forest plants. J. Ecol. 2016, 104, 1638–1648. [Google Scholar] [CrossRef]
- Reinmann, A.; Susser, J.; Demaria, E.; Templer, P. Declines in northern forest tree growth following snowpack decline and soil freezing. Glob. Chang. Biol. 2019, 25, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Piao, S.; Ciais, P.; Fang, J.; Wang, X. Change in winter snow depth and its impacts on vegetation in China. Glob. Chang. Biol. 2010, 16, 3004–3013. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Liu, H.; Ciais, P.; Li, Y.; Xu, C.; Babst, F.; Guo, W.; Hao, B.; Wang, P.; et al. Uneven winter snow influence on tree growth across temperate China. Glob. Chang. Biol. 2019, 25, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaganov, E.; Hughes, M.; Kirdyanov, A.; Schweingruber, F.; Silkin, P. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar] [CrossRef]
- Hänninen, H. Climate warming and the risk of frost damage to boreal forest trees: Identification of critical ecophysiological traits. Tree Physiol. 2006, 26, 889–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shugart, H.; Shugart, H.; Leemans, R.; Bonan, G. A Systems Analysis of the Global Boreal Forest; Cambridge University Press: Cambridge, UK, 1992; pp. 1–10. [Google Scholar]
- Su, Y.; Guo, Q.; Hu, T.; Guan, H.; Jin, S.; An, S.; Ma, K. An updated vegetation map of China (1:1000,000). Sci. Bull. 2020, 65, 1125–1136. [Google Scholar] [CrossRef]
- Tian, H.; Xu, H.; Hall, A.S. Pattern and change of a boreal forest landscape in northern China. Water Air Soil Pollut. 1995, 82, 465–476. [Google Scholar] [CrossRef]
- Coomes, D.; Allen, R. Effects of size, competition and altitude on tree growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Forrester, D. Linking forest growth with stand structure: Tree size inequality, tree growth or resource partitioning and the asymmetry of competition. For. Ecol. Manag. 2019, 447, 139–157. [Google Scholar] [CrossRef]
- De Martonne, E. Aerisme, et índices d’aridite. Comptesrendus L’Academie Sci. 1926, 182, 1395–1398. [Google Scholar]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Du, E.; Fang, J. Linking belowground and aboveground phenology in two boreal forests in Northeast China. Oecologia 2014, 176, 883–892. [Google Scholar] [CrossRef]
- Beck, P.; Atzberger, C.; Høgda, K.; Johansen, B.; Skidmore, A. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Yang, B.; He, M.; Shishov, V.; Tychkov, I.; Vaganov, E.; Rossi, S.; Ljungqvist, F.; Bräuning, A.; Grießinger, J. New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar] [CrossRef] [Green Version]
- Rosbakh, S.; Hartig, F.; Sandanov, D.V.; Bukharova, E.V.; Miller, T.K.; Primack, R.B. Siberian plants shift their phenology in response to climate change. Glob. Chang. Biol. 2021, 27, 4435–4448. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Alexander, R.; Trouet, V.; Frank, D. Toward consistent measurements of carbon accumulation: A multi-site assessment of biomass and basal area increment across Europe. Dendrochronologia 2014, 32, 153–161. [Google Scholar] [CrossRef]
- Fu, L.; Sharma, R.; Zhu, G.; Li, H.; Hong, L.; Guo, H.; Duan, G.; Shen, C.; Lei, Y.; Li, Y.; et al. A basal area increment-based approach of site productivity evaluation for multi-aged and mixed forests. Forests 2017, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Calcagno, V.; de Mazancourt, C. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.; & Anderson, D. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.; Elphick, C. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R J. 2013, 9, 56–71. [Google Scholar] [CrossRef]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 1 December 2020).
- Huang, M.; Piao, S.; Ciais, P.; Peñuelas, J.; Wang, X.; Keenan, T.; Janssens, I. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol. 2019, 3, 772–779. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Ciais, P.; Myneni, R.; Chen, A.; Chevallier, F.; Zeng, H. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 2013, 501, 88–92. [Google Scholar] [CrossRef]
- Wipf, S.; Rixen, C. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Res. 2010, 29, 95–109. [Google Scholar] [CrossRef]
- Harrison, J.; Blagden, M.; Green, M.; Salvucci, G.; Templer, P.H. Water sources for red maple trees in a northern hardwood forest under a changing climate. Ecohydrology 2020, 13, e2248. [Google Scholar] [CrossRef]
- Ffolliott, P.; Gottfried, G.; Baker, M.B., Jr. Water yield from forest snowpack management: Research findings in Arizona and New Mexico. Water Resour. Res. 1989, 25, 1999–2007. [Google Scholar] [CrossRef]
- Langs, L.; Petrone, R.; Pomeroy, J. A δ18O and δ2H stable water isotope analysis of subalpine forest water sources under seasonal and hydrological stress in the Canadian Rocky Mountains. Hydrol. Processes 2020, 34, 5642–5658. [Google Scholar] [CrossRef]
- Carle, J.; Vuorinen, P.; Del Lungo, A. Status and trends in global forest plantation development. For. Prod. J. 2002, 52, 12–23. [Google Scholar]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Feyen, L. Global changes in drought conditions under different levels of warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Keeling, R.; Graven, H.; Welp, L.; Resplandy, L.; Bi, J.; Piper, S.; Meijer, H. Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, 10361–10366. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Stand ID | Latitude (°E) | Longitude (°N) | Elevation (m) | MAT (°C) | MAP (mm) | AI | Mean DBH (cm) | Mean Height (m) | Stand Age (year) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Boreal site | Stand 1 | 120.71 | 50.44 | 804 | −3.3 | 445 | 66 | 24.4 ± 1.6 | 14.8 ± 1.1 | 35 |
| Stand 2 | 120.84 | 50.49 | 815 | −3.3 | 445 | 66 | 21.8 ± 1.2 | 16.2 ± 0.7 | 35 | |
| Stand 3 | 120.96 | 50.41 | 797 | −3.3 | 445 | 66 | 23.2 ± 3.1 | 17.1 ± 0.8 | 32 | |
| Boreal-temperate forest ecotone | Stand 4 | 123.95 | 50.42 | 407 | −0.1 | 551 | 56 | 29.0 ± 2.9 | 16.2 ± 1.0 | 38 |
| Stand 5 | 124.06 | 50.45 | 403 | −0.1 | 551 | 56 | 28.6 ± 2.8 | 16.4 ± 0.3 | 40 | |
| Stand 6 | 124.15 | 50.49 | 408 | −0.1 | 551 | 56 | 28.6 ± 3.1 | 15.7 ± 0.8 | 40 |
| Location | Stand ID | BAImax | b | Age0 | R2 |
|---|---|---|---|---|---|
| Boreal site | Stand 1 | 15.3(1.1) *** | 0.73(0.09) *** | 14.3(1.0) *** | 0.59 |
| Stand 2 | 9.7(0.9) *** | 0.96(0.14) *** | 9.8(1.2) *** | 0.39 | |
| Stand 3 | 14.4(1.3) *** | 0.77(0.11) *** | 12.0(1.0) *** | 0.52 | |
| Boreal-temperate forest ecotone | Stand 4 | 14.6(0.8) *** | 1.01(0.12) *** | 14.4(1.2) *** | 0.56 |
| Stand 5 | 15.7(1.0) *** | 0.73(0.08) *** | 18.2(1.1) *** | 0.64 | |
| Stand 6 | 17.7(1.0) *** | 0.76(0.08) *** | 17.2(0.9) *** | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, E.; Tang, Y. Distinct Climate Effects on Dahurian Larch Growth at an Asian Temperate-Boreal Forest Ecotone and Nearby Boreal Sites. Forests 2022, 13, 27. https://doi.org/10.3390/f13010027
Du E, Tang Y. Distinct Climate Effects on Dahurian Larch Growth at an Asian Temperate-Boreal Forest Ecotone and Nearby Boreal Sites. Forests. 2022; 13(1):27. https://doi.org/10.3390/f13010027
Chicago/Turabian StyleDu, Enzai, and Yang Tang. 2022. "Distinct Climate Effects on Dahurian Larch Growth at an Asian Temperate-Boreal Forest Ecotone and Nearby Boreal Sites" Forests 13, no. 1: 27. https://doi.org/10.3390/f13010027
APA StyleDu, E., & Tang, Y. (2022). Distinct Climate Effects on Dahurian Larch Growth at an Asian Temperate-Boreal Forest Ecotone and Nearby Boreal Sites. Forests, 13(1), 27. https://doi.org/10.3390/f13010027







