Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Soil Sampling and Soil Properties Analyses
2.4. DNA Extraction, PCR Amplification, and Illumina Sequencing
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
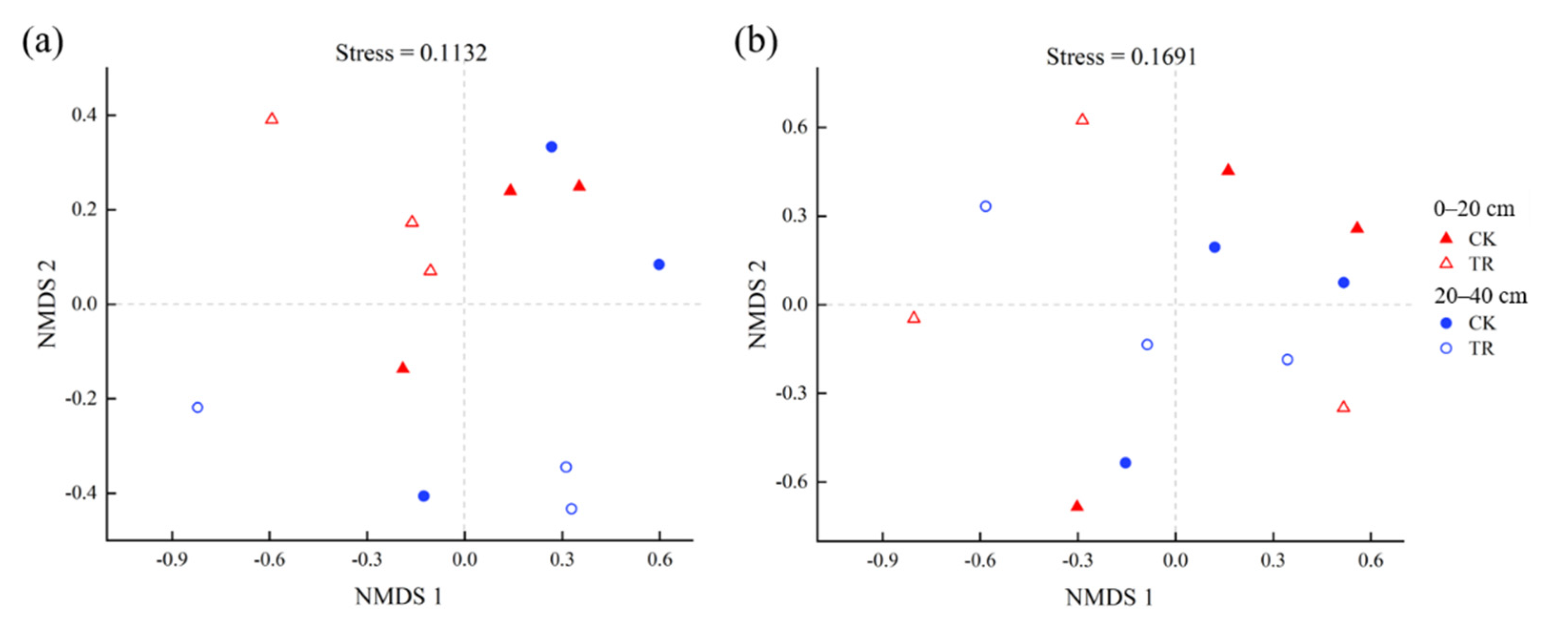
3.2. OTU Cluster and Diversity Analysis
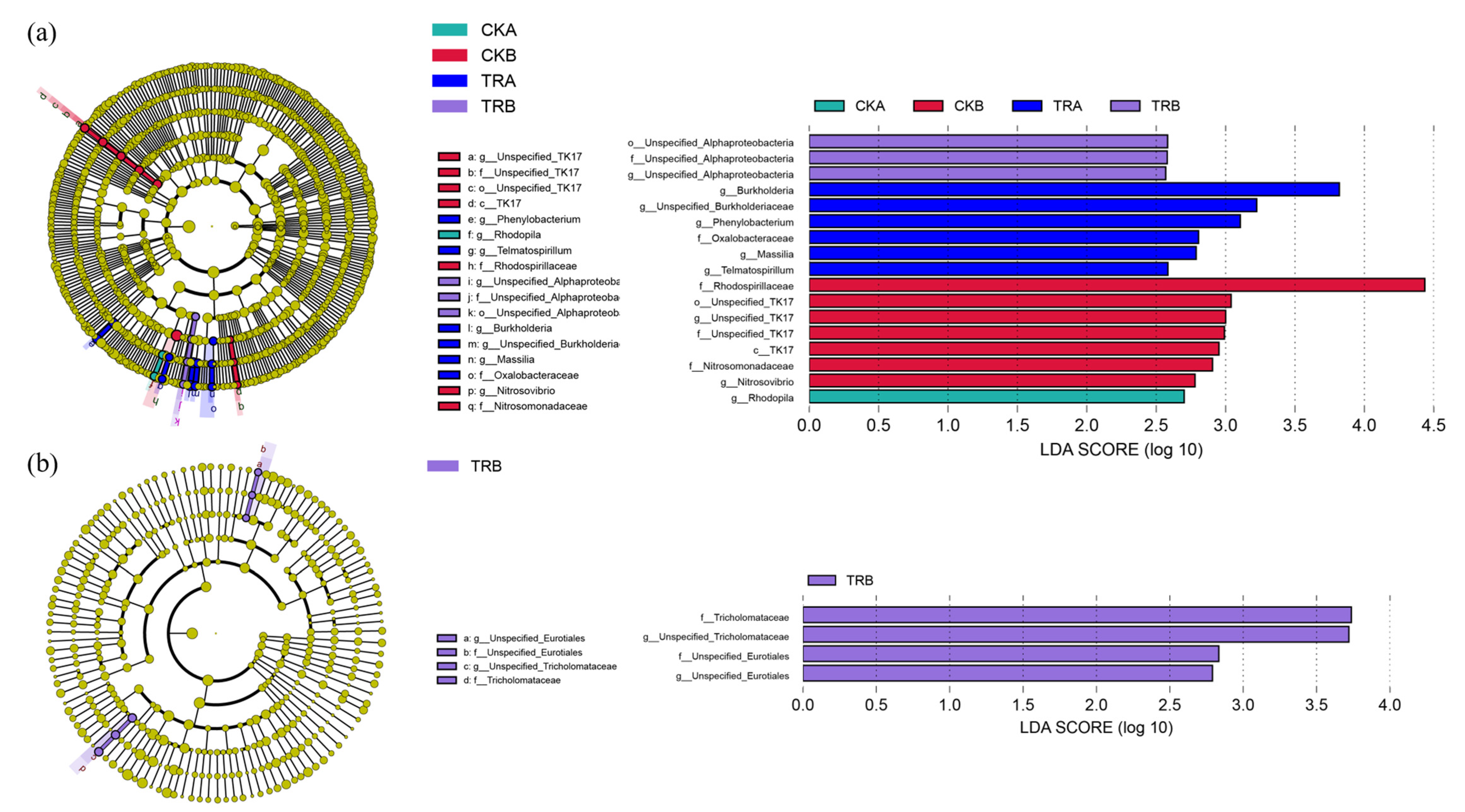
3.3. Difference Analysis of Bacterial and Fungal Community Structure
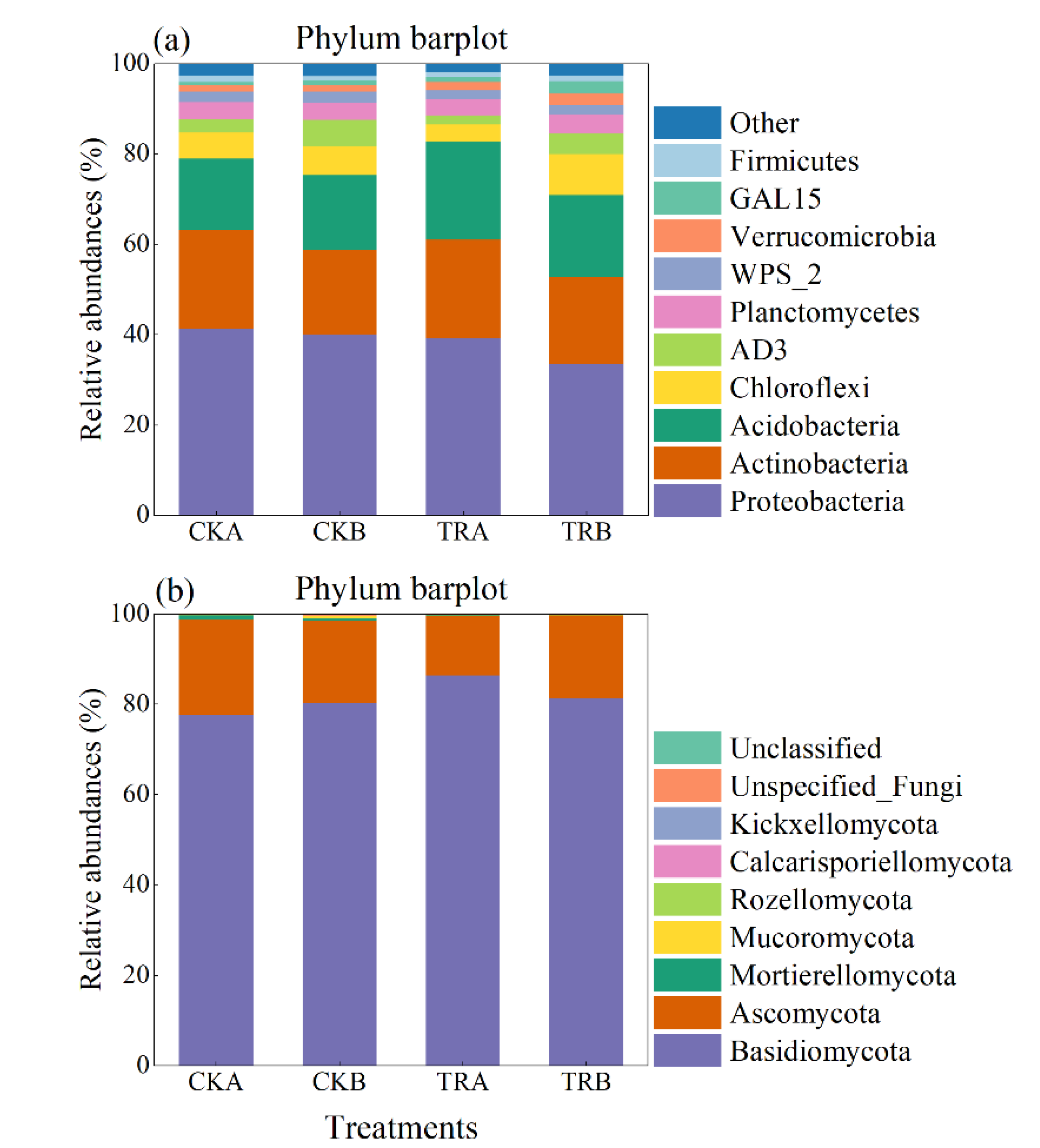
3.4. Diversity and Composition of Bacterial and Fungal Communities
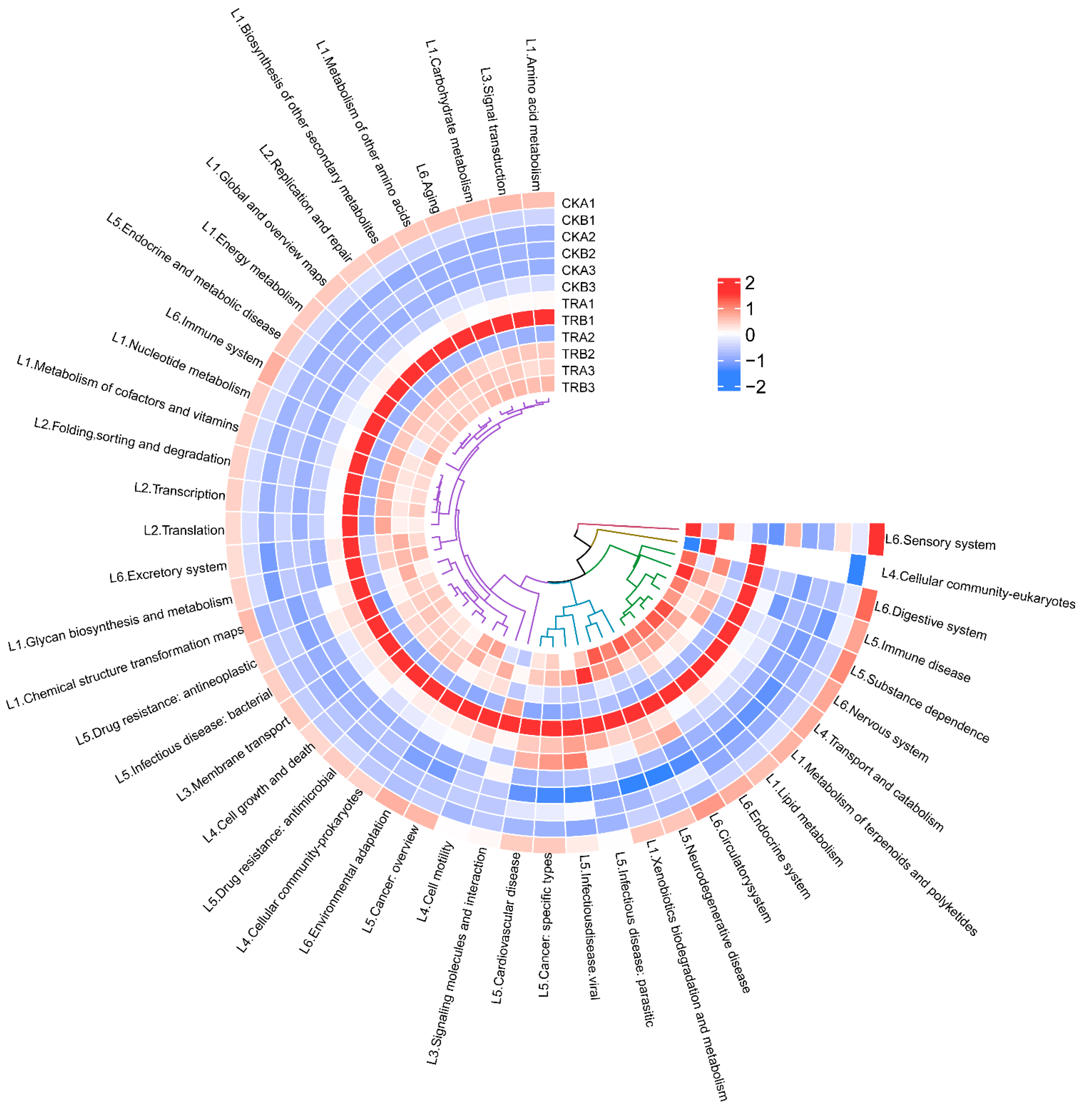
3.5. Response of Functional Groups of Bacterial and Fungal to Throughfall Reduction
4. Discussion
4.1. The Relationship among Throughfall Reduction, Soil Properties, and Microbial Community Changes
4.2. Effects of Throughfall Reduction on Soil Bacterial Community Structure and Diversity
4.3. Effects of Throughfall Reduction on Soil Fungal Community Structure and Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, S.; Zhao, H.; Xing, F.; Bai, Z.; Gao, Y.; Dong, Y.; Zhou, J.; Wu, Y.; Yang, Y. Response of soil microbial community structure to increased precipitation and nitrogen addition in a semiarid meadow steppe. Eur. J. Soil Sci. 2017, 68, 524–536. [Google Scholar] [CrossRef]
- IP, CC. Climate change 2013: The physical science basis. In Proceedings of the , Working Group I Contribution to the IPCC Fifth Assessment Report, Stockholm, Sweden,, 30 September 2013. [Google Scholar]
- Xu, Y.; Huang, X.; Zhang, Y.; Lin, W.; Lin, E. Statistical analyses of climate change scenarios over China in the 21st century. Adv. Clim. Chang. Res. 2005, 1, 80–83. (In Chinese) [Google Scholar]
- Gao, J.Q.; Duan, M.Y.; Zhang, X.Y.; Li, Q.W.; Yu, F.H. Effects of frequency and intensity of drying-rewetting cycles on Hydrocotyle vulgaris growth and greenhouse gas emissions from wetland microcosms. Catena 2018, 164, 44–49. [Google Scholar] [CrossRef]
- Engelhardt, I.C.; Niklaus, P.A.; Bizouard, F.; Breuil, M.C.; Barnard, R.L. Precipitation patterns and N availability alter plant-soil microbial C and N dynamics. Plant Soil 2021, 466, 151–163. [Google Scholar] [CrossRef]
- Beier, C.; Beierkuhnlein, C.; Wohlgemuth, T.; Penuelas, J.; Emmett, B.; Korner, C.; Boeck, H.; Christensen, J.H.; Leuzinger, S.; Janssens, I.A.; et al. Precipitation manipulation experiments-challenges and recommendations for the future. Ecol. Lett. 2012, 15, 899–911. [Google Scholar] [CrossRef]
- Carney, K.M.; Matson, P.A. Plant communities, soil microorganisms, and soil carbon cycling: Does altering the world belowground matter to ecosystem functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef]
- Diaz-Pereira, E.; Marin Sanleandro, P.; Asencio, A.D. Effects of drought and water pulses on microbial functionality and the role of Cyanoprokaryota in the rhizospheres of gypsophytes. Sci. Total Environ. 2019, 691, 919–932. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Castro, H.F.; Classen, A.T.; Austin, E.E.; Norby, R.J.; Schadt, C.W. Soil microbial community responses to multiple experimental climate change drivers. Appl. Environ. Microbiol. 2010, 76, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.; Andreae, M.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosc. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Yu, S.; Mo, Q.; Li, Y.; Zou, B.; Xia, H.; Li, Z.; Wang, F. Changes in seasonal precipitation distribution but not annual amount affect litter decomposition in a secondary tropical forest. Ecol. Evol. 2019, 9, 11344–11352. [Google Scholar] [CrossRef]
- Frossard, A.; Ramond, J.B.; Seely, M.; Cowan, D.A. Water regime history drives responses of soil Namib Desert microbial communities to wetting events. Sci. Rep. 2015, 5, 12263. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; Snoek, B.L.; Nesme, J.; Dent, E.; Jacquiod, S.; Classen, A.T.; Prieme, A. Soil microbial legacies differ following drying-rewetting and freezing-thawing cycles. ISME J. 2021, 15, 1207–1221. [Google Scholar] [CrossRef]
- Zhao, Q.; Jian, S.; Nunan, N.; Maestre, F.T.; Tedersoo, L.; He, J.; Wei, H.; Tan, X.; Shen, W. Altered precipitation seasonality impacts the dominant fungal but rare bacterial taxa in subtropical forest soils. Biol. Fertil. Soils 2017, 53, 1–15. [Google Scholar] [CrossRef]
- Arnold, R.J.; Xie, Y.J.; Luo, J.Z.; Wang, H.R.; Midgley, S.J. A tale of two genera: Exotic Eucalyptus and Acacia species in China. 1. domestication and research. Int. For. Rev. 2020, 22, 1–18. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, S.R.; Wang, H.; Chen, L.; Lu, L.H.; Cai, D.X. Reduction in throughfall reduces soil aggregate stability in two subtropical plantations. Eur. J. Soil. Sci. 2018, 70, 301–310. [Google Scholar] [CrossRef]
- Fang, Y.T.; Gundersen, P.; Mo, J.M.; Zhu, W.X. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 2008, 5, 339–352. [Google Scholar] [CrossRef]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef]
- Lai, X.; Cao, L.; Tan, H.; Fang, S.; Huang, Y.; Zhou, S. Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J. 2007, 1, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J 2011, 5, 169–172. [Google Scholar] [CrossRef]
- R Development Core Team. Development a Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Su, X.L.; Su, X.; Zhou, G.; Du, Z.; Deng, J. Drought accelerated recalcitrant carbon loss by changing soil aggregation and microbial communities in a subtropical forest. Soil Biol. Biochem. 2020, 148, 107898. [Google Scholar] [CrossRef]
- Olatunji, O.A.; Luo, H.; Pan, K.; Tariq, A.; Sun, X.; Chen, W.; Wu, X.; Zhang, L.; Xiong, Q.; Li, Z. Influence of phosphorus application and water deficit on the soil microbiota of N2-fixing and non-N-fixing tree. Ecosphere 2018, 9, e02276. [Google Scholar] [CrossRef]
- Nicole, W.; Yvette, P.; Ding, G.C. PhyloChip hybridization uncovered an enormous bacterial diversity in the rhizosphere of different potato cultivars: Many common and few cultivar-dependent taxa. FEMS Microbiol. Ecol. 2011, 75, 497–506. [Google Scholar]
- Wu, T.H.; Dan, O.C.; Graham, J.H.; Martin, K.J.; Rosskopf, E.N. Comparison of soil bacterial communities under diverse agricultural land management and crop production practices. Microb. Ecol. 2008, 55, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Schutter, M.E.; Sandeno, J.M.; Dick, R.P. Seasonal, soil type, and alternative management influences on microbial communities of vegetable cropping systems. Biol. Fertil. Soils 2001, 34, 397–410. [Google Scholar]
- Deng, L.; Peng, C.; Li, J.W.; Liu, Y.L.; Hai, X.Y.; Liu, Q.Y.; Huang, C.B.; Shangguan, Z.P.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; Garcia-Gomez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, X.; Guo, Q.Y. Relationship of soil bacterial community composition from different ecosystems on Qinghai-Tibet Plateau with environment factors. Agric. Res. Arid Areas 2019, 37, 18–26. (In Chinese) [Google Scholar]
- Wang, N.N.; Yang, X.; Li, S.L.; Sui, X.; Han, S.J.; Feng, F.J. Effects of precipitation variation on the distribution pattern of soil fungal diversity in broadleaved Korean pine mixed forest. Chin. J. Appl. Ecol. 2013, 7, 1985–1990. (In Chinese) [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; Van, W.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- McHugh, T.A.; Koch, G.W.; Schwartz, E. Minor changes in soil bacterial and fungal community composition occur in response to monsoon precipitation in a semiarid grassland. Microb. Ecol. 2014, 68, 370–378. [Google Scholar] [CrossRef]
- Bouskill, N.J.; Lim, H.C.; Borglin, S.; Salve, R.; Wood, T.E.; Silver, W.L.; Brodie, E.L. Pre-exposure to drought increases the resistance of tropical forest soil bacterial communities to extended drought. ISME J. 2013, 7, 384–394. [Google Scholar] [CrossRef]
- Naether, A.; Foesel, B.U.; Naegele, V.; Wust, P.K.; Weinert, J.; Bonkowski, M.; Alt, F.; Oelmann, Y.; Polle, A.; Lohaus, G.; et al. Environmental factors affect Acidobacterial communities below the subgroup level in grassland and forest soils. Appl. Environ. Microbiol. 2012, 78, 7398–7406. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- Barns, S.M.; Cain, E.C.; Sommerville, L.; Kuske, C.R. Acidobacteria phylum sequences in uranium-contaminated subsurface sediments greatly expand the known diversity within the phylum. Appl. Environ. Microbiol. 2007, 73, 3113–3116. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Kuske, C.R.; Schmidt, T.M. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria. Appl. Environ. Microbiol. 2011, 77, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, Z.; He, J.; Sun, H.; Zhang, L. Evaluation outcome of actinobacteria diversity in saline environment influenced by different DNA extraction methods. Acta Microbiol. Sin. 2013, 53, 746–757. (In Chinese) [Google Scholar]
- Galachyants, A.D.; Krasnopeev, A.Y.; Podlesnaya, G.V.; Potapov, S.A.; Sukhanova, E.V.; Tikhonova, I.V.; Zimens, E.A.; Kabilov, M.R.; Zhuchenko, N.A.; Gorshkova, A.S.; et al. Diversity of aerobic anoxygenic phototrophs and rhodopsin-containing bacteria in the surface microlayer, water column and epilithic biofilms of Lake Baikal. Microorganisms 2021, 9, 842–863. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wichern, F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol. Biochem. 2008, 40, 2977–2991. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Haugwitz, M.S.; Prieme, A.; Nielsen, C.S.; Elberling, B.; Michelsen, A.; Grogan, P.; Blok, D. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Chang. Biol. 2017, 23, 406–420. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Chang. Biol. 2015, 17, 1637–1645. [Google Scholar] [CrossRef]
- Weber, C.F.; Rytas, V.; Kuske, C.R. Changes in fungal community composition in response to elevated atmospheric CO2 and nitrogen fertilization varies with soil horizon. Front. Microbiol. 2013, 4, 78–92. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhao, B.; Yan, P.; Zhou, G.; Xin, X. Effects of straw amendment and moisture on microbial communities in Chinese fluvo-aquic soil. J. Soils Sediments 2014, 14, 1829–1840. [Google Scholar] [CrossRef]
- He, F.; Yang, B.; Wang, H.; Yan, Q.; Cao, Y.; He, X. Changes in composition and diversity of fungal communities along Quercus Mongolica forests developments in Northeast China. Appl. Soil Ecol. 2016, 100, 162–171. [Google Scholar] [CrossRef]
- Chen, Z.J.; Gao, S.K.; Chen, Y.; He, P.H.; He, Q.; Qiu, Q.; Li, J.Y. Effects of short-term fertilization on soil fungal community structure and functional group in Eucalyptus artificial forest. Acta Ecol. Sin. 2020, 40, 3813–3821. (In Chinese) [Google Scholar]
- Pena, R.; Polle, A. Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J. 2013, 8, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mcguire, K.L.; Zak, D.R.; Edwards, I.P.; Blackwood, C.B.; Upchurch, R. Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest. Oecologia 2010, 164, 785–795. [Google Scholar] [CrossRef] [PubMed]



| Soil Parameter | 0–20 cm | 20–40 cm | ||
|---|---|---|---|---|
| CK | TR | CK | TR | |
| TN (g/kg) | 0.53 ± 0.07 | 0.56 ± 0.03 | 0.34 ± 0.03 | 0.30 ± 0.01 |
| TP (g/kg) | 0.29 ± 0.06 | 0.24 ± 0.03 | 0.30 ± 0.03 | 0.26 ± 0.03 |
| AHN (mg/kg) | 65.26 ± 1.49 | 68.64 ± 2.02 | 53.65 ± 3.21 | 43.99 ± 2.00 |
| AP (mg/kg) | 17.57 ± 0.61 a | 9.52 ± 0.48 b | 13.01 ± 0.29 a | 5.49 ± 0.21 b |
| C:N ratio | 1.77 ± 0.21 | 1.76 ± 0.06 | 2.15 ± 0.14 | 2.31 ± 0.07 |
| TOC (%) | 0.93 ± 0.03 | 0.98 ± 0.05 | 0.71 ± 0.02 | 0.60 ± 0.01 |
| SOM (g/kg) | 17.72 ± 0.48 | 18.51 ± 0.97 | 13.43 ± 0.46 | 11.44 ± 0.27 |
| Soil Depth | Treatments | Chao1 | ACE | Shannon | Simpson | Pielou | |
|---|---|---|---|---|---|---|---|
| Bacterial | 0–20 cm | CK | 876.876 ± 78.848 | 876.851 ± 78.709 | 6.318 ± 0.090 | 0.997 ± 0.000 | 0.933 ± 0.001 |
| TR | 849.704 ± 55.311 | 849.279 ± 54.769 | 6.276 ± 0.059 | 0.997 ± 0.000 | 0.931 ± 0.003 | ||
| 20–40 cm | CK | 875.495 ± 29.869 | 875.690 ± 29.843 | 6.284 ± 0.053 | 0.997 ± 0.000 | 0.928 ± 0.003 | |
| TR | 1017.267 ± 217.043 | 1017.153 ± 216.812 | 6.219 ± 0.360 | 0.995 ± 0.003 | 0.904 ± 0.023 | ||
| Fungi | 0–20 cm | CK | 67.000 ± 16.523 | 66.848 ± 16.440 | 2.395 ± 0.276 | 0.811 ± 0.046 | 0.579 ± 0.037 a |
| TR | 104.489 ± 18.071 | 103.401 ± 18.060 | 1.886 ± 0.322 | 0.709 ± 0.081 | 0.418 ± 0.060 b | ||
| 20–40 cm | CK | 70.292 ± 10.286 | 70.668 ± 10.314 | 2.407 ± 0.373 | 0.807 ± 0.052 | 0.565 ± 0.069 | |
| TR | 112.432 ± 30.579 | 107.190 ± 27.193 | 2.111 ± 0.340 | 0.758 ± 0.054 | 0.466 ± 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Kong, J.; Yang, L.; He, Q.; Su, Y.; Li, J.; Wang, G.; Qiu, Q. Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China. Forests 2022, 13, 37. https://doi.org/10.3390/f13010037
Lin Y, Kong J, Yang L, He Q, Su Y, Li J, Wang G, Qiu Q. Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China. Forests. 2022; 13(1):37. https://doi.org/10.3390/f13010037
Chicago/Turabian StyleLin, Yubiao, Jiejun Kong, Ling Yang, Qian He, Yan Su, Jiyue Li, Guangyu Wang, and Quan Qiu. 2022. "Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China" Forests 13, no. 1: 37. https://doi.org/10.3390/f13010037
APA StyleLin, Y., Kong, J., Yang, L., He, Q., Su, Y., Li, J., Wang, G., & Qiu, Q. (2022). Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China. Forests, 13(1), 37. https://doi.org/10.3390/f13010037








