Evaluation of Aerogel Spheres Derived from Salix psammophila in Removal of Heavy Metal Ions in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
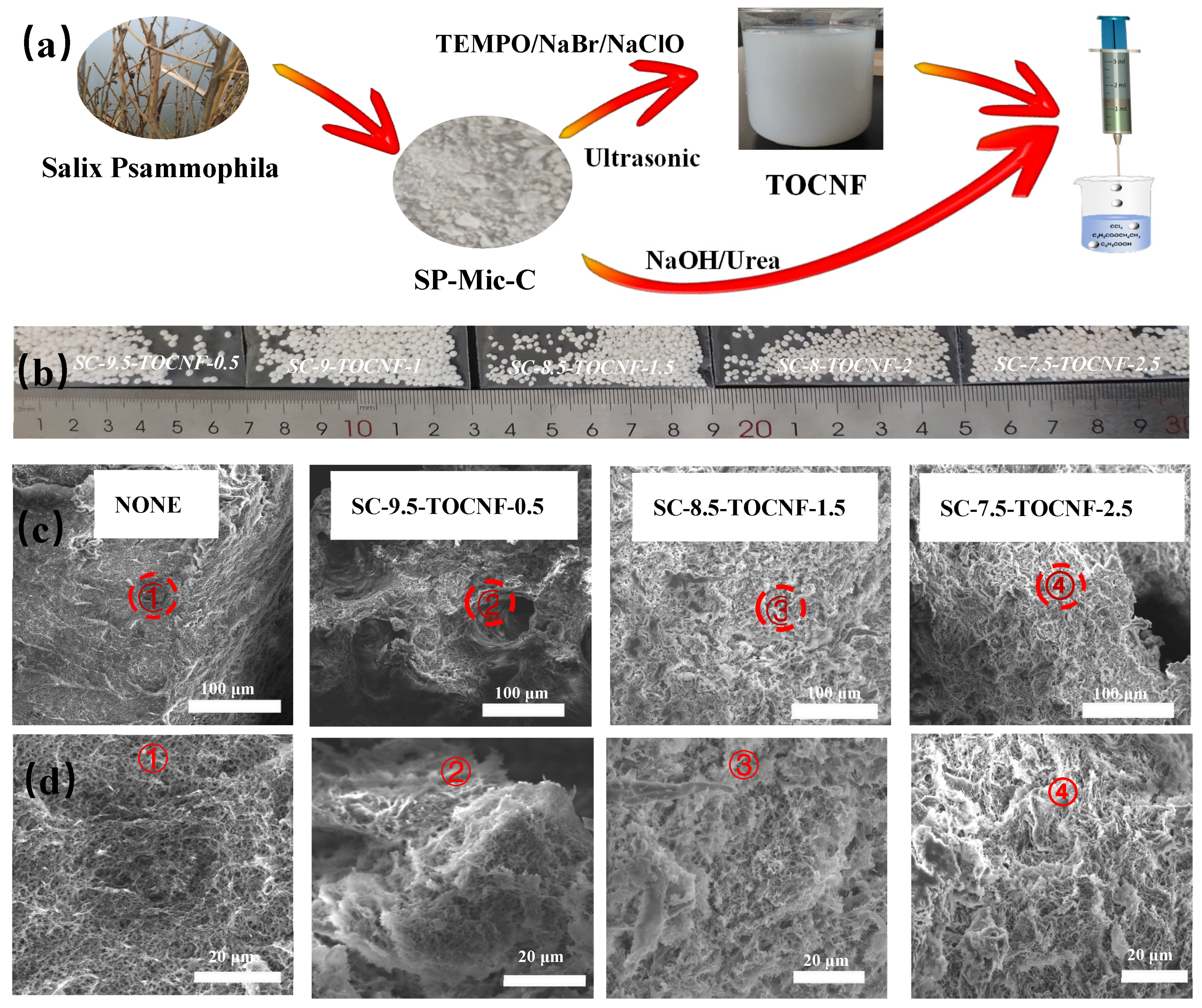
2.2. Preparation of Salix psammophila Microcrystalline Cellulose
2.3. Preparation of Salix psammophila Nanofibers
2.4. Preparation of Salix psammophila Microcrystalline Cellulose/Salix psammophila Nanofiber Aerogel Spheres
2.5. Heavy Metal Adsorption
2.6. Adsorption Kinetics
2.7. Characterization
3. Results and Discussion
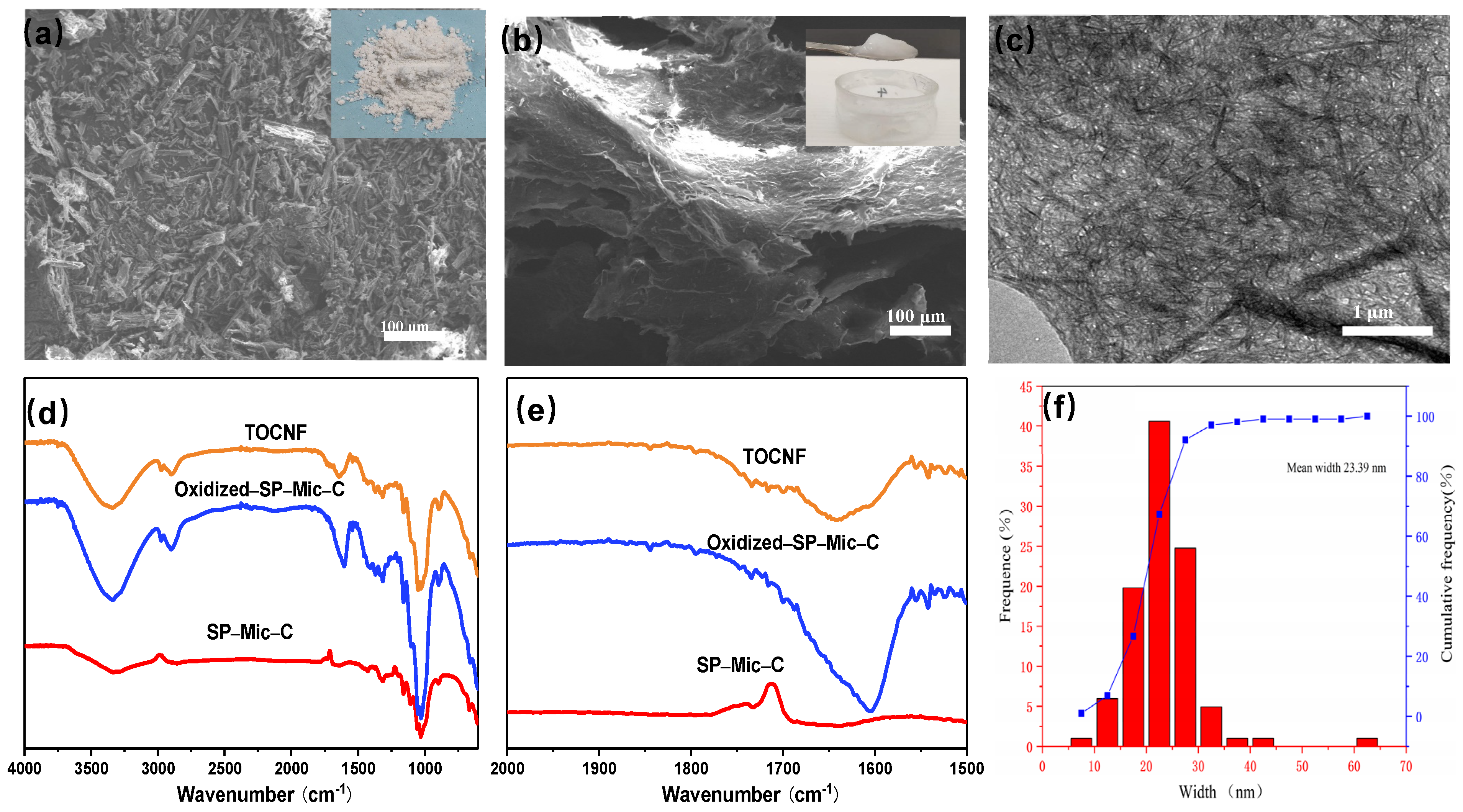
3.1. Characterization of the Salix psammophila Nanofibers
3.2. Characterization of the Aerogel Spheres
3.2.1. Scanning Electron Microscopy (SEM) of Aerogel Spheres
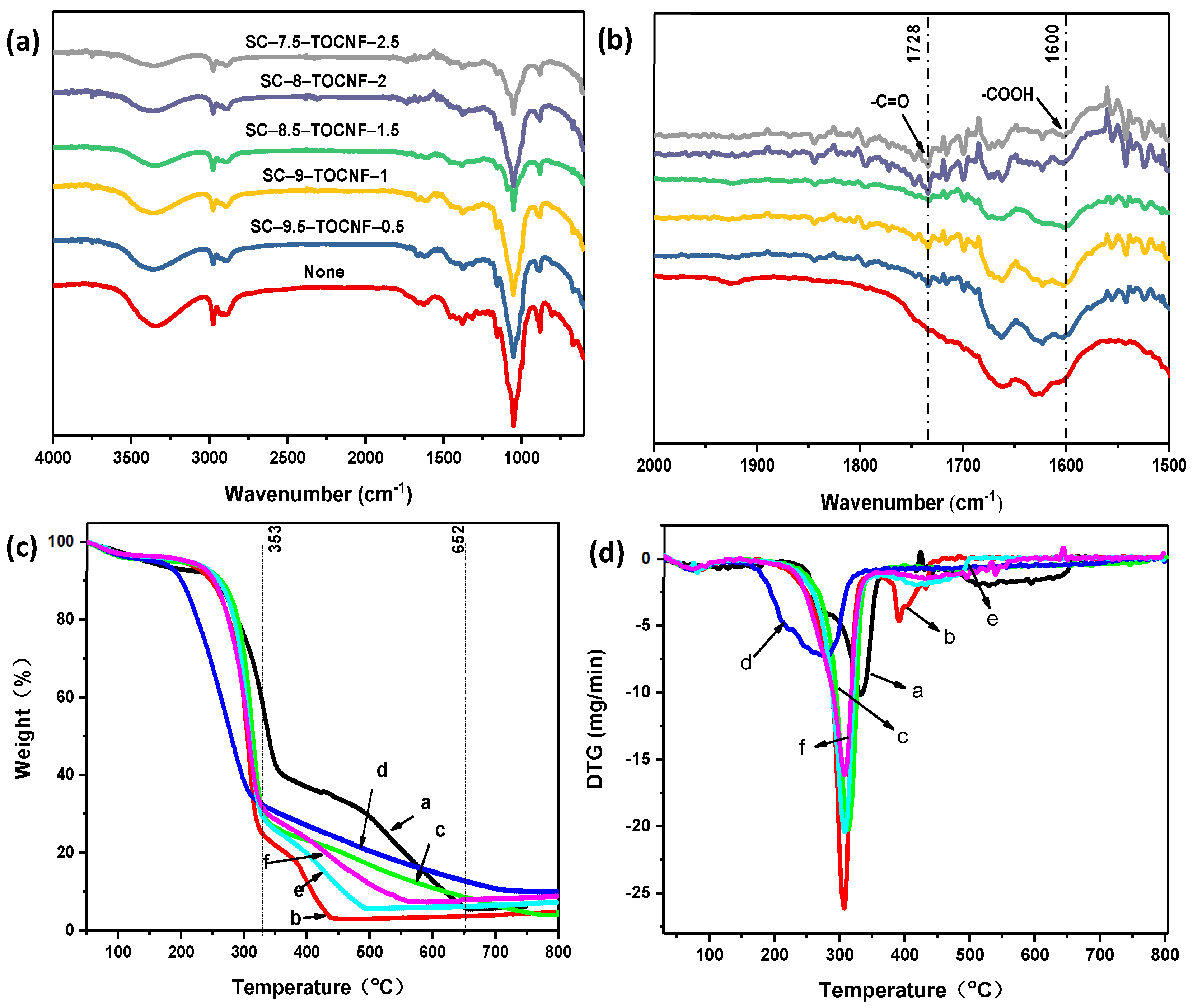
3.2.2. Fourier-Transform Infrared Spectroscopy (FTIR) of Aerogel Spheres
3.2.3. Thermogravimetric Analysis (TGA) of Aerogel Spheres
3.2.4. The N2 Adsorption–Desorption Isotherms (BET)
3.3. Heavy Metal Adsorption
3.3.1. Effect of Initial Heavy Metal Concentration on Adsorption
3.3.2. Effect of Temperature on Adsorption
3.3.3. Effect of pH Values on Adsorption
3.3.4. Effect of Time on Adsorption
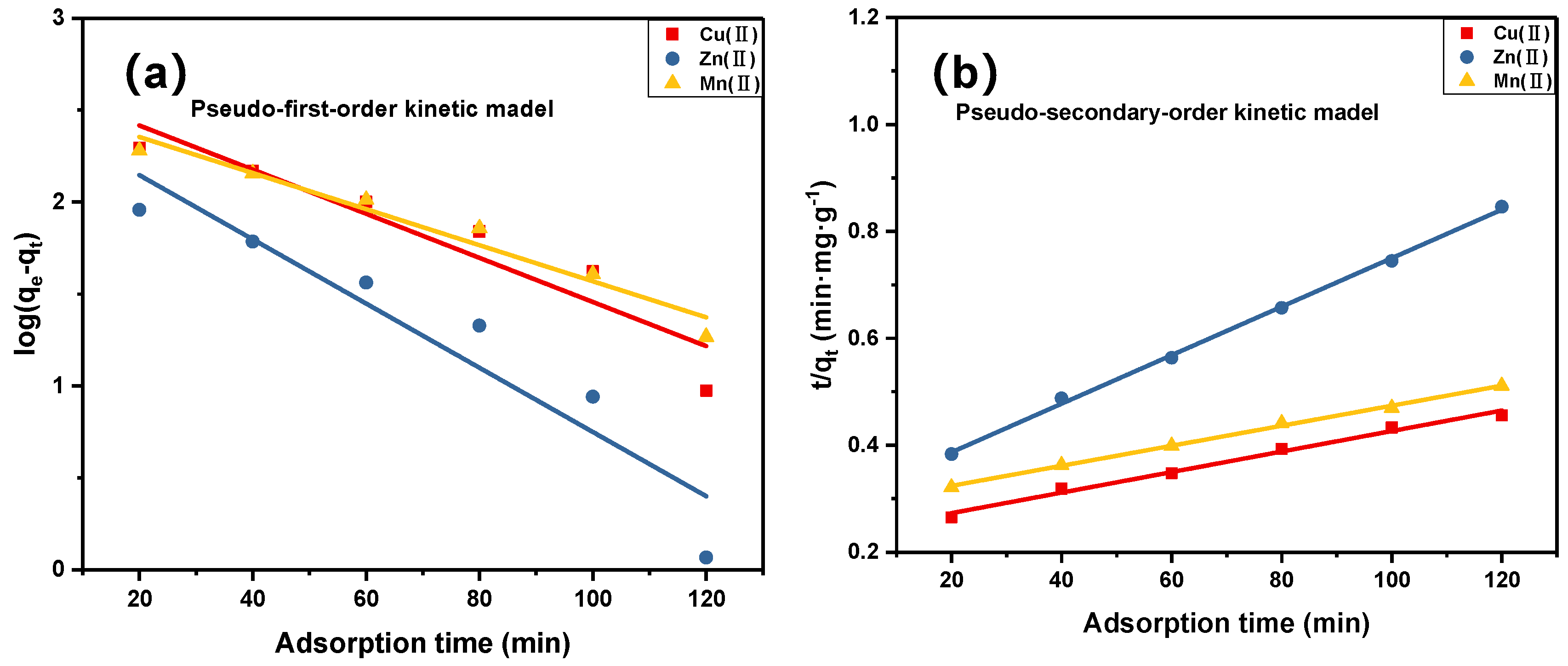
3.4. Kinetic Studies
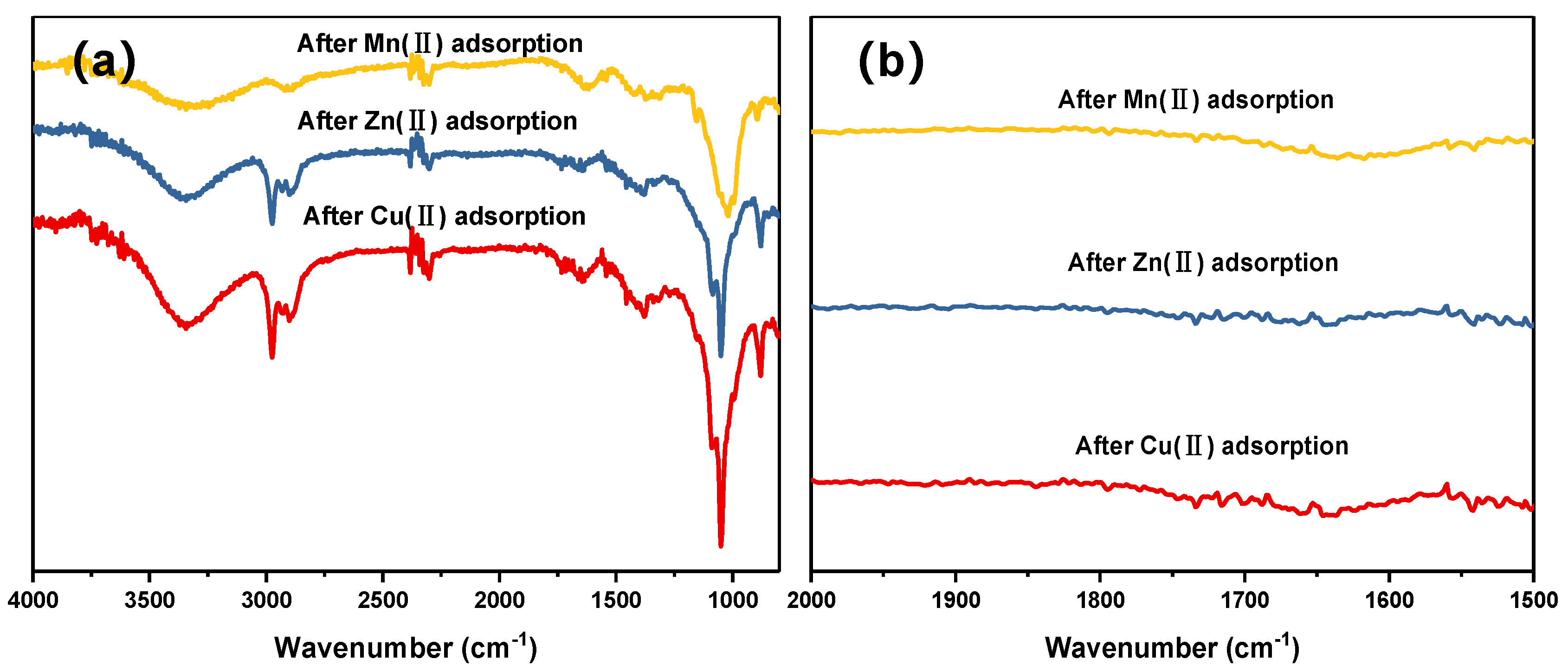
3.5. FTIR following Heavy Metal Adsorption
3.6. Comparison with Previously Reported Data for Cu(II), Mn(II), and Zn(II)
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.F.; Li, Y.A.; Wang, J.Q.; Zhang, G.S.; Wang, S.S.; Zhang, X. Identification of fasciation phenotype and anatomic structure analysis in Salix psammophila. J. Desert Res. 2021, 41, 45–50. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.; Yun, T.; Zha, T.S.; Jia, X.; Bourque, C.P.A.; Wu, Y.J.; Bai, Y.J.; Ma, J.Y.; Zhang, M.Y. Light Energy Partitioning and Photoprotection in an Exotic Species (Salix psammophila) Grown in a Semi-Arid Area of Northwestern China. Forests 2018, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Zhai, Y.; Zhang, G.; Lu, D.Y.; Huang, H.G. Efficient Fingerprinting of the Tetraploid Salix psammophila Using SSR Markers. Forests 2020, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.Y.; Liu, Z.G.; Pei, C.H.; Duan, M.Z.; Liu, P.F. Determination and Analysis on Physical and Mechanical Properties of Salix. China For. Prod. Ind. 2020, 57, 12–14. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Wang, W.L.; Chang, J.M. Pyrolysis Characteristics of Salix Compounds by TG-FTIR. J. Northeast. For. Univ. 2016, 44, 49–52. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, R.; Okita, R.; Akira, I. Thermal stabilization of TEMPO-oxidized cellulose. Polym. Degrad. Stab. 2010, 95, 1502–1508. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Akira, I. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485. [Google Scholar] [CrossRef] [PubMed]
- Jaka, L.; Vladimira, P.S.; Peter, N.; Ida, P.; Primoz, O.; Antti, H. Analyzing TEMPO-Oxidized Cellulose Fiber Morphology: New Insights into Optimization of the Oxidation Process and Nanocellulose Dispersion Quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: Recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.H.; Zhang, X.J.; Zhang, J.L.; Yan, X.X. Influence of Sonomechanical Treatment on the Structure of Cellulose Micro/Nano Fibrils. Key Eng. Mater. 2014, 609–610, 526–530. [Google Scholar] [CrossRef]
- Lee, J.A.; Yoon, M.J.; Lee, E.S.; Lim, D.Y.; Kim, K.Y. Preparation and characterization of cellulose nanofibers (CNFs) from microcrystalline cellulose (MCC) and CNF/polyamide 6 composites. Macromol. Res. 2014, 22, 738–745. [Google Scholar] [CrossRef]
- Jozala, A.F.; Lencastre-Novaes, L.C.; Lopes, A.M.; Santos, V.D.C.; Mazzola, P.G.; Pessoa, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, D.J.; Oporto, G.S.; Mills, R. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Li, Q.; Zhao, J.S.; Wang, W. Study on Formation and Mechanical Properties of Recycled PP/Salix Composites. Plast. Sci. Technol. 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Liu, X.; Wan, Y.; Liu, P.; Zhao, L.; Zou, W.H. Optimization of sulfamethazine sodium adsorption onto activated carbon-based Salix psammophila: Investigation of adsorption behavior and mechanism. J. Dispers. Sci. Technol. 2019, 40, 507–518. [Google Scholar] [CrossRef]
- Wen, J.F. A Study on Preparation of Activated Carbon from Salix psammophila by Different Activators. J. Yulin Univ. 2019, 9–14. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.E.; Wei, F.; Liu, T. Polymer/carbon-based hybrid aerogels: Preparation, properties and applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J. Advances on Dimensional Structure Designs and Functional Applications of Aerogel. Acta Chim. Sin. 2021, 79, 430–442. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.H.; Duan, G.G.; Jiang, S.H.; Zhang, K. Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wu, P. Preparation of Hydrophobic Cellulose Aerogel Beads and Its Adsorption Performance. Chem. Ind. For. Prod. 2018, 38, 9–17. [Google Scholar] [CrossRef]
- Pająk, M.; Gąsiorek, M.; Jasik, M.; Halecki, W.; Otremba, K.; Pietrzykowski, M. Risk Assessment of Potential Food Chain Threats from Edible Wild Mushrooms Collected in Forest Ecosystems with Heavy Metal Pollution in Upper Silesia, Poland. Forests 2020, 11, 1240. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bhattacharjee, S.; Kumar, V.; Singh, A.K. A study on the interdependence of heavy metals while contributing to groundwater pollution index. Environ. Sci. Pollut. Res. 2021, 28, 25798–25807. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Seubsai, A. Removal of heavy metal ions using modified celluloses prepared from pineapple leaf fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef] [PubMed]
- Guiza, S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 2017, 99, 134–140. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, K.B.; Liu, Y.L.; Wang, X. Preparation and characterization of Salix psammophila cellulose and Mic-cellulose under the pretreatment of two kinds of acid. J. Phys. Conf. Ser. 2020, 1605, 012165. [Google Scholar] [CrossRef]
- Zhang, X.T. Study on the Preparation and Adsorption Property of Lignocellulose/layered Silicate Nanocomposite. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2018. [Google Scholar]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nano-cellulose participating as crosslinking aid for alginate-basedsponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Wu, B.; Lu, M.T.; Shao, F.N.; Chen, M.; Xie, L.; Xu, Z.H.; Wen, H.E. Effect of NaClO addition on structure and properties of TEMPO oxidized cellulose nanofibrils. J. For. Eng. 2019, 4, 94–100. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G. One-shot TEMPO-periodate oxidation of native cellulose. Carbohydr. Polym. 2019, 226, 115292. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Rao, J.; Guan, Y.; Li, W.Q.; Zhang, M.C.; Shu, T.; Lv, Z.W. Investigation of the thermo-mechanical properties of blend films based on hemicelluloses and cellulose. Int. J. Polym. Sci. 2018, 2018, 9620346. [Google Scholar] [CrossRef]
- Yeasmin, S.; Yeum, J.H.; Yang, S.B. Fabrication and characterization of pullulan-based nanocomposites reinforced with montmorillonite and TEMPO cellulose nanofibril. Carbohydr. Polym. 2020, 240, 116307. [Google Scholar] [CrossRef] [PubMed]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhong, K.; Liu, W.; Cui, S.; Jiang, S. Preparation and oil adsorption properties of hydrophobic microcrystalline cellulose aerogel. Cellulose 2020, 27, 7663–7675. [Google Scholar] [CrossRef]
- Li, J.F.; Yan, W.J.; Fang, T.; Gu, D.K.; Hu, C.F.; Du, Z.F.; Chen, W. Preparation of C6 carboxylic cellulose and adsorption for Cu2+. Acta Mater. Compos. Sin. 2022, 39, 1–11. [Google Scholar] [CrossRef]
- Jiang, P.P.; Gong, Y.M.; Chang, L.L.; Zhao, T.Y. Modification of chinar cellulose and the adsorption of copper ions. J. Dalian Polytech. Univ. 2020, 39, 365–369. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, R.; Yang, F.; Sun, L.; Li, Y.; Xu, J.Z. Fabrication of polyethylenimine functionalized magnetic cellulose nanofibers for the sorption of Ni (II), Cu (II) and Cd (II) in single-component and multi-component systems. Int. J. Biol. Macromol. 2021, 184, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.M.; Fang, Z.Q.; Zhou, J.; Sun, C.; Qiu, X. Efficient removal of Cu2+ in water by carboxymethylated cellulose nanofibrils: Performance and mechanism. Biomacromolecules 2019, 20, 4466–4475. [Google Scholar] [CrossRef]
- Li, K.C.; Lei, F.H.; Lu, J.F.; Zhou, J.; Zhao, Y. Preparation of Core-shell Magnetic Rosin-based Polymer Microspheres and Their Adsorption Kinetics and Thermodynamics of Mn2+. Chem. Ind. For. Prod. 2018, 38, 87–94. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, J.; Duan, H.; Yin, X.H. Static magnetic field-assisted synthesis of Fe3O4 nanoparticles and their adsorption of Mn (II) in aqueous solution. Chin. J. Chem. Eng. 2017, 25, 32–36. [Google Scholar] [CrossRef]
- Deng, H.; Li, Y.F.; Kang, C.Y.; Li, Q.Y.; Li, S.L.; Li, S.S. Adsorption Characteristics of Cu (II) and Zn (II) in Water on Sulfhydryl Modified Cassava Straw. Water Treat. Technol. 2021, 47, 22–27. [Google Scholar] [CrossRef]
- Zhang, X.T.; Hao, Y.N.; Chen, Z.J.; An, Y.H.; Wang, X.M. Lignocellulose @activated clay nanocomposite with hierarchical nanostructure enhancing the removal of aqueous Zn (II). Polymers 2019, 11, 1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
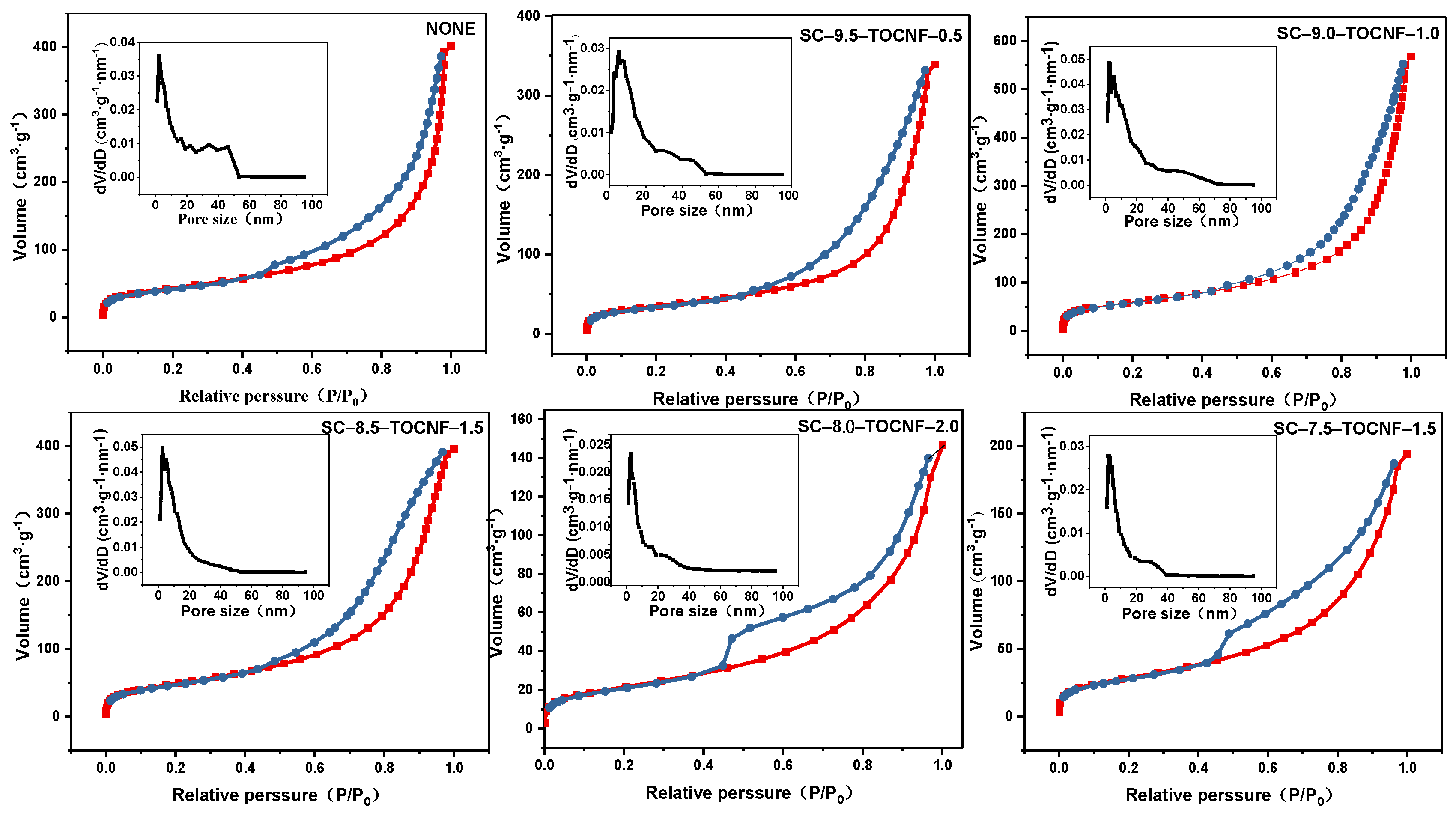

| Sample | BET Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| NONE | 157.42 | 0.8996 | 22.860 |
| SC-9.5-TOCNF-0.5 | 115.90 | 0.5169 | 17.841 |
| SC-9.0-TOCNF-1.0 | 204.84 | 0.8613 | 16.819 |
| SC-8.5-TOCNF-1.5 | 171.76 | 0.6070 | 14.136 |
| SC-8.0-TOCNF-2.0 | 74.142 | 0.2175 | 11.736 |
| SC-7.5-TOCNF-2.5 | 100.11 | 0.2958 | 11.818 |
| Metal | Pseudo-First-Order Kinetic Model | Pseudo-Secondary-Order Kinetic Model | ||
|---|---|---|---|---|
| K1 (min−1) | R2 | K2 (min−1) | R2 | |
| Cu(II) | 0.0636 | 0.8612 | 0.00006 | 0.9895 |
| Zn(II) | 0.0925 | 0.8694 | 0.00017 | 0.9986 |
| Mn(II) | 0.0520 | 0.9484 | 0.00006 | 0.9980 |
| Sample | Cu(II) (mg/g) | Mn(II) (mg/g) | Zn(II) (mg/g) | Author |
|---|---|---|---|---|
| This work | 272.69 | 253.25 | 143.00 | This work |
| C6 carboxylic microcrystalline cellulose | 165.5 0 | --- | --- | Jifeng L [35] |
| Chinar cellulose-graft-poly amidoxime | 84.00 | --- | --- | Panpan J [36] |
| Polyethyleneimine functionalized cellulose nanofiber magnetic composites | 93.71 | ---- | --- | Guo Z [37] |
| Carboxymethylated CNFs | 115.3 | --- | --- | Qin F [38] |
| Core-shell magnetic rosin-based polymer Fe3O4@ RPM microspheres | --- | 45.00 | --- | Kechun L [39] |
| Fe3O4 nanoparticles | --- | 36.81 | --- | Liu Y [40] |
| Sulfhydryl-modified cassava straw | --- | --- | 60.24 | Deng H [41] |
| Lignocellulose@ activated clay nanocomposite | --- | --- | 315.90 | Zhang X [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; An, Y.; Wang, K.; Zhang, W.; Hu, Z.; Chen, Z.; Wang, S.; Wang, B.; Wang, X.; Li, X.; et al. Evaluation of Aerogel Spheres Derived from Salix psammophila in Removal of Heavy Metal Ions in Aqueous Solution. Forests 2022, 13, 61. https://doi.org/10.3390/f13010061
Zhong Y, An Y, Wang K, Zhang W, Hu Z, Chen Z, Wang S, Wang B, Wang X, Li X, et al. Evaluation of Aerogel Spheres Derived from Salix psammophila in Removal of Heavy Metal Ions in Aqueous Solution. Forests. 2022; 13(1):61. https://doi.org/10.3390/f13010061
Chicago/Turabian StyleZhong, Yuan, Yuhong An, Kebing Wang, Wanqi Zhang, Zichu Hu, Zhangjing Chen, Sunguo Wang, Boyun Wang, Xiao Wang, Xinran Li, and et al. 2022. "Evaluation of Aerogel Spheres Derived from Salix psammophila in Removal of Heavy Metal Ions in Aqueous Solution" Forests 13, no. 1: 61. https://doi.org/10.3390/f13010061
APA StyleZhong, Y., An, Y., Wang, K., Zhang, W., Hu, Z., Chen, Z., Wang, S., Wang, B., Wang, X., Li, X., Zhang, X., & Wang, X. (2022). Evaluation of Aerogel Spheres Derived from Salix psammophila in Removal of Heavy Metal Ions in Aqueous Solution. Forests, 13(1), 61. https://doi.org/10.3390/f13010061






