Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Needle Gas Exchange Measurements
2.3. Soil Water Content Measurements
2.4. Needle Relative Water Content Measurements
2.5. Biochemical Assays
2.6. Data Analysis
3. Results
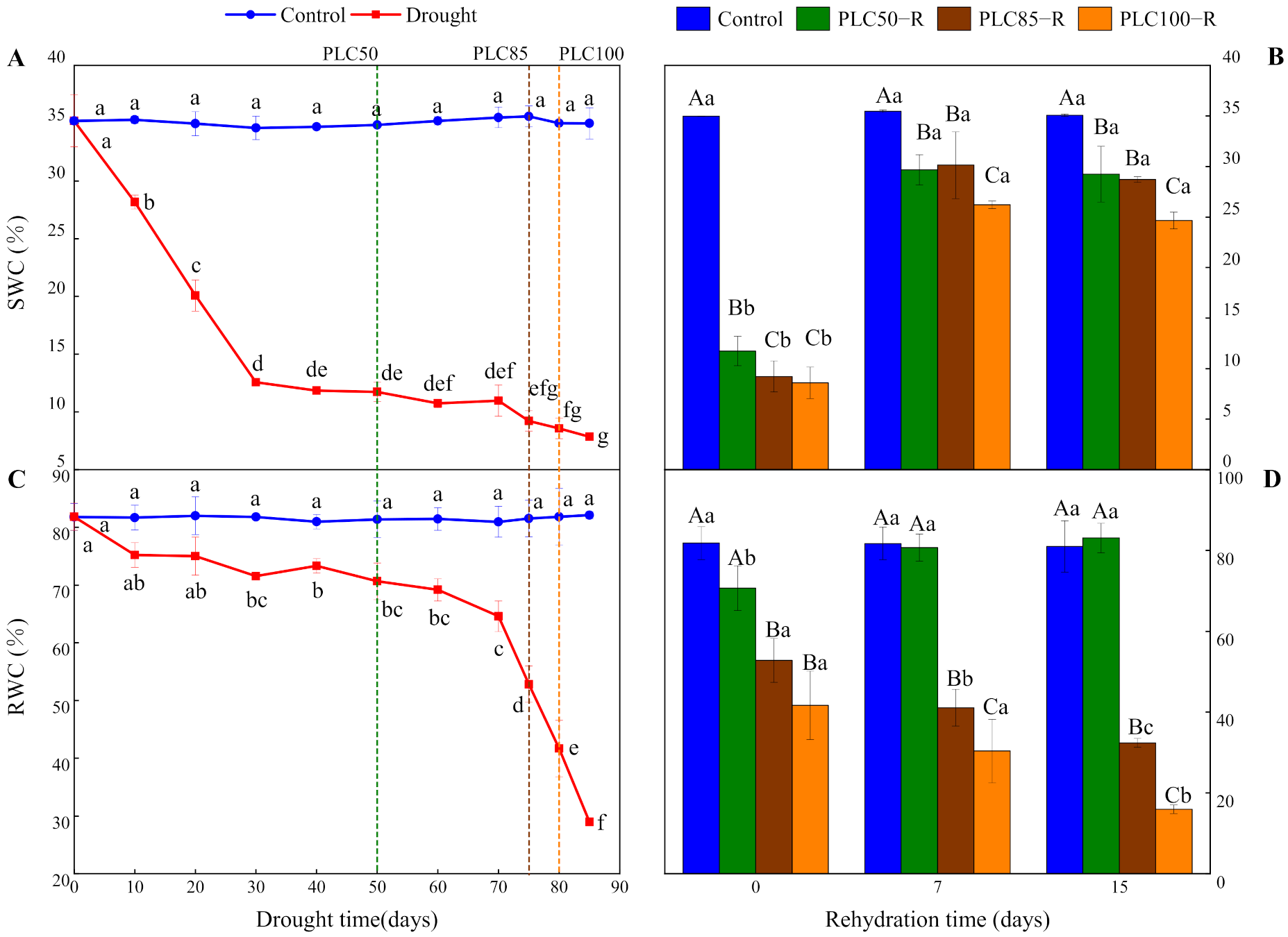
3.1. Soil and Needle Water Status Responses
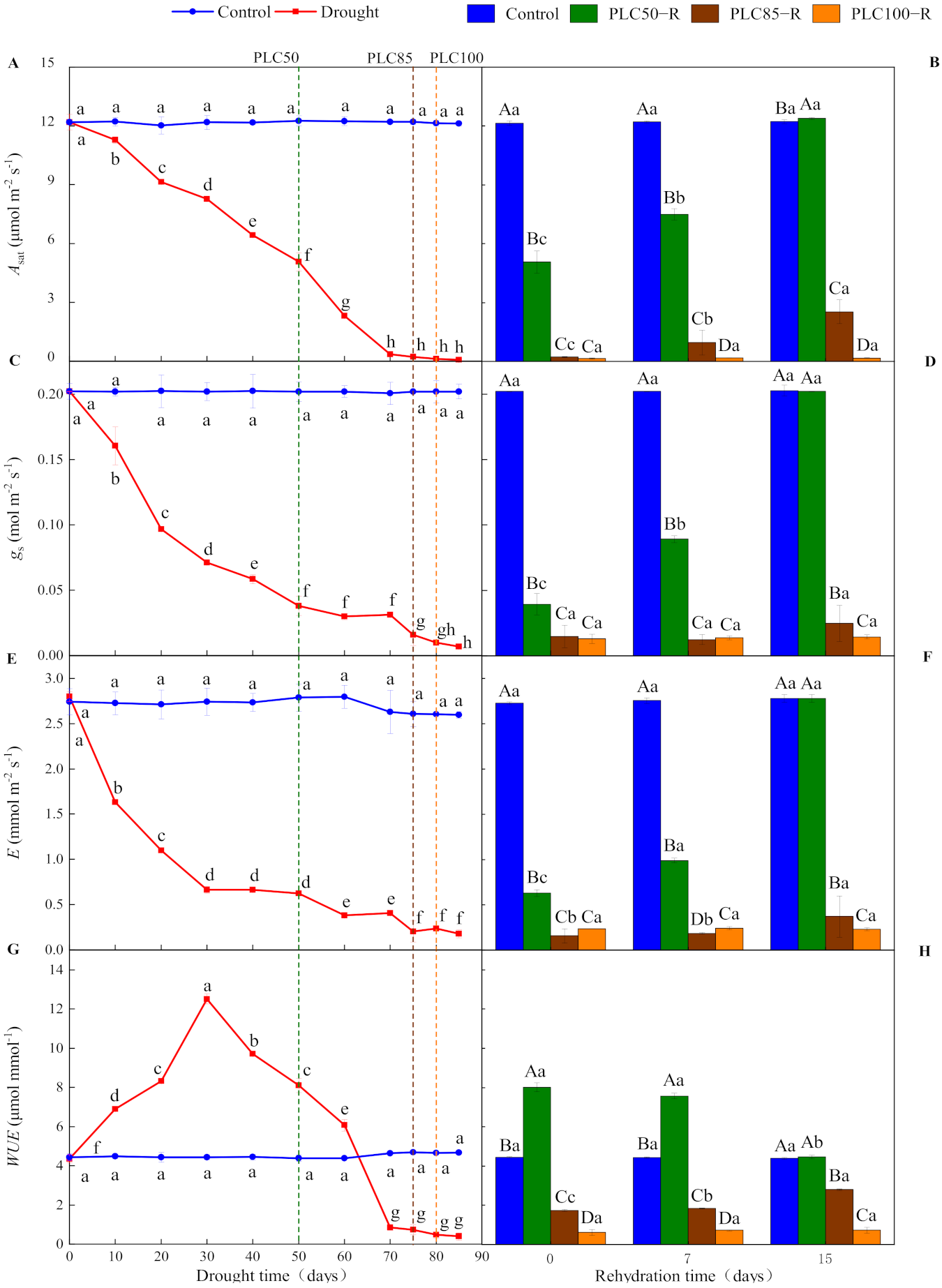
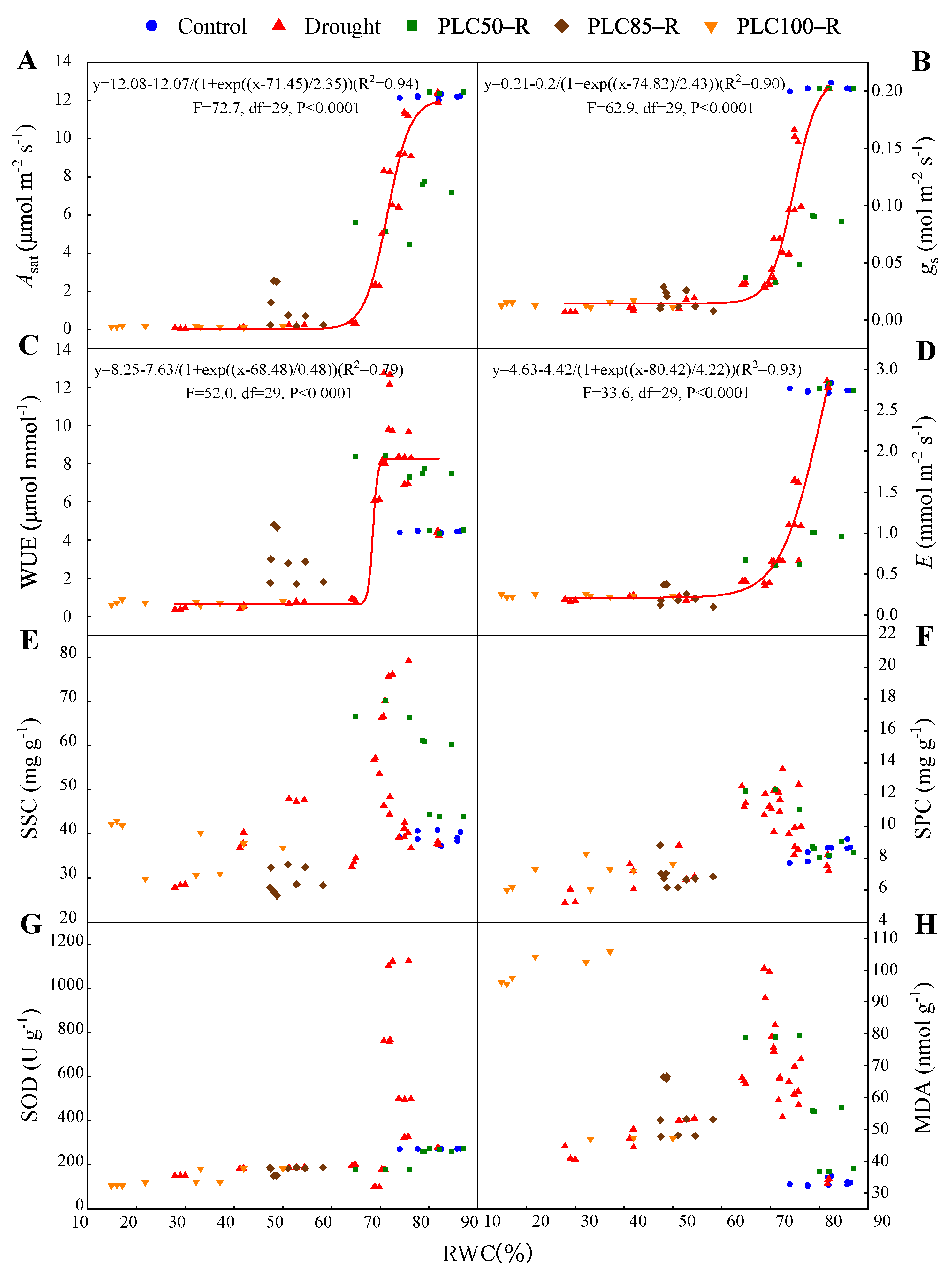
3.2. Gas Exchange Responses
3.3. Soluble Sugar and Soluble Protein Responses
3.4. Malondialdehyde (MDA) Responses
3.5. Superoxide Dismutase Activity (SOD) Responses
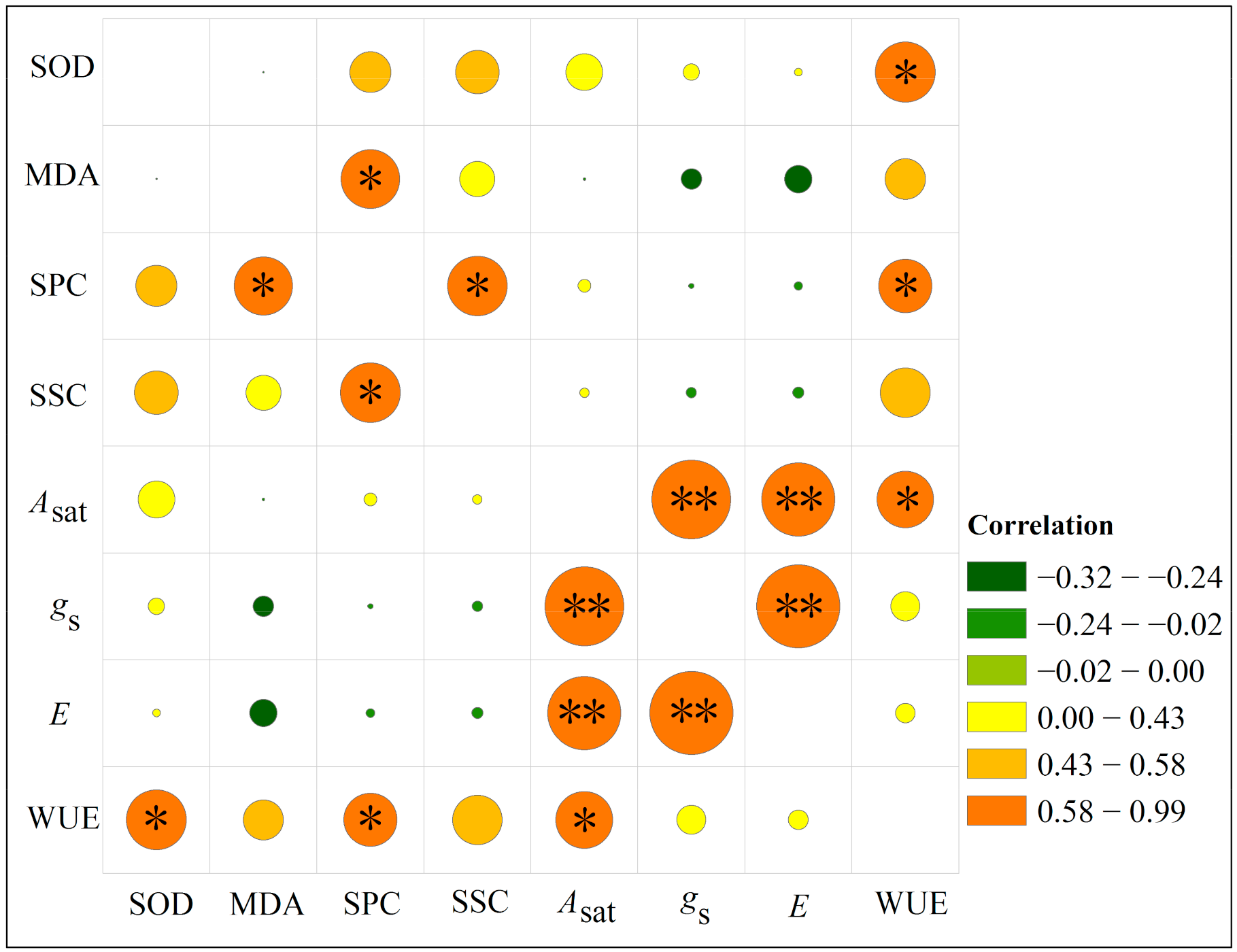
3.6. Correlations among Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucía, D.; Maxime, D.; Frank, C.; Steven, S.; Koen, J.; Tuomas, R.E.M.; Christof, A.M.; Julio, B.; Katarina, C.J.; Gillner, J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Tsamir, M.; Gottlieb, S.; Preisler, Y.; Rotenberg, E.; Tatarinov, F.; Yakir, D.; Tague, C.; Klein, T. Stand density effects on carbon and water fluxes in a semi-arid forest, from leaf to stand–scale. For. Ecol. Manag. 2019, 453, 117573. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, P.; Ouyang, L.; Zhu, L.; Ni, G.; Schafer, K.V.R. Whole-plant water hydraulic integrity to predict drought-induced Eucalyptus urophylla mortality under drought stress. For. Ecol. Manag. 2020, 468, 118179. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Anderegg, W.R.L.; Sapes, G.; Sala, A. Greater focus on water pools may improve our ability to understand and anticipate drought-induced mortality in plants. New Phytol. 2019, 223, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Gaylord, M.L.; Kolb, T.E.; McDowell, N.G. Mechanisms of piñon pine mortality after severe drought: A retrospective study of mature trees. Tree Physiol. 2015, 35, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Wu, L.; Chen, Y.; Gao, M.; Jiang, X.; Li, Q.; Huang, S.; Wang, Y. Response of white oak seedling leaves from different provenances to drought stress and drought resistance evaluation. Chin. J. Ecol. 2020, 39, 3924–3933. [Google Scholar]
- Duan, H.; Wang, D.; Wei, X.; Huang, G.; Fan, H.; Zhou, S.; Wu, J.; Liu, W.; Tissue, D.T.; Wan, S. The decoupling between gas exchange and water potential of Cinnamomum camphora seedlings during drought recovery and its relation to ABA accumulation in leaves. J. Plant Ecol. 2020, 6, 683–692. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, P.; Sun, X.; Hu, X.; Ji, K. Effects of drought on photosynthesis and related physiology of Pinus massoniana seedlings. Chin. Agric. Sci. Bull. 2021, 37, 32–38. [Google Scholar]
- He, F.; Liu, P.; Wang, L.; Du, L.; Qing, J.; Du, Q.; Du, H. Effects of drought stress and rehydration on physiological characteristics of Eucommia ulmoides seedlings. Acta Phytophysiol. 2021, 57, 661–671. [Google Scholar]
- Wang, F.; Zhang, F.; Gou, X.; Fonti, P.; Xia, J.; Cao, Z.; Wang, Y.; Zhang, J. Seasonal variations in leaf-level photosynthesis and water use efficiency of three isohydric to anisohydric conifers on the Tibetan Plateau. Agric. For. Meteorol. 2021, 308–309, 108581. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Ouyang, S.; Gessler, A.; Saurer, M.; Hagedorn, F.; Schnbeck, L. Root carbon and nutrient homeostasis determines downy oak sapling survival and recovery from drought. Tree Physiol. 2021, 41, 1400–1412. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Wang, M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- Ding, G.J.; Zhou, Z.C.; Wang, Z.R. Masson pine Pulpwood Forest Cultivation and Utilization; China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- Yuan, X.; Wang, L.; Wu, P.; Ji, P.; Sheffield, J.; Zhang, M. Anthropogenic shift towards higher risk of flash drought over China. Nat. Commun. 2019, 10, 4661. [Google Scholar] [CrossRef] [Green Version]
- Quan, W.; Ding, G. Changes of volatile substances and endogenous hormones in the needles of Masson pine seedlings under drought stress. For. Sci. 2017, 53, 49–55. [Google Scholar]
- Mo, R.; Ding, G.; Luo, X.; Chen, L. Responses of Pinus massoniana seedlings from different families to persistent drought. J. For. Environ. 2018, 38, 473–480. [Google Scholar]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined Effects of Drought and Shading on Growth and Non-Structural Carbohydrates in Pinus massoniana Lamb. Seedlings. Forests 2019, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Shi, Z.; Xiao, W.; Zeng, L.; Lei, L. Effects of drought and shading on the growth and photosynthetic characteristics of Masson pine seedlings. Acta Ecol. Sin. 2020, 40, 2735–2742. [Google Scholar]
- Zhu, P.; Ma, Y.; Zhu, L.; Chen, Y.; Li, R.; Ji, K. Selection of Suitable Reference Genes in Pinus massoniana Lamb. Under Different Abiotic Stresses for qPCR Normalization. Forests 2019, 10, 632. [Google Scholar] [CrossRef] [Green Version]
- Choat, B.; Brodribb, T.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Li, H. The Principle and Technology of Plant Physiology and Biochemistry Experiment; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Ranjbar, A.; Imani, A.; Piri, S.; Abdoosi, V. Drought effects on photosynthetic parameters, gas exchanges and water use efficiency in almond cultivars on different rootstocks. Plant Physiol. Rep. 2021, 26, 95–108. [Google Scholar] [CrossRef]
- Hasan, M.M.; Gong, L.; Nie, Z.; Li, F.; Ahammed, G.J. ABA-induced stomatal movements in vascular plants during dehydration and rehydration. Environ. Exp. Bot. 2021, 186, 104436. [Google Scholar] [CrossRef]
- Shin-Taro, S.; Yuho, A.; Kenichi, Y.; Hiroyuki, T. Drought Hardening Contributes to the Maintenance of Proportions of Non-Embolized Xylem and Cambium Status during Consecutive Dry Treatment in Container-Grown Seedling of Japanese Cedar (Cryptomeria japonica). Forests 2020, 11, 441. [Google Scholar] [CrossRef]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.K.; McElrone, A.J. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Jifon, J.L.; Santos, C.M.D.; Jadoski, C.J.; Silva, J.A.G. Photosynthetic Capacity and Water Use Efficiency in Sugarcane Genotypes Subject to Water Deficit During Early Growth Phase. Braz. Arch. Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Li, Y.; Xu, Y.; Zhou, S.; Liu, J.; Tissue, D.T.; Liu, J. Contrasting drought sensitivity and post-drought resilience among three co-occurring tree species in subtropical China. Agric. For. Meteorol. 2019, 272, 55–68. [Google Scholar] [CrossRef]
- Jia, Y.; Xiao, W.; Ye, Y.; Wang, X.; Liu, X.; Wang, G.; Li, G.; Wang, Y. Response of Photosynthetic Performance to Drought Duration and Re-Watering in Maize. Agronomy 2020, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhou, G.; He, Q. Critical Leaf Water Content for Maize Photosynthesis under Drought Stress and Its Response to Rewatering. Sustainability 2021, 13, 7218. [Google Scholar] [CrossRef]
- Abdalla, M.; Carminati, A.; Cai, G.; Javaux, M.; Ali, A. Stomatal closure of tomato under drought is driven by an increase in soil-root hydraulic resistance. Plant Cell Environ. 2020, 44, 425–431. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2010, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.A.; Gonzalez, G.S.; Aranda, S.M.N.; Rodriguez, R.M.P.; Venema, K.; Palma, J.M.; Corpas, F.J. Loss of function of the chloroplast membrane K+/H+ antiporters AtKEA1 and AtKEA2 alters the ROS and NO metabolism but promotes drought stress resilience. Plant Physiol. Biochem. 2021, 160, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Sui, X.; Wang, Y.; Jin, G.; Wang, K.; An, S. Effects of drought stress and rehydration on the growth and physiological characteristics of Seriphis yili seedlings. Acta Grassl. 2020, 28, 1216–1225. [Google Scholar]
- Lai, X.; Yan, Y.; Yan, L.; Jiang, L.; Xiang, G. Effects of drought stress on the growth and physiological characteristics of Dalbergia lutea seedlings. J. Northeast For. Univ. 2020, 48, 1–6. [Google Scholar]
- Yang, B.; Peng, C.H.; Zhang, X.; Liu, W.; Duan, M.; Wang, M. Effects of drought stress on leaf nitrogen Concentration, photosynthetic rate and non-structural carbohydrates of Robinia pseudoacacia seedlings. Chin. J. Appl. Environ. Biol. 2019, 25, 1261–1269. [Google Scholar]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Falchi, R.; Petrussa, E.; Braidot, E.; Sivilotti, P.; Boscutti, F.; Vuerich, M.; Calligaro, C.; Filippi, A.; Herrera, J.C.; Sabbatini, P. Analysis of Non-Structural Carbohydrates and Xylem Anatomy of Leaf Petioles Offers New Insights in the Drought Response of Two Grapevine Cultivars. Int. J. Mol. Sci. 2020, 21, 1457. [Google Scholar] [CrossRef] [Green Version]
- Mantova, M.; Herbette, S.; Cochard, H.; Torres-Ruiz, J.M. Hydraulic failure and tree mortality: From corre-lation to causation. Trends Plant Sci. 2021, 9, 1360–1385. [Google Scholar] [CrossRef]
- Morar, I.M.T.; González-Orenga, S.; Boscaiu, M.; Plazas, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E. Responses to Water Deficit and Salt Stress in Silver Fir (Abies alba Mill.) Seedlings. Forests 2020, 11, 395. [Google Scholar]
- Duan, Q.; Tian, Y.; E, X.; Qin, G.; Zhang, J. Gender differences in the growth and physiological characteristics of southern black poplar in response to continuous drought and rehydration. Chin. J. Ecol. 2020, 39, 2140–2150. [Google Scholar]
- Johnson, K.M.; Brodersen, C.R.; Carins-Murphy, M.R.; Choat, B.; Brodribb, T.J.; Timothy, J. Xylem embolism spreads by single-conduit events in three dry forest angiosperm stems. Plant Physiol. 2020, 184, 212–222. [Google Scholar] [CrossRef]
- John, S.S.; David, M.L. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 2015, 207, 14–27. [Google Scholar]
- Liu, J.; Gu, L.; Yu, Y.; Huang, P.; Wu, Z.; Zhang, Q.; Qian, Y.; Wan, X. Corticular photosynthesis drives bark water uptake to refill embolized vessels in dehydrated branches of Salix matsudana. Plant Cell Environ. 2019, 42, 2584–2596. [Google Scholar] [CrossRef]
- Holmlund, H.I.; Pratt, R.B.; Jacobsen, A.L.; Davis, S.D.; Pittermann, J. High-resolution computed tomography reveals dynamics of desiccation and rehydration in fern petioles of a desiccation-tolerant fern. New Phytol. 2019, 224, 97–105. [Google Scholar] [CrossRef]
- Klein, T.; Melanie, J.B.Z.; William, R.L.A.; Bloemen, J.; Martin, G.D.K.; Hudson, P.; Nadine, K.R.; Thomas, L.P.; Arx, G.; Nardini, A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: Processes and trade-offs. Ecol. Res. 2018, 33, 839–855. [Google Scholar] [CrossRef]
- Choat, B. Predicting thresholds of drought-induced mortality in woody plant species. Tree Physiol. 2013, 33, 669–671. [Google Scholar] [CrossRef] [Green Version]
- Hammond, W.M.; Yu, K.; Wilson, L.A.; Will, R.E.; Anderegg, W.R.L.; Adams, H.D. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol. 2019, 223, 1834–1843. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Effects | Drought Stage | Rehydration Stage | ||||
|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | ||
| SWC (%) | Water | 1 | 5589 | <0.0001 | 3 | 608 | <0.0001 |
| Time | 10 | 102 | <0.0001 | 2 | 1123 | <0.0001 | |
| Water × Time | 10 | 102 | <0.0001 | 6 | 123 | <0.0001 | |
| RWC (%) | Water | 1 | 478 | <0.0001 | 3 | 233 | <0.0001 |
| Time | 10 | 37 | <0.0001 | 2 | 9 | 0.0014 | |
| Water × Time | 10 | 38 | <0.0001 | 6 | 9 | <0.0001 | |
| Asat (μmol m−2 s−1) | Water | 1 | 27,315 | <0.0001 | 3 | 5988 | <0.0001 |
| Time | 10 | 1076 | <0.0001 | 2 | 370 | <0.0001 | |
| Water × Time | 10 | 1080 | <0.0001 | 6 | 184 | <0.0001 | |
| gs (mol m−2 s−1) | Water | 1 | 8816 | <0.0001 | 3 | 3911 | <0.0001 |
| Time | 10 | 177 | <0.0001 | 2 | 331 | <0.0001 | |
| Water × Time | 10 | 175 | <0.0001 | 6 | 268 | <0.0001 | |
| E (mmol m−2 s−1) | Water | 1 | 2914 | <0.0001 | 3 | 10,223 | <0.0001 |
| Time | 10 | 50 | <0.0001 | 2 | 974 | <0.0001 | |
| Water × Time | 10 | 43 | <0.0001 | 6 | 727 | <0.0001 | |
| WUE (μmol mmol−1) | Water | 1 | 638 | <0.0001 | 3 | 1585 | <0.0001 |
| Time | 10 | 1412 | <0.0001 | 2 | 34 | <0.0001 | |
| Water × Time | 10 | 1454 | <0.0001 | 6 | 93 | <0.0001 | |
| SSC (mg g−1) | Water | 1 | 1092 | <0.0001 | 3 | 869 | <0.0001 |
| Time | 10 | 299 | <0.0001 | 2 | 291 | <0.0001 | |
| Water × Time | 10 | 301 | <0.0001 | 6 | 183 | <0.0001 | |
| SPC (mg g−1) | Water | 1 | 37,033 | <0.0001 | 3 | 35 | <0.0001 |
| Time | 10 | 25 | <0.0001 | 2 | 14 | <0.0001 | |
| Water × Time | 10 | 23 | <0.0001 | 6 | 7 | <0.0001 | |
| MDA (mmol g−1) | Water | 1 | 2666 | <0.0001 | 3 | 77 | <0.0001 |
| Time | 10 | 99 | <0.0001 | 2 | 4 | 0.04 | |
| Water × Time | 10 | 96 | <0.0001 | 6 | 30 | <0.0001 | |
| SOD (U g−1) | Water | 1 | 11,898 | <0.0001 | 3 | 79,744 | <0.0001 |
| Time | 10 | 14,534 | <0.0001 | 2 | 578 | <0.0001 | |
| Water × Time | 10 | 14,519 | <0.0001 | 6 | 11,147 | <0.0001 | |
| Treatment | PLC50 | PLC50−R | PLC85 | PLC85−R | PLC100 | PLC100−R |
|---|---|---|---|---|---|---|
| Parameter | ||||||
| Asat (μmol m−2 s−1) | −59% *** | +1% | −98% *** | −79% *** | −99% *** | −99% *** |
| gs (mol m−2 s−1) | −81% *** | −0% | −92% *** | −88% *** | −95% *** | −93% *** |
| E (mmol m−2 s−1) | −78% *** | +0% | −93% *** | −87% *** | −93% *** | −92% *** |
| WUE (μmol mmol−1) | +86% *** | +1% | −83% *** | −36% *** | −89% *** | −83% *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Duan, H.; Ding, G.; Luo, X.; Fu, Y.; Lou, Q. Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery. Forests 2022, 13, 65. https://doi.org/10.3390/f13010065
Shao C, Duan H, Ding G, Luo X, Fu Y, Lou Q. Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery. Forests. 2022; 13(1):65. https://doi.org/10.3390/f13010065
Chicago/Turabian StyleShao, Changchang, Honglang Duan, Guijie Ding, Xianying Luo, Yuanhong Fu, and Qing Lou. 2022. "Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery" Forests 13, no. 1: 65. https://doi.org/10.3390/f13010065
APA StyleShao, C., Duan, H., Ding, G., Luo, X., Fu, Y., & Lou, Q. (2022). Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery. Forests, 13(1), 65. https://doi.org/10.3390/f13010065







