Insect Community Response Following Wildfire in an Eastern North American Pine Barrens
Abstract
:1. Introduction
2. Materials and Methods
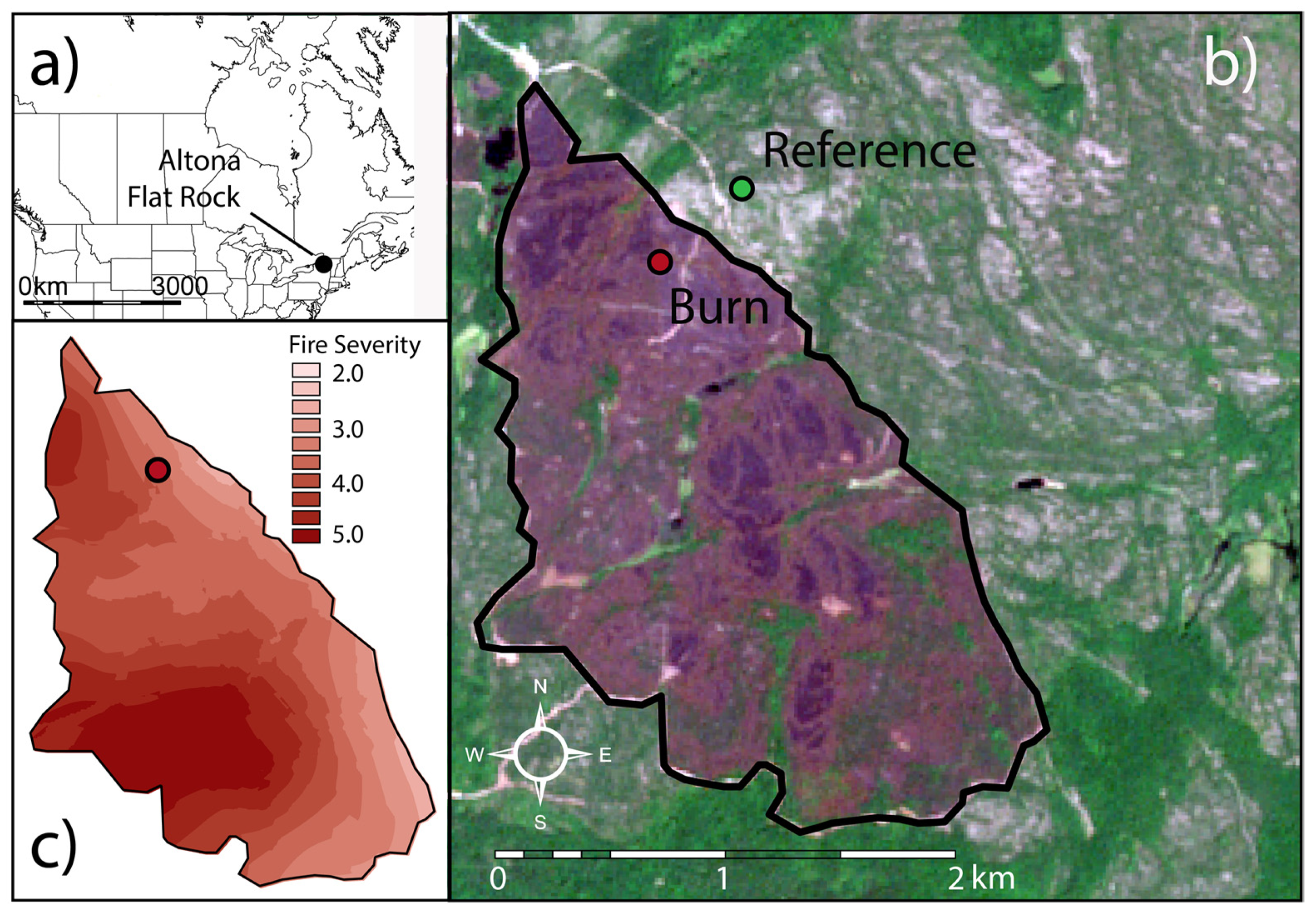
2.1. Study Site
2.2. Sample Collection
2.3. Identification
2.4. Data Analysis
3. Results
3.1. General Patterns in Insect Abundance and Diversity
3.2. Patterns in Insect Community Composition
4. Discussion
4.1. Fire-Adapted Responses
4.2. Fire-Vulnerable Responses
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schowalter, T.D. Insect Responses to Major Landscape-Level Disturbance. Annu. Rev. Entomol. 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Elia, M.; Lafortezza, R.; Tarasco, E.; Colangelo, G.; Sanesi, G. The Spatial and Temporal Effects of Fire on Insect Abundance in Mediterranean Forest Ecosystems. For. Ecol. Manag. 2012, 263, 262–267. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.W.; et al. Changing Disturbance Regimes, Ecological Memory, and Forest Resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Turner, M.G.; Baker, W.L.; Peterson, C.J.; Peet, R.K. Factors Influencing Succession: Lessons from Large, Infrequent Natural Disturbances. Ecosystems 1998, 1, 511–523. [Google Scholar] [CrossRef]
- Lentile, L.B.; Morgan, P.; Hudak, A.T.; Bobbitt, M.J.; Lewis, S.A.; Smith, A.M.S.; Robichaud, P.R.; Morgan, P.; Hudak, A.T.; Bobbitt, M.J.; et al. Post-Fire Burn Severity and Vegetation Response Following Eight Large Wildfires Across the Western United States. Fire Ecol. Spec. Issue 2007, 3, 91–108. [Google Scholar] [CrossRef]
- Connell, J. Diversity in Tropical Rain Forest and Coral Reefs (Disturbio Intermedio). Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Kranz, C.; Whitman, T. Short Communication: Surface Charring from Prescribed Burning Has Minimal Effects on Soil Bacterial Community Composition Two Weeks Post-Fire in Jack Pine Barrens. Appl. Soil Ecol. 2019, 144, 134–138. [Google Scholar] [CrossRef]
- Quigley, K.M.; Kolka, R.; Sturtevant, B.R.; Dickinson, M.B.; Kern, C.C.; Donner, D.M.; Miesel, J.R. Prescribed burn frequency, vegetation cover, and management legacies influence soil fertility: Implications for restoration of imperiled pine barrens habitat. For. Ecol. Manag. 2020, 470, 118163. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Miller, C. The Hidden Consequences of Fire Suppression. Park Sci. 2011, 28, 75–80. [Google Scholar]
- Calkin, D.E.; Thompson, M.P.; Finney, M.A. Negative consequences of positive feedbacks in US wildfire management. For. Ecosyst. 2015, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Buddle, C.M.; Langor, D.W.; Pohl, G.R.; Spence, J.R. Arthropod responses to harvesting and wildfire: Implications for emulation of natural disturbance in forest management. Biol. Conserv. 2006, 128, 346–357. [Google Scholar] [CrossRef]
- Bull, E.L.; Aubry, K.B.; Wales, B.C. Effects of Disturbance on Forest Carnivores of Conservation Concern in Eastern Oregon and Washington. Northwest Sci. 2001, 75, 180–184. [Google Scholar]
- Fisher, J.T.; Wilkinson, L. The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Rev. 2005, 35, 51–81. [Google Scholar] [CrossRef]
- Hood, G.A.; Bayley, S.E.; Olson, W. Effects of prescribed fire on habitat of beaver (Castor canadensis) in Elk Island National Park, Canada. For. Ecol. Manag. 2007, 239, 200–209. [Google Scholar] [CrossRef]
- Lyon, L.J.; Huff, M.H.; Hooper, R.G.; Telfer, E.S.; Schreiner, D.S. Wildland Fire in Ecosystems Effects of Fire on Fauna. USDA-FS Rocky Mountain Research Station; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000; Volume 7. [Google Scholar]
- Komarek, E.V. Fire and Animal Behavior. In Proceedings of the Tall Timbers Fire Ecology Conference 9; Tall Timbers Research Station: Tallahassee, FL, USA, 1969. Available online: https://www.talltimbers.org/wp-content/uploads/2014/03/Komarek1969_op.pdf (accessed on 28 October 2021).
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Johnson, S.D.; Horn, K.C.; Savage, A.M.; Windhager, S.; Simmons, M.T.; Rudgers, J.A. Timing of Prescribed Burns Affects Abundance and Composition of Arthropods in the Texas Hill Country. Southwest Nat. 2008, 53, 137–145. [Google Scholar] [CrossRef]
- Ferrenberg, S.M.; Schwilk, D.W.; Knapp, E.E.; Groth, E.; Keeley, J.E. Fire decreases arthropod abundance but increases diversity: Early and late season prescribed fire effects in a Sierra Nevada mixed-conifer forest. Fire Ecol. 2006, 2, 79–102. [Google Scholar] [CrossRef]
- Christiansen, T.; Lavigne, R. Effects of the 1988 Fires in Yellowstone National Park, USA, on the Ant Populations (Hymenoptera: Formicidae). J. Entomol. Res. Soc. 2010, 12, 29–37. [Google Scholar]
- Ruchin, A.B.; Alekseev, S.K.; Khapugin, A.A. Post-Fire Fauna of Carabid Beetles (Coleoptera, Carabidae) in Forests of the Mordovia State Nature Reserve (Russia). Nat. Conserv. Res. 2019, 4, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Swengel, A.B. A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodivers. Conserv. 2001, 10, 1141–1169. [Google Scholar] [CrossRef]
- Boulanger, Y.; Sirois, L. Postfire Succession of Saproxylic Arthropods, with Emphasis on Coleoptera, in the North Boreal Forest of Quebec. Environ. Èntomol. 2007, 36, 128–141. [Google Scholar] [CrossRef]
- Evans, W.G. Attraction of Insects to Forest Fires. In Tall Timbers Conference on Ecological Animal Control by Habitat Management 3; Tall Timbers Research Station: Tallahasse, FL, USA, 1972; Volume 3, pp. 115–127. [Google Scholar]
- Wikars, L.-O. Dependence on Fire in Wood-living Insects: An Experiment with Burned and Unburned Spruce and Birch Logs. J. Insect Conserv. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Adedoja, O.; Dormann, C.F.; Kehinde, T.; Samways, M.J. Refuges from fire maintain pollinator–plant interaction networks. Ecol. Evol. 2019, 9, 5777–5786. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; York, A.; Christie, F.; McCarthy, M. Effects of fire on pollinators and pollination. J. Appl. Ecol. 2016, 54, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Lazarina, M.; Devalez, J.; Neokosmidis, L.; Sgardelis, S.P.; Kallimanis, A.S.; Tscheulin, T.; Tsalkatis, P.; Kourtidou, M.; Mizerakis, V.; Nakas, G.; et al. Moderate fire severity is best for the diversity of most of the pollinator guilds in Mediterranean pine forests. Ecology 2019, 100, e02615. [Google Scholar] [CrossRef]
- Swengel, A.B.; Swengel, S.R. Benefit of permanent non-fire refugia for Lepidoptera conservation in fire-managed sites. J. Insect Conserv. 2006, 11, 263–279. [Google Scholar] [CrossRef] [Green Version]
- Kwilosz, J.R.; Knutson, R.L. Prescribed Fire Management of Karner Blue Butterfly Habitat at Indiana Dunes National Lakeshore. Nat. Areas J. 1999, 19, 98–108. [Google Scholar]
- York, N.; Gifford, N.A.; O’brien, K. Karner Blue Butterfly Recovery Plan for the Albany Pine Bush Meta-Population Recovery Unit; NYS DEC & USFWS: Albany, NY, USA, 2010; Available online: https://albanypinebush.org/fckimages/uploads/1525371775_App%20D%20ABP%20Karner%20blue%20butterfly%20Recovery%20Plan.pdf (accessed on 28 October 2021).
- Stewart, M.M.; Rossi, J. The Albany Pine Bush: A Northern Outpost for Southern Species of Amphibians and Reptiles in New York. Am. Midl. Nat. 1981, 106, 282. [Google Scholar] [CrossRef]
- Gifford, N.A.; Deppen, J.M.; Bried, J.T. Importance of an urban pine barrens for the conservation of early-successional shrubland birds. Landsc. Urban Plan. 2010, 94, 54–62. [Google Scholar] [CrossRef]
- Cave, H.; Adams, M.; Jaeger, T.; Peet, T.; Staats, L.; Garneau, D.; Lesser, M. Wildlife Response to Wildfire in a Northern New York Jack Pine Barrens. Forests 2021, 12, 676. [Google Scholar] [CrossRef]
- Meigs, G.W.; Zald, H.; Campbell, J.L.; Keeton, W.S.; E Kennedy, R. Do insect outbreaks reduce the severity of subsequent forest fires? Environ. Res. Lett. 2016, 11, 045008. [Google Scholar] [CrossRef]
- Meigs, G.W.; Campbell, J.L.; Zald, H.; Bailey, J.D.; Shaw, D.C.; Kennedy, R.E. Does wildfire likelihood increase following insect outbreaks in conifer forests? Ecosphere 2015, 6, 1–24. [Google Scholar] [CrossRef]
- McCullough, D.G. A review of factors affecting the population dynamics of jack pine budworm (Choristoneura pinus pinus Freeman). Popul. Ecol. 2000, 42, 243–256. [Google Scholar] [CrossRef]
- Nealis, V.G.; Magnussen, S.; Hopkin, A.A. A lagged, density-dependent relationship between jack pine budworm Choristoneura pinus pinusand its host tree Pinusbanksiana. Ecol. Èntomol. 2003, 28, 183–192. [Google Scholar] [CrossRef]
- Tucker, E.; Rehan, S.M. Wild Bees (Hymenoptera: Apoidea) of the Ossipee Pine Barrens. Northeast Nat. 2019, 26, 379–391. [Google Scholar] [CrossRef]
- Mihuc, T.B.; Minshall, G.W. Trophic Generalists vs. Trophic Specialists: Implications for Food Web Dynamics in Post-Fire Streams. Ecology 1995, 76, 2361–2372. [Google Scholar] [CrossRef]
- Malison, R.; Baxter, C.V. Effects of wildfire of varying severity on benthic stream insect assemblages and emergence. J. North Am. Benthol. Soc. 2010, 29, 1324–1338. [Google Scholar] [CrossRef]
- Siemann, E. Experimental Tests of Effects of Plant Productivity and Diversity on Grassland Arthropod Diversity. Ecology 1998, 79, 2057–2070. [Google Scholar] [CrossRef]
- Pausas, J.G.; Belliure, J.; Mínguez, E.; Montagud, S. Fire benefits flower beetles in a Mediterranean ecosystem. PLoS ONE 2018, 13, e0198951. [Google Scholar] [CrossRef]
- McCullough, D.G.; Kulman, H.M. Differences in foliage quality of young jack pine (Pinus banksiana Lamb.) on burned and clearcut sites: Effects on jack pine budworm (Choristoneura pinus pinus Freeman). Oecologia 1991, 87, 135–145. [Google Scholar] [CrossRef]
- Dharmawan, A.; Sueb; Wedhanto, S.; Purwanto; Suhadi; Nafiah, K.; Kaffah, S. Impact of Savanna Fires on Soil Insect Communities in Baluran National Park. In AIP Conference Proceedings; AIP Publishing LLC, Melville, NY, USA, Volume 2120.
- Siemann, E.; Haarstad, J.; Tilman, D. Short-term and Long-term Effects of Burning on Oak Savanna Arthropods. Am. Midl. Nat. 1997, 137, 349. [Google Scholar] [CrossRef]
- Murphy, S.M.; Richards, L.A.; Wimp, G.M. Editorial: Arthropod Interactions and Responses to Disturbance in a Changing World. Front. Ecol. Evol. 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, P.; Barton, P.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Persson, T. Recovery of soil macrofauna after wildfires in boreal forests. Soil Biol. Biochem. 2013, 57, 182–191. [Google Scholar] [CrossRef]
- Franzi, D.A.; Adams, K.B. The Altona Flat Rock Jack Pine Barrens: A Legacy of Fire and Ice. Vt. Geol. Soc. 1993, 7, 43–61. [Google Scholar]
- Reschke, C. Barrens and Woodlands: Sandstone pavement pine barrens. In Ecological Communities of New York State, 2nd ed.; Edinger, G.J., Evans, D.J., Gebauer, S., Howard, T.G., Hunt, D.M., Olivero, A.M., Eds.; New York Natural Heritage Program, N.Y.S. Department of Environmental Conservation: Albany, NY, USA, 1990; p. 102. [Google Scholar]
- McCabe, T.L. Insect Biodiversity of a Jack Pine Barrens; A Report Prepared for the Biodiversity Research Institute: Albany, NY, USA, 2004; Available online: https://guides.nynhp.org/jack-pine-looper/ (accessed on 28 October 2021).
- Stanton, E.J. Report on 1995 Lepidoptera Inventory in Clinton County, New York Jack Pine Pavement Barrens; SUNY ESF: Syracuse, NY, USA, 1996. [Google Scholar]
- Marshall, S.A. Insects: Their Natural History and Diversity. In With a Photographic Guide to Insects of Eastern North America, 4th ed.; Firefly Books: Buffalo, NY, USA, 2006. [Google Scholar]
- Murray, T. Insects of New England and New York, 1st ed.; Kollath + Stensaas: Duluth, MN, USA, 2012. [Google Scholar]
- Marshall, S.A. The Natural History and Diversity of Diptera, 1st ed.; Firefly Books: Buffalo, NY, USA, 2012. [Google Scholar]
- Jewiss-Gaines, A.; Marshall, S.A.; Whitworth, T.L. Cluster Flies (Calliphoridae: Polleniinae: Pollenia) of North America. Can. J. Arthropod Identif. 2012, 19, 1–19. [Google Scholar] [CrossRef]
- Stireman, J.O.; O’Hara, J.E.; Wood, D.M. TACHINIDAE: Evolution, Behavior, and Ecology. Annu. Rev. Èntomol. 2006, 51, 525–555. [Google Scholar] [CrossRef] [Green Version]
- Borror, D.J.; White, R.E. A Field Guide to Insects. America North of Mexico. In Houghton Mifflin; Houghton Mifflin Harcourt: Boston, MA, USA, 1998. [Google Scholar]
- Sabrosky, C.W. The Chloropidae of Kansas (Diptera). Trans. Am. Entomol. Soc. 1935, 61, 207–268. [Google Scholar]
- Ross, H. Arnett American Insects: A Handbook of the Insects of America North of Mexico, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Pollet, M.A.; Brooks, S.E.; Cumming, J.M. Catalog of the Dolichopodidae (Diptera) of America North of Mexico. Bull. Am. Mus. Nat. Hist. 2004, 283, 1–114. [Google Scholar] [CrossRef]









| Site Data | Reference | Burn |
|---|---|---|
| Fire severity | 1 | 3 |
| Soil organic matter | 32% | 18.534% |
| Soil moisture | 27.258% | 11.77% |
| Canopy cover | 0.16 | 0 |
| Number of samples | 2018: 4 (biweekly) | 2018: 4 (biweekly) |
| 2019: 4 (biweekly) | 2019: 4 (biweekly) | |
| 2020: 3 (triweekly) | 2020: 3 (triweekly) | |
| Total abundance | 13,860 | 5879 |
| Richness | Mean: 23.64 ± 5.464 | Mean: 22.64 ± 3.931 |
| Maximum: 29 | Maximum: 29 | |
| (August–September 2020) | (June–July 2019) | |
| Mean Sørensen’s (all years) | 0.599 ± 0.049 | --- |
| Common Name | Taxon | Post-Fire Response | Ecological Traits (Environmental Niche Dimensions, Life History Traits, Habitat/Food Preferences) |
|---|---|---|---|
| Leafmining flies | Diptera (Agromyzidae) | + | Phytophagous; oviparous; requirements: vegetation (host plant specific) |
| Root-maggot flies | Diptera (Anthomyiidae) | + | Pollinator, phytophagous, some parasitic; oviparous; requirements: moist vegetated/wooded habitat and sometimes open habitat [60] |
| Robber/Assassin flies | Diptera (Asilidae) | + | Predatory; oviparous; requirements: prey species, dry open habitat; Perching behaviour require vegetation or elevated substrates, decaying matter (larval prey species) [57] |
| Frit/Grass flies | Diptera (Chloropidae) | + | Varied feeding based on species, grass feeding, stem mining, saprophagous, scavenger, or parasitic; oviparous; requirments: low-lying vegetation, open habitat [61] |
| Vinegar/fruit flies | Diptera (Drosophilidae) | + | Saprophageous, fungivore, pollinator; oviparous; requirements: adult and larval food source (typically decaying fruit) [57] |
| Dance flies | Diptera (Empididae) | + | Predatory, pollinator, saprophagous; oviparous; requirements: prey species, decaying matter, moisture [62] |
| House flies | Diptera (Muscidae) | + | Saprophagous; oviparous; requirements: adult and larval food source [57] |
| Cluster flies | Diptera (Polleniidae) | + | Pollinator, parasitic (earthworms, some sp. parasitize caterpillars and bees); oviparous; prefer warm, open habitat [58] |
| Beetles | Coleoptera | + | Pollinator, xylophageous, predatory, etc.; oviparous; varied requirements [55] |
| True Bugs | Hemiptera | + | Phytophageous; oviparous; requirements: vegetation [55] |
| Grasshoppers and Katydids | Orthoptera | + | Phytophagous; oviparous; requirements: vegetation, open habitat [55] |
| Lance flies | Diptera (Lonchaeidae) | ~ | Oviparous; feed on decaying plant material [57] |
| Flesh flies | Diptera (Sarcophagidae) | ~ | Parasitic; viviparous [57] |
| Flower/Hover flies | Diptera (Syrphidae) | ~ | Pollinator, predatory, saprophagous; oviparous; requirements: flowering vegetation, larval food source; open habitat [57] |
| Parasitic flies | Diptera (Tachinidae) | ~ | koinobiont parasitoid (caterpillars common); pollinator, saprophagous; oviparous; requirements: adult and larval food source, open habitat for mating [59] |
| Bee flies | Diptera (Bombyliidae) | − | Pollinator and parasitic (bees); oviparous; requirements: host species and flowering vegetation [57] |
| Long-legged flies | Diptera (Dolichopodidae) | − | Predatory; oviparous; requirements: prey species, vegetation, moisture [57,63] |
| Scuttle flies | Diptera (Phoridae) | − | Saprophagous, fungivorous; oviparous; requirements: moisture, organic matter [57] |
| Big-eye flies | Diptera (Pipunculidae) | − | Parasitic (Hemiptera); oviparous; requirements: host species [57] |
| Wasps, bees, ants, sawflies | Hymenoptera | − | Pollinator (bees and wasps), parasitic (wasps), predatory (wasps), generalists (wasps and ants); oviparous; requirements: nesting habitat, adult and larval food sources [55] |
| Moths and butterflies | Lepidoptera | − | Pollinator, phytophagous; oviparpous; requirements: adult and larval food sources, relatively open habitat [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, H.M.; Lesser, M.R.; Myers, L.; Mihuc, T.B. Insect Community Response Following Wildfire in an Eastern North American Pine Barrens. Forests 2022, 13, 66. https://doi.org/10.3390/f13010066
Thompson HM, Lesser MR, Myers L, Mihuc TB. Insect Community Response Following Wildfire in an Eastern North American Pine Barrens. Forests. 2022; 13(1):66. https://doi.org/10.3390/f13010066
Chicago/Turabian StyleThompson, Heather M., Mark R. Lesser, Luke Myers, and Timothy B. Mihuc. 2022. "Insect Community Response Following Wildfire in an Eastern North American Pine Barrens" Forests 13, no. 1: 66. https://doi.org/10.3390/f13010066
APA StyleThompson, H. M., Lesser, M. R., Myers, L., & Mihuc, T. B. (2022). Insect Community Response Following Wildfire in an Eastern North American Pine Barrens. Forests, 13(1), 66. https://doi.org/10.3390/f13010066







