The Use of Bryophytes, Lichens and Bromeliads for Evaluating Air and Water Pollution in an Andean City
Abstract
:1. Introduction
2. Materials and Methods
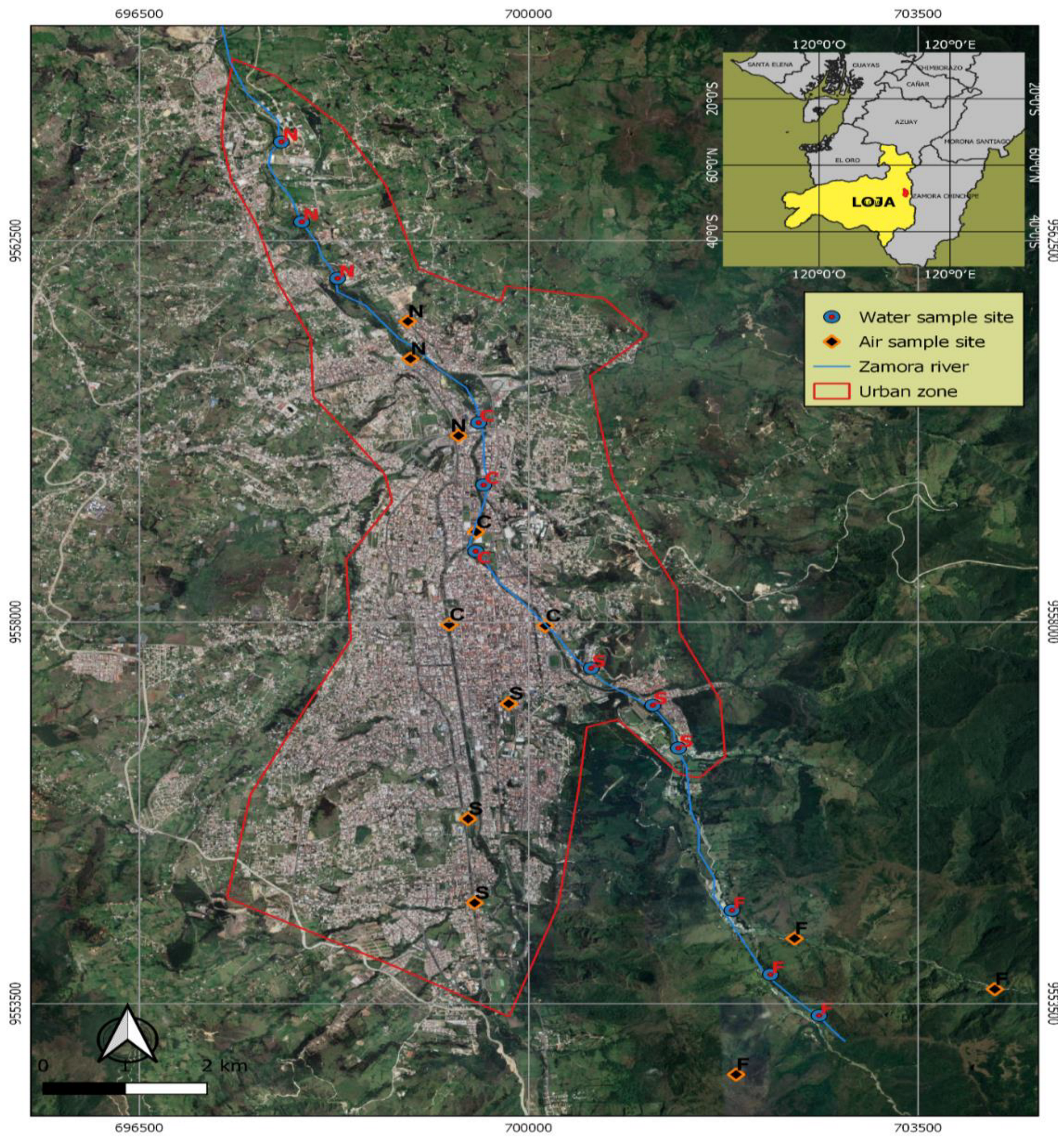
2.1. Study Area
2.2. Design and Data Collection
2.3. Chemical Analysis of Metals and Metalloid
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme. Perspectivas del Medio Ambiente Urbano-GEO Loja; Programa de las Naciones Unidas para el Medio Ambiente: Loja, Ecuador, 2007. [Google Scholar]
- Lijteroff, R.; Lima, L.; Prieri, B. Uso de líquenes como bioindicadores de contaminación atmosférica en la ciudad de San Luis, Argentina. Rev. Int. Contam. Ambient. 2009, 25, 111–120. [Google Scholar]
- Ștefanuț, S.; Ollerer, K.; Ion, M.; Florescu, L.; Constantin, M.; Banciu, C.; Onete, M.; Manu, M.; Vicol, L.; Moldoveanu, M.; et al. Country-scale complementary passive and active biomonitoring of airborne trace elements for environmental risk assessment. Ecol. Indic. 2021, 126, 107357. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Rodrigues, N.; D’Souza, R.; Varun, M.; Paul, M.S. Metal(loid) accumulation in aquatic plants of a mining area: Potential for water quality biomonitoring and biogeochemical prospecting. Chemosphere 2018, 194, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, M.W.; Din, L.; Zakaria, Z.; Latip, J.; Lihan, T.; Jemain, A.A.; Samsudin, F. Measuring Air Quality using Lichen Mapping at Universiti Kebangsaan Malaysia (UKM) Campus. Procedia Soc. Behav. Sci. 2012, 59, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Muotka, T.; Laasonen, P. Ecosystem recovery in restored headwater streams: The role of enhanced leaf retention. J. Appl. Ecol. 2002, 39, 145–156. [Google Scholar] [CrossRef]
- Anze, R.; Franken, M.; Zaballa, M.; Pinto, M.R.; Zeballos, G. Bioindicadores en la detección de la contaminación atmosférica en Bolivia. Rev. Virtual REDESMA 2007, 1, 53–74. [Google Scholar]
- Schweitzer, L.; James, N. Water Contamination and Pollution. In Green Chem; Chapter 3.6; Springer: Berlin/Heidelberg, Germany, 2018; pp. 261–290. [Google Scholar] [CrossRef]
- Berend, N. Contribution of air pollution to COPD and small airway dysfunction. Respirology 2016, 22, 237–244. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Climate Change and Health. 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 11 January 2022).
- Balti, E.V.; Echouffo-Tcheugui, J.B.; Yako, Y.Y.; Kengne, A.P. Air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 161–172. [Google Scholar] [CrossRef]
- Calle, G.P.; Gómez, J.C. Índices de calidad del agua de fuentes superficiales y aspectos toxicológicos, evaluación del Río Burgay. Maskana 2014, 5, 165–176. [Google Scholar]
- Gecheva, G.; Yurukova, L. Water pollutant monitoring with aquatic bryophytes: A review. Environ. Chem. Lett. 2014, 12, 49–61. [Google Scholar] [CrossRef]
- World Health Organization. Water. World Health Organization. (WHO/HSE/PHE/AMR/08.01.01); World Health Organization: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/handle/10665/336876 (accessed on 1 January 2022).
- Giampaoli, P.; Tavares, A.R.; Domingos, M. Physiological responses of two different epiphytic bromeliads exposed in a polluted subtropical region in southeast Brazil characterized by seasonal climate. Ecol. Indic. 2021, 120, 106945. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Lipski, B.; Da Silva Santos, J.J.; Piazzetta, K.D.; De Melo Rodrigues, J.; Leal, L. Atmospheric Pollutants and the Occurrence of Bromeliads in Electric Power Distribution Network. Int. J. Environ. Pollut. Remediat. 2017, 6, 32–40. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Štork, F.; Hedbavny, J. Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application. J. Hazard. Mater. 2012, 239–240, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Benítez, Á.; Armijos, L.; Calva, J. Monitoring Air Quality with Transplanted Bryophytes in a Neotropical Andean City. Life 2021, 11, 821. [Google Scholar] [CrossRef]
- Ecke, F. The added value of bryophytes and macroalgae in ecological assessment of lakes. Ecol. Indic. 2018, 85, 487–492. [Google Scholar] [CrossRef]
- Debén, S.; Aboal, J.R.; Carballeira, A.; Cesa, M.; Fernández, J.A. Monitoring river water quality with transplanted bryophytes: A methodological review. Ecol. Indic. 2017, 81, 461–470. [Google Scholar] [CrossRef]
- Debén, S.; Aboal, J.R.; Carballeira, A.; Cesa, M.; Real, C.; Fernández, J.A. Inland water quality monitoring with native bryophytes: A methodological review. Ecol. Indic. 2015, 53, 115–124. [Google Scholar] [CrossRef]
- Burton, M.A.S. Terrestrial and aquatic bryophytes as monitors of environmental contaminants in urban and industrial habitats. Bot. J. Linn. Soc. 1990, 104, 267–280. [Google Scholar] [CrossRef]
- Abas, A.; Mazlan, S.M.; Latif, M.T.; Aiyub, K.; Muhammad, N.; Nadzir, M.S.M. Lichens reveal the quality of indoor air in Selangor, Malaysia. Ecol. Process. 2021, 10, 3. [Google Scholar] [CrossRef]
- Benítez, Á.; Torres, S.; Morocho, R.; Carrillo, W.; Donoso, D.A.; Calva, J. Platyhypnidium aquaticum as Bioindicator of Metal and Metalloid Contamination of River Water in a Neotropical Mountain City. Plants 2020, 9, 974. [Google Scholar] [CrossRef]
- Bargagli, R. Moss and lichen biomonitoring of atmospheric mercury: A review. Sci. Total Environ. 2016, 572, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chardi, A. Biomonitoring potential of five sympatric Tillandsia species for evaluating urban metal pollution (Cd, Hg and Pb). Atmos. Environ. 2016, 131, 352–359. [Google Scholar] [CrossRef]
- Pescott, O.L.; Simkin, J.M.; August, T.A.; Randle, Z.; Dore, A.J.; Botham, M.S. Air pollution and its effects on lichens, bryophytes, and lichen-feeding Lepidoptera: Review and evidence from biological records. Biol. J. Linn. Soc. 2015, 115, 611–635. [Google Scholar] [CrossRef]
- Coskun, M.; Steinnes, E.; Coskun, M.; Cayir, A. Comparison of epigeic moss (Hypnum cupressiforme) and lichen (Cladonia rangiformis) as biomonitor species of atmospheric metal deposition. Bull. Environ. Contam. Toxicol. 2009, 82, 1–5. [Google Scholar] [CrossRef]
- Bonanno, G.; Borg, J.A.; Di Martino, V. Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: A comparative assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef]
- Simijaca, D.F.; Morales, M.E.; Vargas, D. Use of Non Vascular Plant Organisms as Indicators of Urban Air Pollution (Tunja, Boyacá, Colombiano). Acta Biol. Colomb. 2014, 19, 221. [Google Scholar] [CrossRef]
- Capozzi, F.; Di Palma, A.; Sorrentino, M.C.; Adamo, P.; Giordano, S.; Spagnuolo, V. Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves. Atmosphere 2020, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Vuković, G.; Urošević, M.A.; Goryainova, Z.; Pergal, M.; Škrivanj, S.; Samson, R.; Popović, A. Active moss biomonitoring for extensive screening of urban air pollution: Magnetic and chemical analyses. Sci. Total Environ. 2015, 521–522, 200–210. [Google Scholar] [CrossRef]
- Rivera, M.; Zechmeister, H.; Medina-Ramón, M.; Basagaña, X.; Foraster, M.; Bouso, L.; Moreno, T.; Solanas, P.; Ramos, R.; Köllensperger, G.; et al. Monitoring of heavy metal concentrations in home outdoor air using moss bags. Environ. Pollut. 2011, 159, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, K.; Viteri, F.; Rybarczyk, Y.; Guevara Andino, J.E.; Zalakeviciute, R. Biomonitoring of metal levels in urban areas with different vehicular traffic intensity by using Araucaria heterophylla needles. Ecol. Indic. 2020, 117, 106701. [Google Scholar] [CrossRef]
- Baldantoni, D.; Bellino, A.; Lofrano, G.; Libralato, G.; Pucci, L.; Carotenuto, M. Biomonitoring of nutrient and toxic element concentrations in the Sarno River through aquatic plants. Ecotox. Environ. Saf. 2018, 148, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Markert, B.; Breure, A.; Zechmeister, H. Bioindicators & Biomonitors: Principles. Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 2003; pp. 285–419. [Google Scholar]
- Herzig, R.; Liebendörfer, L.; Urech, M.; Ammann, K.; Cuecheva, M.; Landolt, W. Passive Biomonitoring with Lichens as a Part of an Integrated Biological Measuring System for Monitoring Air Pollution in Switzerland. Int. J. Environ. Anal. Chem. 2006, 35, 43–57. [Google Scholar] [CrossRef]
- Yatawara, M.; Dayananda, N. Use of corticolous lichens for the assessment of ambient air quality along rural–urban ecosystems of tropics: A study in Sri Lanka. Environ. Monit. Assess. 2019, 191, 179. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, H.G.; Hann, S.; Koellensperger, G. Monitoring of Platinum Group Element Deposition by Bryophytes. In Platinum Metals in the Environment. Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 339–349. [Google Scholar] [CrossRef]
- Salemaa, M.; Derome, J.; Helmisaari, H.-S.; Nieminen, T.; Vanha-Majamaa, I. Element accumulation in boreal bryophytes, lichens and vascular plants exposed to heavy metal and sulfur deposition in Finland. Sci. Total Environ. 2004, 324, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Dolegowska, S.; Galuszka, A.; Migaszewski, Z. Significance of the long-term biomonitoring studies for understanding the impact of pollutants on the environment based on a synthesis of 25-year biomonitoring in the Holy Cross Mountains, Poland. Environ. Sci. Pollut. Res. 2021, 28, 10413–10435. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, M.; Hu, R.; Zhao, J.; Wu, Y. Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas. Environ. Health 2018, 15, 1105. [Google Scholar] [CrossRef] [Green Version]
- Krömer, T. Epífitas vasculares como bioindicadoras de la calidad forestal: Impacto antrópico sobre su diversidad y composición. In Bioindicadores: Guardianes de Nuestro Futuro Ambienta; Instituto Nacional de Ecología y Cambio Climático (INECC), México: Campeche, Mexico, 2014; pp. 606–623. [Google Scholar]
- Nali, C.; Lorenzini, G. Plants as indicators of urban air pollution (ozone and trace elements) in Pisa, Italy. J. Environ. Monit. 2004, 6, 636–645. [Google Scholar] [CrossRef]
- Ceschin, S.; Aleffi, M.; Bisceglie, S.; Savo, V.; Zuccarello, V. Aquatic bryophytes as ecological indicators of the water quality status in the Tiber River basin (Italy). Ecol. Indic. 2012, 14, 74–81. [Google Scholar] [CrossRef]
- Samecka, A.; Kempers, A.; Kolon, K. Concentrations of heavy metals in aquatic bryophytes used for biomonitoring in rhyolite and trachybasalt areas: A case study with Platyhypnidium rusciforme from the Sudety Mountains. Ann. Bot. Fenn. 2000, 37, 95–104. [Google Scholar]
- Vásquez, C.; Calva, J.; Morocho, R.; Donoso, D.A.; Benítez, Á. Bryophyte Communities along a Tropical Urban River Respond to Heavy Metal and Arsenic Pollution. Water 2019, 11, 813. [Google Scholar] [CrossRef] [Green Version]
- Kłos, A.; Ziembik, Z.; Rajfur, M.; Dołhańczuk-Śródka, A.; Bochenek, Z.; Bjerke, J.W.; Tømmervik, H.; Zagajewski, B.; Ziółkowski, D.; Jerz, D.; et al. Using moss and lichens in biomonitoring of heavy-metal contamination of forest areas in southern and north-eastern Poland. Sci. Total Environ. 2018, 627, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, F.; Yang, W.; Tan, B.; Chang, C.; Wang, Q.; Cao, R.; Tang, G. Effect of Gap Position on the Heavy Metal Contents of Epiphytic Mosses and Lichens on the Fallen Logs and Standing Trees in an Alpine Forest. Forests 2018, 9, 383. [Google Scholar] [CrossRef]
- Benítez, Á.; Medina, J.; Vásquez, C.; Loaiza, T.; Luzuriaga, Y.; Calva, J. Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador. Diversity 2019, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Monna, F.; Marques, A.N.; Guillon, R.; Losno, R.; Couette, S.; Navarro, N.; Dongarra, G.; Tamburo, E.; Varrica, D.; Chateau, C.; et al. Perturbation vectors to evaluate air quality using lichens and bromeliads: A Brazilian case study. Environ. Monit. Assess. 2017, 189, 566. [Google Scholar] [CrossRef] [PubMed]
- Puczko, K.; Zieliński, P.; Jusik, S.; Kołakowska, A.; Jekatierynczuk-Rudczyk, E. Vascular plant and bryophyte species richness in response to water quality in lowland spring niches with different anthropogenic impacts. Environ. Monit. Assess. 2018, 190, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvereva, E.L.; Toivonen, E.; Kozlov, M.V. Changes in species richness of vascular plants under the impact of air pollution: A global perspective. Glob. Ecol. Biogeogr. 2008, 17, 305–319. [Google Scholar] [CrossRef]
- Deacon, J.R.; Spahr, N.E.; Mize, S.V.; Boulger, R.W. Using water, bryophytes, and macroinvertebrates to assess trace element concentrations in the Upper Colorado River Basin. Hydrobiologia 2001, 455, 29–39. [Google Scholar] [CrossRef]
- Nelson, S.M.; Campbell, S.G. Integrated Assessment of Metals Contamination in a Lotic System Using Water Chemistry, Transplanted Bryophytes, and Macroinvertebrates. J. Freshw. Ecol. 1995, 10, 409–420. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública. Programa Nacional Municipios y Mercados Saludables. 2021. Available online: https://www.salud.gob.ec/programa-nacional-de-municipios-saludables/ (accessed on 10 February 2022).
- Abas, A. A systematic review on biomonitoring using lichen as the biological indicator: A decade of practices, progress and challenges. Ecol. Indic 2021, 121, 107197. [Google Scholar] [CrossRef]
- Equipo RStudio. RStudio: Desarrollo Integrado Para R; RStudio, PBC: Boston, MA, USA, 2020; Available online: https://www.R-project.org/ (accessed on 11 January 2022).
- Hernandez, J.; Arguella, E.; Fernandez, R.; Galarraga, F.; Roschman, G.; Benzo, Z. Evaluación del uso bromelias y líquenes como bioindicadores de metales pesados (Pb, Cu, Zn, Ni, V y Cr) en la ciudad de Caracas. In Proceedings of the XVIII Congreso Venezolana de Botánica, Barquisimeto, Venezuela, 17–22 May 2009. [Google Scholar] [CrossRef]
- Pyatt, F.B.; Grattan, J.P.; Lacy, D.; Seaward, M.R.D. Comparative Effectiveness of Tillandsia usneoides L. and Parmotrema praesorediosum (Nyl.) Hale as Bio-Indicators of Atmospheric Pollution in Louisiana (U.S.A.). Water Air Soil Pollut. 1999, 111, 317–326. [Google Scholar] [CrossRef]
- Debén, S.; Fernández, J.A.; Giráldez, P.; Vázquez Arias, A.; Aboal, J.R. Methodological advances to biomonitor water quality with transplanted aquatic mosses. Sci. Total Environ. 2020, 706, 136082. [Google Scholar] [CrossRef]
- Loppi, S.; Bonini, I. Lichens and mosses as biomonitors of trace elements in areas with thermal springs and fumarole activity (Mt. Amiata, central Italy). Chemosphere 2000, 41, 1333–1336. [Google Scholar] [CrossRef]
- Gałuszka, A. The Chemistry of Soils, Rocks and Plant Bioindicators in Three Ecosystems of the Holy Cross Mountains, Poland. Environ. Monit. Assess. 2005, 110, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Giampaoli, P.; Wannaz, E.D.; Tavares, A.R.; Domingos, M. Suitability of Tillandsia usneoides and Aechmea fasciata for biomonitoring toxic elements under tropical seasonal climate. Chemosphere 2016, 149, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Techato, K.; Salaeh, A.; Van Beem, N.C. Use of Atmospheric Epiphyte Tillandsia usneoides (Bromeliaceae) as Biomonitor. APCBEE Procedia 2014, 10, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Brighigna, L.; Ravanelli, M.; Minelli, A.; Ercoli, L. The use of an epiphyte (Tillandsia caput-medusae morren) as bioindicator of air pollution in Costa Rica. Sci. Total Environ. 1997, 198, 175–180. [Google Scholar] [CrossRef]
- Safitri, R.; Sudirman, L.; Koesmary, Y. Exploration of Parmotrema tinctorum and Leptogium sp. lichens as bioindicator of air quality in Mekarsari fruit park. IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012006. [Google Scholar] [CrossRef]
- Adamo, P.; Bargagli, R.; Giordano, S.; Modenesi, P.; Monaci, F.; Pittao, E.; Spagnuolo, V.; Tretiach, M. Natural and pre-treatments induced variability in the chemical composition and morphology of lichens and mosses selected for active monitoring of airborne elements. Environ. Pollut. 2008, 152, 11–19. [Google Scholar] [CrossRef]
- Cardoso-Gustavson, P.; Fernandes, F.F.; Alves, E.S.; Victorio, M.P.; Moura, B.B.; Domingos, M.; Rodrigues, C.A.; Ribeiro, A.P.; Nievola, C.C.; Figueiredo, A.M.G. Tillandsia usneoides: A successful alternative for biomonitoring changes in air quality due to a new highway in São Paulo, Brazil. Environ. Sci. Pollut. Res. 2017, 24, 12015. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, G.; Rodriguez, J.; Pignata, M. Comparison of the air pollution biomonitoring ability of three Tillandsia species and the lichen Ramalina celastri in Argentina. Environ. Res. 2009, 109, 6–14. [Google Scholar] [CrossRef]
- Vianna, N.A.; Gonçalves, D.; Brandão, F.; de Barros, R.P.; Filho, G.M.A.; Meire, R.O.; Torres, J.P.M.; Malm, O.; Júnior, A.D.; Andrade, L.R. Assessment of heavy metals in the particulate matter of two Brazilian metropolitan areas by using Tillandsia usneoides as atmospheric biomonitor. Environ. Sci. Pollut. Res. 2011, 18, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Sanches, L.V.C. Desenvolvimento de Aechmea Fasciata (Bromeliaceae) em Função de Diferentes Saturações por Bases no Substrato e Modos de Aplicação da Fertirrigação; UNESP: São Paulo, Brazil, 2009. [Google Scholar]
- Scatena, V.L.; Segecin, S. Anatomia foliar de Tillandsia L. (Bromeliaceae) dos Campos Gerais, Paraná, Brasil. Rev. Bras. Bot. 2005, 28, 635–649. [Google Scholar] [CrossRef] [Green Version]
- Kosior, G.; Steinnes, E.; Samecka-Cymerman, A.; Lierhagen, S.; Kolon, K.; Dołhańczuk-Śródka, A.; Ziembik, Z. Trace elements in native and transplanted Fontinalis antipyretica and Platyhypnidium riparioides from rivers polluted by uranium mining. Chemosphere 2017, 171, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Cesa, M.; Bertossi, A.; Cherubini, G.; Gava, E.; Mazzilis, D.; Piccoli, E.; Verardo, P.; Nimis, P.L. Development of a standard protocol for monitoring trace elements in continental waters with moss bags: Inter- and intraspecific differences. Environ. Sci. Pollut. Res. 2015, 22, 5030–5040. [Google Scholar] [CrossRef] [PubMed]
- Nimis, P.L.; Fumagalli, F.; Bizzotto, A.; Codogno, M.; Skert, N. Bryophytes as indicators of trace metals pollution in the River Brenta, Italy. Sci. Total Environ. 2002, 286, 233–242. [Google Scholar] [CrossRef]
- Vanderpoorten, A. Aquatic bryophytes for a spatio-temporal monitoring of the water pollution of the rivers Meuse and Sambre (Belgium). Environ. Pollut. 1999, 104, 401–410. [Google Scholar] [CrossRef]
- Vitt, D.; Glime, J. The structural adaptations of aquatic Musci. Lindbergia 1984, 10, 95–110. Available online: http://www.jstor.org/stable/20149514 (accessed on 25 May 2022).
- Becerra, D.; Cardenas, K.; Alvaro, W. Diversidad de briófitos acuáticos en un río de alta montaña tropical. Caldasia 2020, 42, 294–305. [Google Scholar] [CrossRef]
- Castello, M.A. Comparison Between Two Moss Species Used as Transplants for Airborne Trace Element Biomonitoring in NE Italy. Environ. Monit. Assess. 2007, 133, 267–276. [Google Scholar] [CrossRef]
- Cesa, M.; Baldisseri, A.; Bertolini, G.; Dainese, E.; Col, M.D.; Vecchia, U.D.; Marchesini, P.; Nimis, P.L. Implementation of an active ‘bryomonitoring’ network for chemical status and temporal trend assessment under the Water Framework Directive in the Chiampo Valley’s tannery district (NE Italy). J. Environ. Manag. 2013, 114, 303–315. [Google Scholar] [CrossRef]
- Figueira, R.; Ribeiro, T. Transplants of aquatic mosses as biomonitors of metals released by a mine effluent. Environ. Pollut. 2005, 136, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Adamo, P.; Monaci, F.; Pittao, E.; Tretiach, M.; Bargagli, R. Bags with oven-dried moss for the active monitoring of airborne trace elements in urban areas. Environ. Pollut. 2009, 157, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Barrera Zambrano, V.A.; Ceusters, J.; Shorrock, K. The photosynthetic plasticity of crassulacean acid metabolism: An evolutionary innovation for sustainable productivity in a changing world. New Phytol. 2011, 191, 619–633. [Google Scholar] [CrossRef] [PubMed]



| Species | Heavy Metal | Forest | South | Center | North |
|---|---|---|---|---|---|
| Parmotrema arnoldii (mg/g) | Cd | 0.60 ± 0.805 | 30.83 ± 19.124 | 34.66 ± 9.221 | 27.99 ± 9.058 |
| Pb | 7.14 ± 3.053 | 39.48 ± 14.659 | 25.29 ± 9.464 | 42.95 ± 18.030 | |
| Cu | 10.41 ± 7.367 | 21.27 ± 3.928 | 25.41 ± 4.441 | 31.02 ± 4.129 | |
| Mn | 12.30 ± 3.430 | 53.49 ± 18.971 | 20.03 ± 8.422 | 56.81 ± 25.589 | |
| Zn | 16.19 ± 3.688 | 91.37 ± 35.781 | 100.54 ± 23.921 | 44.46 ± 26.487 | |
| Tillandsia usneoides (mg/g) | Cd | 1.10 ± 1.272 | 49.16 ± 6.927 | 28.93 ± 12.158 | 28.11 ± 8.170 |
| Pb | 12.29 ± 3.677 | 35.53 ± 10.754 | 27.74 ± 14.525 | 49.93 ± 10.811 | |
| Cu | 9.08 ± 5.976 | 27.57 ± 11.550 | 22.36 ± 9.685 | 28.44 ± 5.974 | |
| Mn | 15.60 ± 2.522 | 71.43 ± 34.935 | 43.27 ± 22.314 | 96.12 ± 29.603 | |
| Zn | 54.65 ± 13.001 | 70.97 ± 33.702 | 89.54 ± 24.085 | 30.11 ± 8.491 | |
| Marchantia polymorpha (µg g−1) | Cd | 0.01 ± 1.742 | 0.01 ± 001 | 0.01 ± 010 | 0.01 ± 003 |
| Zn | 3.08 ± 167 | 11.64 ± 1.242 | 13.02 ± 1.901 | 10.71 ± 0.497 | |
| Fe | 0.34 ± 0.342 | 8.84 ± 1.531 | 7.61 ± 1.282 | 6.03 ± 0.583 | |
| Al | 7.82 ± 1.741 | 11.43 ± 2.100 | 13.21 ± 1.510 | 8.31 ± 0.874 | |
| Pb | 0.04 ± 0.036 | 0.04 ± 001 | 0.04 ± 000 | 0.03 ± 010 | |
| Mn | 0.23 ± 0.230 | 0.75 ± 0.093 | 0.54 ± 023 | 0.37 ± 0.077 | |
| As | 0 ± 000 | 0.42 ± 1.120 | 4.28 ± 4.691 | 3.94 ± 4.375 | |
| Platyhypnidium aquaticum (µg g−1) | Cd | 0.72 ± 0.751 | 4.92 ± 1.230 | 5.25 ± 0.656 | 6.64 ± 0.806 |
| Zn | 185.08 ± 138.384 | 913.87 ± 256.436 | 640.4 ± 245.748 | 559.55 ± 107.930 | |
| Fe | 510.13 ± 164.041 | 1179.52 ± 301.680 | 977.03 ± 320.574 | 1076.6 ± 197.255 | |
| Al | 1355.12 ± 343.114 | 1390.17 ± 671.933 | 1505.45 ± 407.687 | 1370.42 ± 516.179 | |
| Pb | 10.62 ± 6.970 | 10.39 ± 8.864 | 12.86 ± 10.676 | 12.74 ± 9.675 | |
| Mn | 0.4 ± 1.023 | 2412.21 ± 1301.376 | 936.49 ± 1032.782 | 536.62 ± 549.811 | |
| As | 6.81 ± 3.585 | 22.2 ± 5.193 | 17.25 ± 5.664 | 21.95 ± 8.190 |
| Metal | W | p Value |
|---|---|---|
| Cd | 1082.5 | 0.1486 |
| Pb | 1497 | 0.1848 |
| Cu | 1326 | 0.8565 |
| Mn | 881 | 0.0152 |
| Zn | 1126 | 0.2493 |
| Metal | W | p Value |
|---|---|---|
| Cd | 1716 | 0.3576 |
| Zn | 254 | <0.0001 |
| Fe | 350 | <0.0001 |
| Al | 140 | <0.0001 |
| Pb | 267 | <0.0001 |
| Mn | 832 | <0.0001 |
| As | 182 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, W.; Calva, J.; Benítez, Á. The Use of Bryophytes, Lichens and Bromeliads for Evaluating Air and Water Pollution in an Andean City. Forests 2022, 13, 1607. https://doi.org/10.3390/f13101607
Carrillo W, Calva J, Benítez Á. The Use of Bryophytes, Lichens and Bromeliads for Evaluating Air and Water Pollution in an Andean City. Forests. 2022; 13(10):1607. https://doi.org/10.3390/f13101607
Chicago/Turabian StyleCarrillo, Washington, James Calva, and Ángel Benítez. 2022. "The Use of Bryophytes, Lichens and Bromeliads for Evaluating Air and Water Pollution in an Andean City" Forests 13, no. 10: 1607. https://doi.org/10.3390/f13101607






