Multi-Directional Rather Than Unidirectional Northward-Dominant Range Shifts Predicted under Climate Change for 99 Chinese Tree Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Species Occurrence Data
2.3. Climatic Variables and Layers
2.4. Simulation of Species Ranges and Evaluation Processes
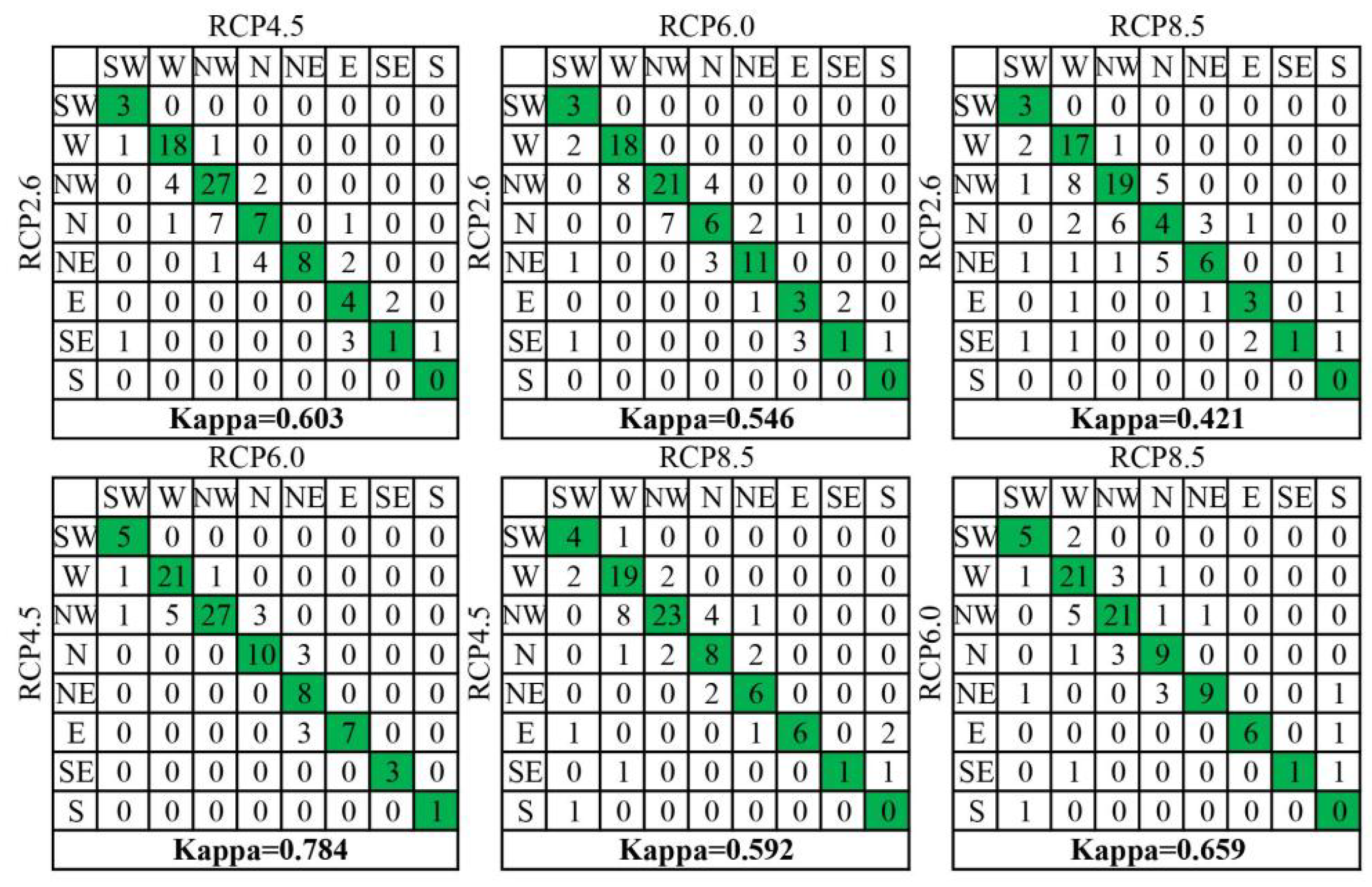
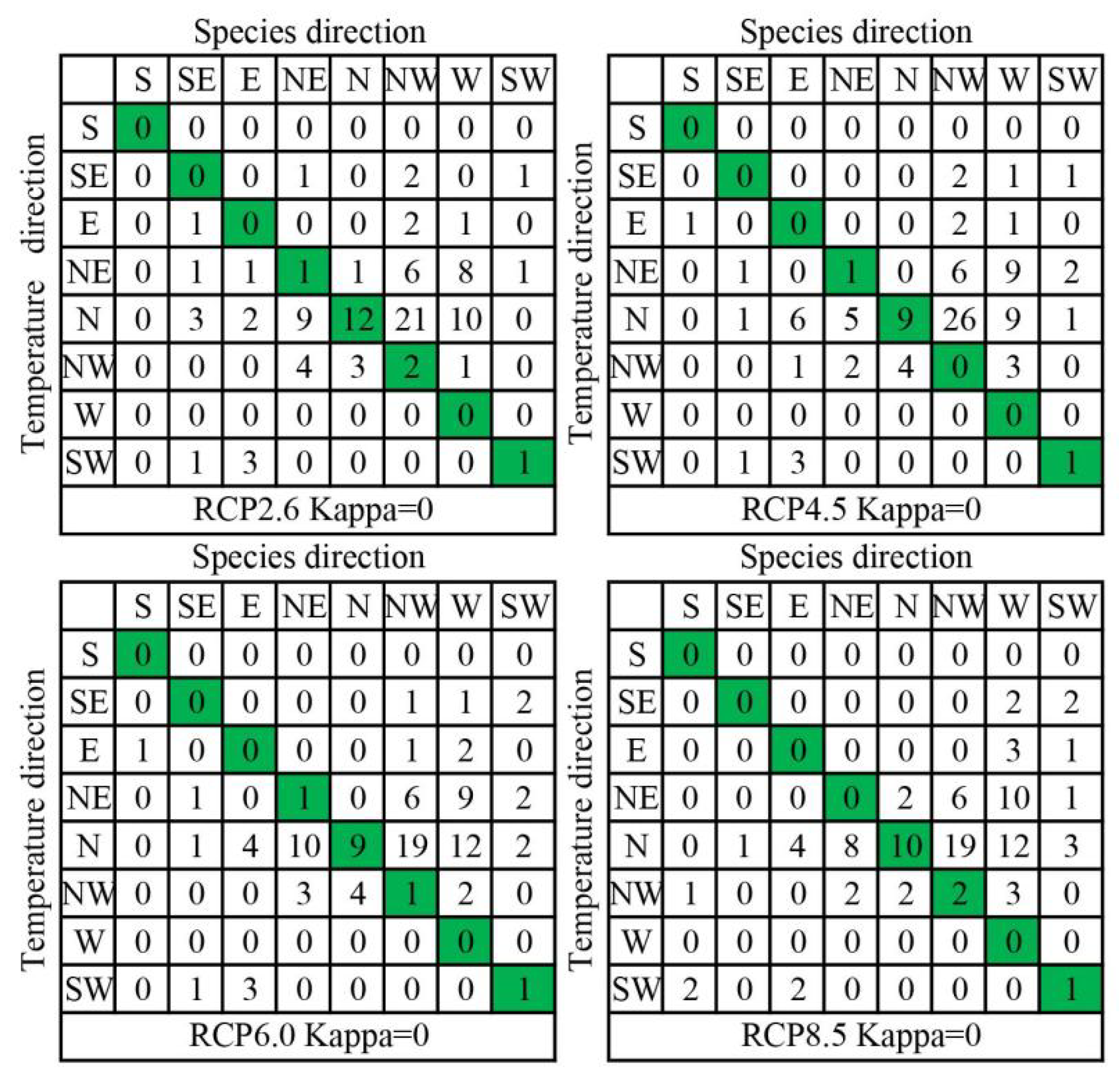
2.5. Calculation of Species Velocity and Hypothesis Testing
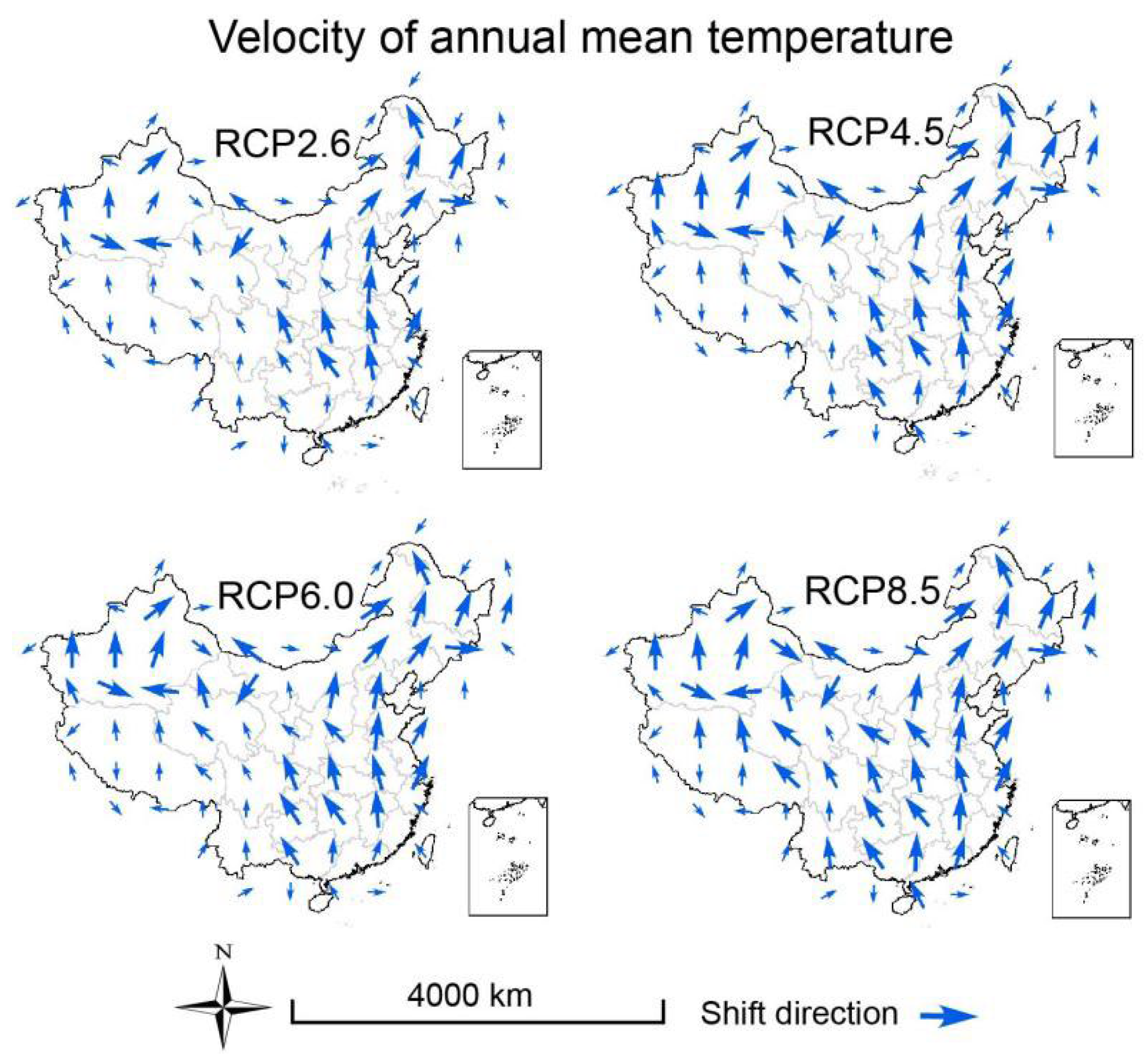
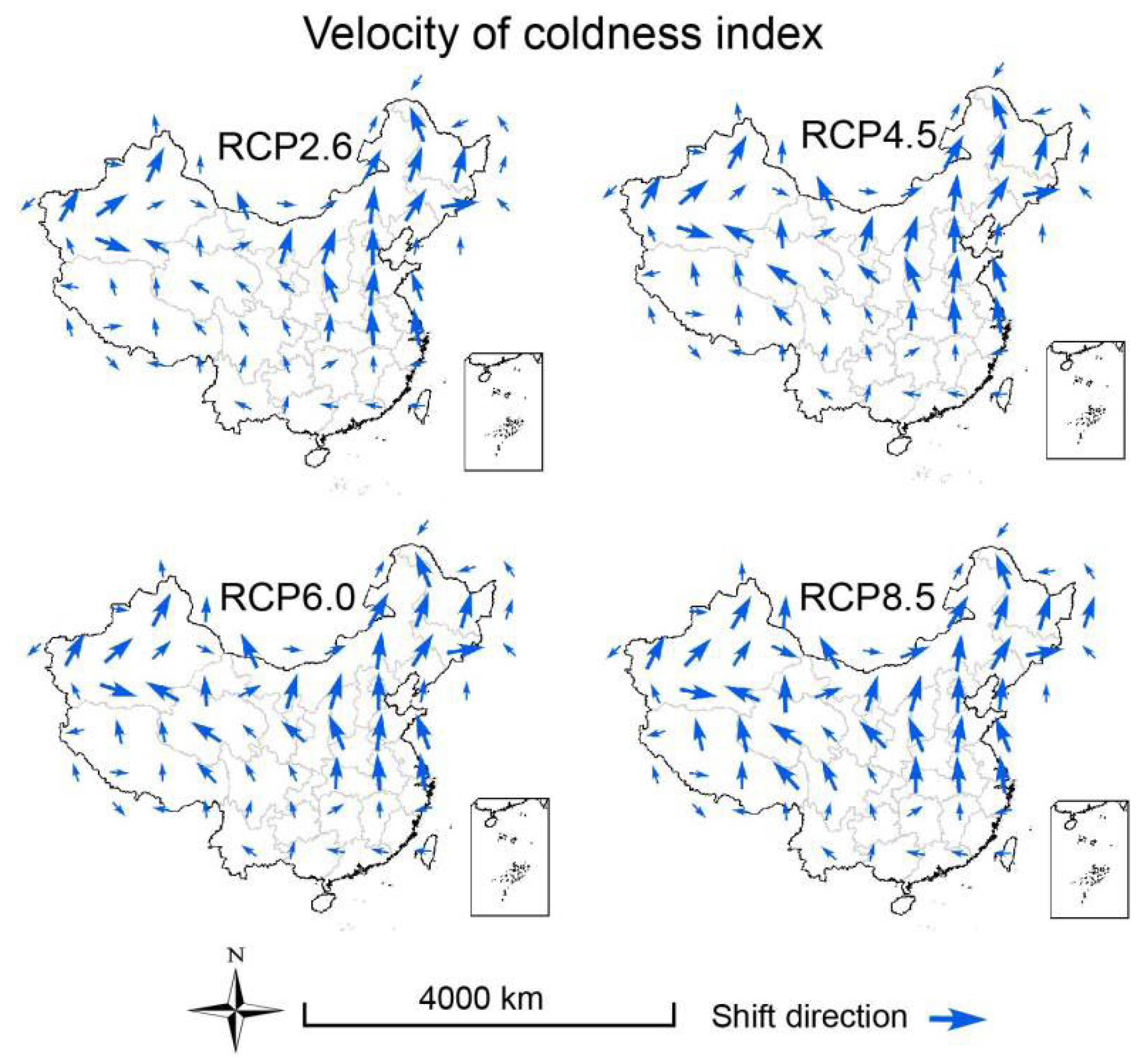
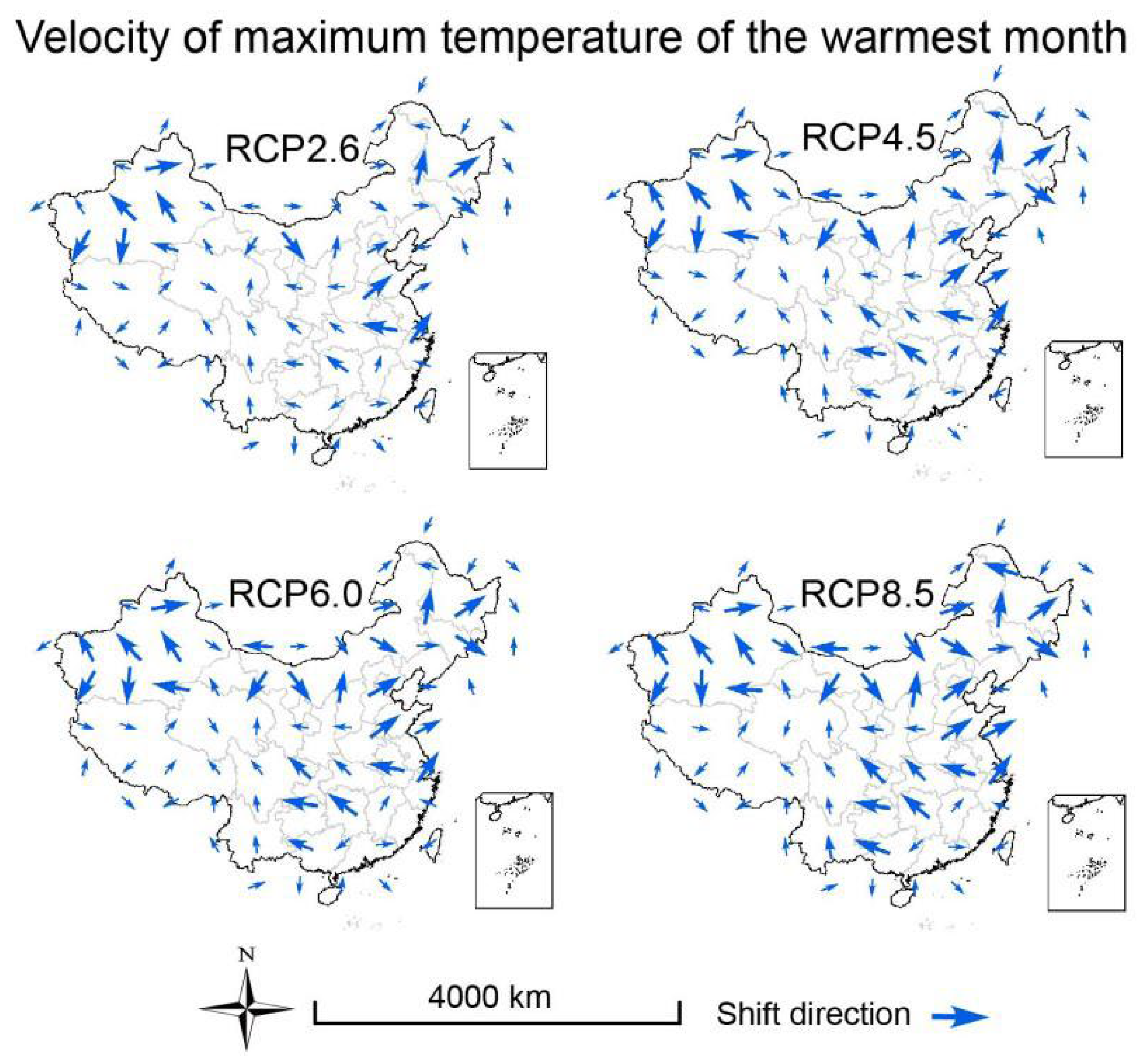
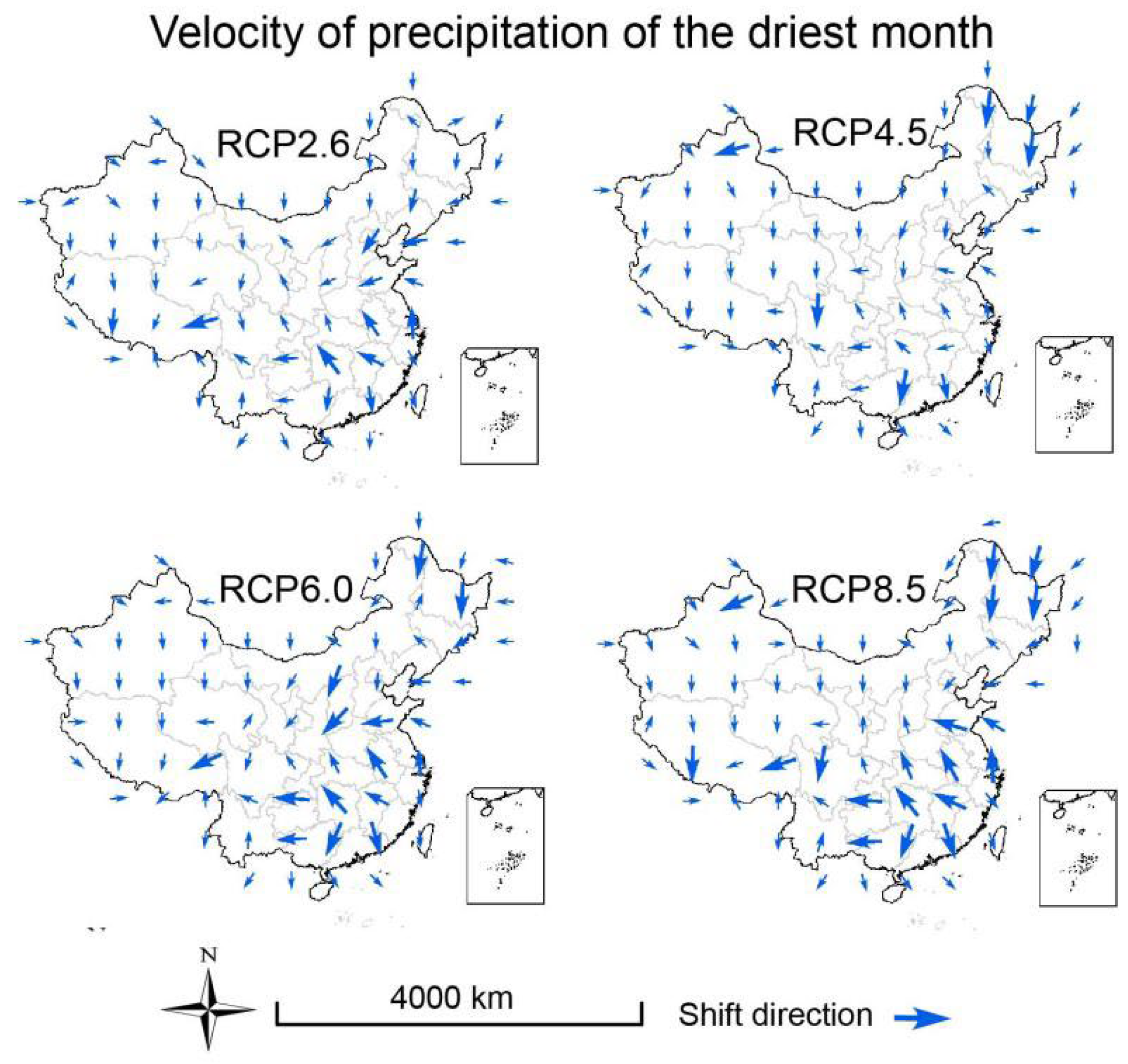
2.6. Calculation of Climate Velocity and Hypothesis Testing
3. Results
3.1. Model Performance and Important Climatic Variables
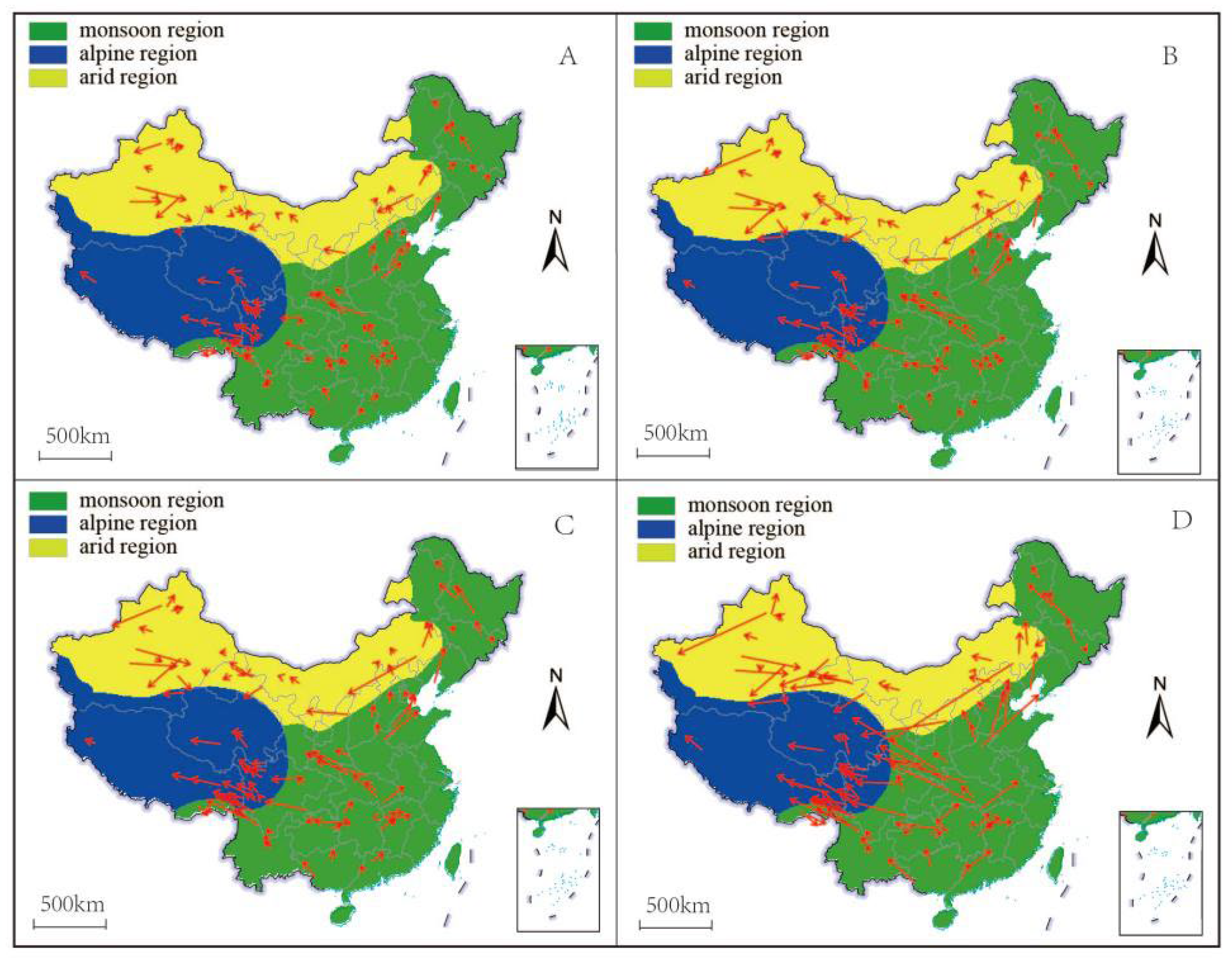
3.2. Velocity of Species Habitat Range Shifts
3.3. Velocities of Climate Change
4. Discussion
4.1. Species-Projected Range Shifts Are Predominantly Multidirectional
4.2. Explaining Species Velocity Using Climate Velocity
4.3. Research Limitations and Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A







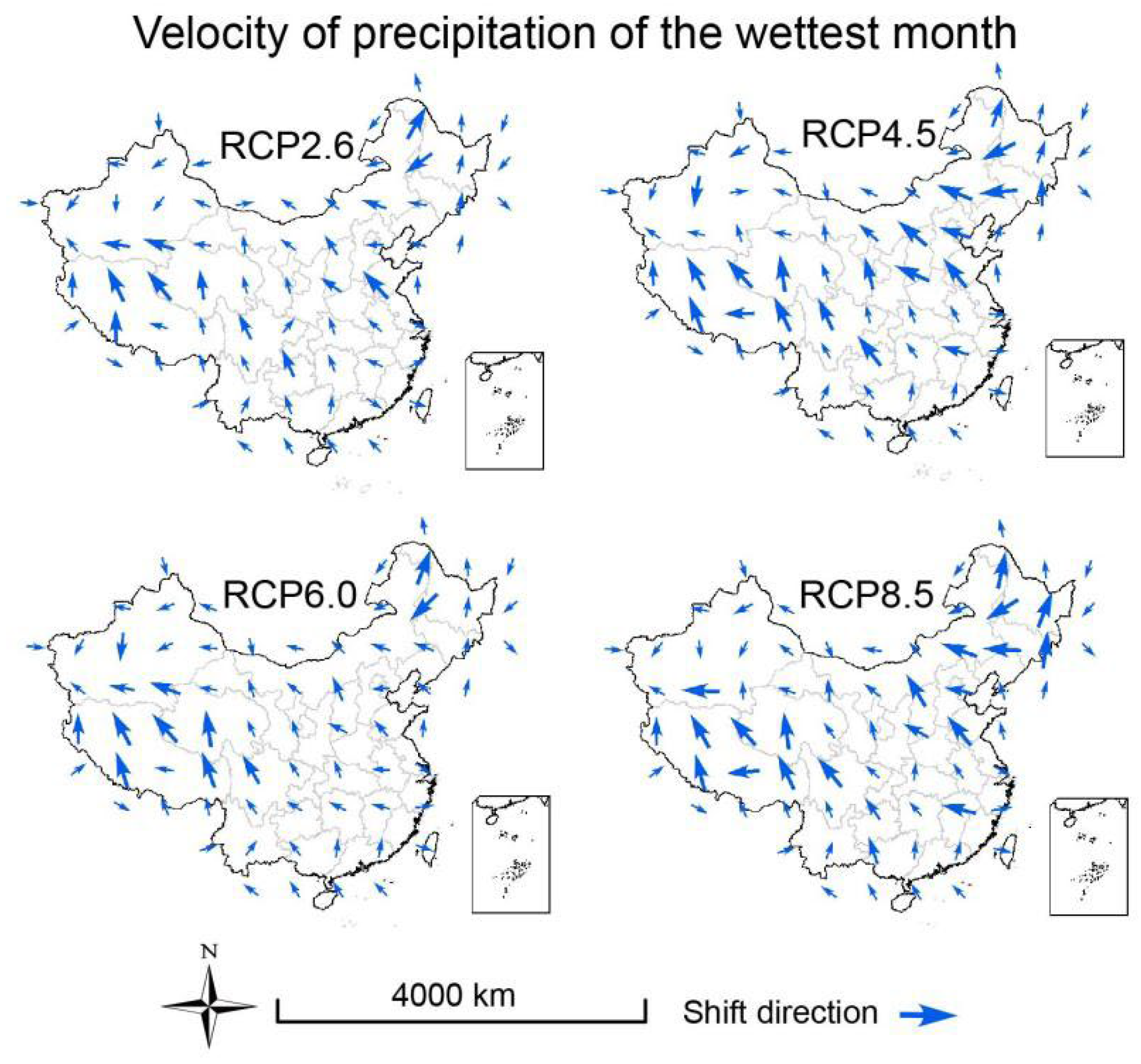
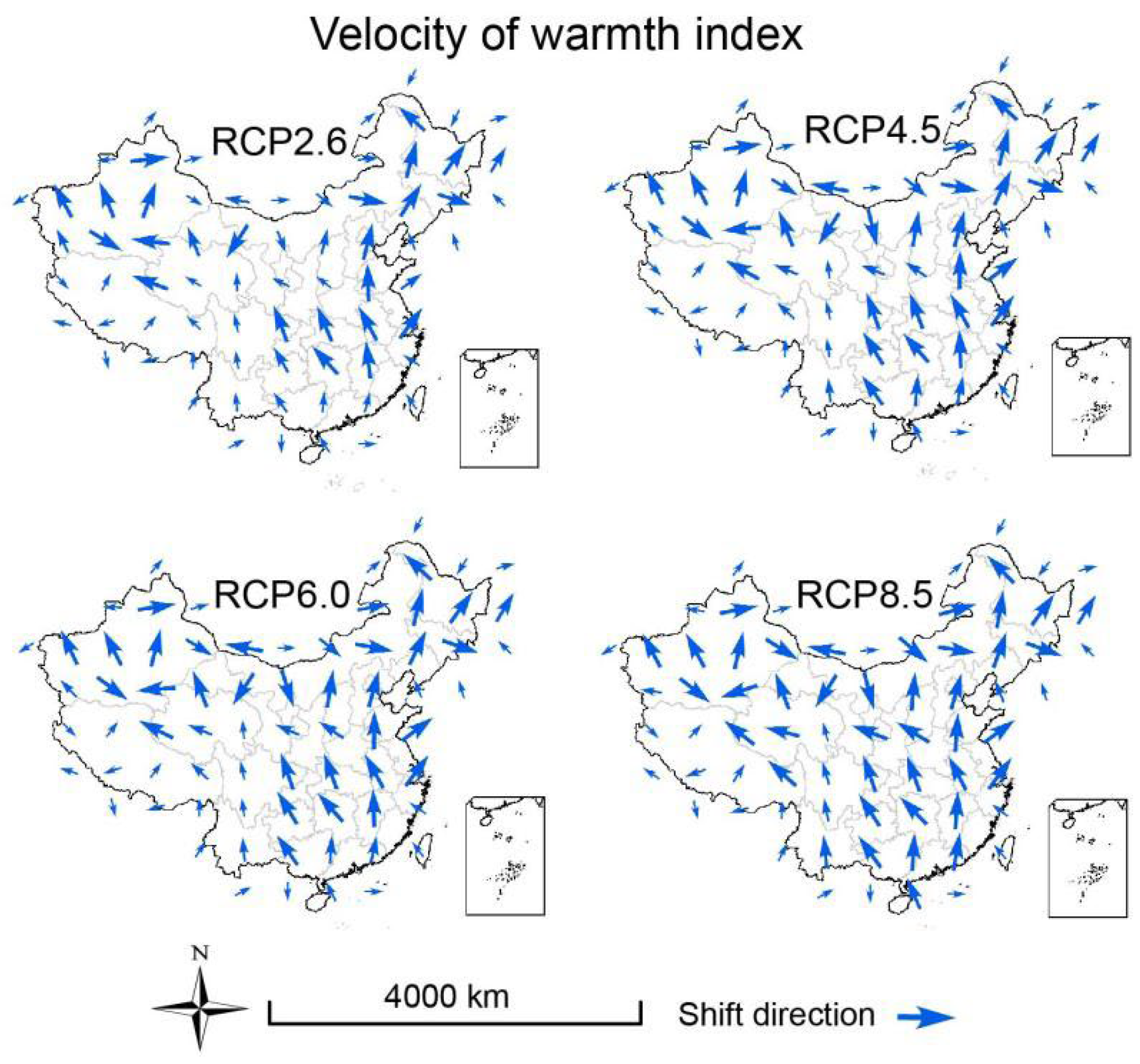
References
- IPCC. Summary for policymakers. In Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Qian, W.; Zhu, Y. Climate Change in China from 1880 to 1998 and its impact on the environmental condition. Clim. Chang. 2001, 50, 419–444. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, G.; Shi, G.; Gong, P.; Zheng, X.; Zhai, P.; Zhang, D.; Zhao, Z.; Wang, S.; Wang, H.; et al. National Assessment Report of Climate Change (I): Climate change in China and its future trend. Clim. Chang. Res. 2006, 2, 3–8. [Google Scholar]
- Ren, G.; Ding, Y.; Zhao, Z.; Zheng, J.; Wu, T.; Tang, G.; Xu, Y. Recent progress in studies of climate change in China. Adv. Atmos. Sci. 2012, 29, 958–977. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Moat, J.; Gole, T.W.; Davis, A.P. Least concern to endangered: Applying climate change projections profoundly influences the extinction risk assessment for wild arabica coffee. Glob. Chang. Biol. 2019, 25, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, X.S.; Li, B.L. The possible response of life zones in China under global climate change. Glob. Planet. Chang. 2003, 38, 327–337. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, D.; Wu, S. Climate change risk to forests in China associated with warming. Sci. Rep. 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Huang, J.; Guo, K.; Du, S. Projecting species loss and turnover under climate change for 111 Chinese tree species. For. Ecol. Manag. 2020, 477, 118488. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Zhu, F.; Li, X. Climate change may cause distribution areas loss for tree species in southern China. For. Ecol. Manag. 2022, 511, 120134. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Aubin, I.; Munson, A.D.; Cardou, F.; Burton, P.J.; Isabel, N.; Pedlar, J.H.; Paquette, A.; Taylor, A.R.; Delagrange, S.; Kebli, H.; et al. Traits to stay, traits to move: A review of functional traits to access sensitivity and adaptive capacity of temperate and boreal trees to climate change. Environ. Rev. 2016, 24, 164–186. [Google Scholar] [CrossRef]
- Du, H.; Liu, J.; Li, M.H.; Buntgen, U.; Yang, Y.; Wang, L.; Wu, Z.; He, H.S. Warming-induced upward migration of the alpine treeline in the Changbai Mountains, northeast China. Glob. Chang. Biol. 2018, 24, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Zhang, Y.; Zhang, Y.; Zhang, K.; Zhang, Q. Variability and rapid response of subalpine fir (Abies fargesii) to climate warming at upper altitudinal limits in north-centeral China. Trees 2015, 29, 785–795. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Q.; Xie, F.; He, N.; He, R.; Zhang, K.; Zhang, Q.; Dang, H. Disparity in elevational shifts of upper species limits in response to recent climate change warming in the Qinling Mountains, North-central China. Sci. Total Environ. 2020, 706, 135718. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Q.B.; Ma, K. Treeline dynamics in relation to climatic variability in the central Tianshan Mountains, northwestern China. Glob. Ecol. Biogeogr. 2006, 15, 406–415. [Google Scholar] [CrossRef]
- Liang, E.Y.; Wang, Y.F.; Piao, S.L.; Lu, X.M.; Camarero, J.J.; Zhu, H.F.; Zhu, L.P.; Ellison, A.M.; Ciais, P.; Penuelas, J. Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, G.; Li, J.; Zhang, X.; Yan, M.; Du, S. Projecting the Range Shifts in Climatically Suitable Habitat for Chinese Sea Buckthorn under Climate Change Scenarios. Forests 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Paul, C.; Huang, J. Black locust (Robinia pseudoacacia L.) range shifts in China: Application of a global model in climate change futures. Clim. Chang. Ecol. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Kou, X.; Li, Q.; Beierkuhnlein, C.; Zhao, Y.; Liu, S. A new tools for exploring climate change induced range shifts of conifer species in China. PLoS ONE 2014, 9, e98643. [Google Scholar] [CrossRef]
- LaSorte, F.A.; Thompson, F.R. Poleward shifts in winter ranges of North American birds. Ecology 2007, 88, 1803–1812. [Google Scholar]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of species responses to climate change. Sci. Adv. 2017, 3, e1603055. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- VanDerWal, J.; Murphy, H.T.; Kutt, A.S.; Perkins, G.C.; Bateman, B.L.; Perry, J.J.; Reside, A.E. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat. Clim. Chang. 2013, 3, 239–243. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; Perie, C.; Blois, S.D. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 2014, 5, 1–33. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2011, 18, 1042–1052. [Google Scholar] [CrossRef]
- Aubin, I.; Boisvert-Marsh, L.; Kebli, H.; McKenney, D.; Pedlar, J.; Lawrence, K.; Hogg, E.H.; Boulanger, Y.; Gauthier, S.; Ste-Marie, C. Tree vulnerability to climate change: Improving exposure-based assessments using traits as indicators of sensitivity. Ecospher 2018, 9, e02108. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1111. [Google Scholar] [CrossRef]
- Dobrowski, S.Z.; Abatzoglou, J.; Swanson, A.K.; Greenberg, J.A.; Mynsberge, A.R.; Holden, Z.A.; Schwartz, M.K. The climate velocity of the contiguous United States during the 20th century. Glob. Chang. Biol. 2012, 19, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Soberon, J.; Nakamura, M. Niches and distributional area: Concepts, methods, and assumptions. P. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamme, R.; Gotzenberger, L.; Zobel, M.; Bullock, J.M.; Hooftman, D.; Kaasik, A.; Partel, M. Predicting species’ maximum dispersal distances from simple plant traits. Ecology 2014, 95, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Tian, H.; Lange, S.; Yang, J.; Pan, S.; Fu, B.; Reyer, C. Terrestrial biodiversity threatened by increasing a global aridity velocity under high level warming. Proc. Natl. Acad. Sci. USA 2021, 118, e2015552118. [Google Scholar] [CrossRef]
- ECVC (Editorial Committee for Vegetaiton of China). Vegetation of China; Science Press: Beijing, China, 1980. [Google Scholar]
- Hou, X.Y. Vegetation of China with reference to its geographical distribution. Ann. Mo. Bot. Gard. 1983, 70, 509–549. [Google Scholar] [CrossRef]
- Gratzfeld, J.; Wen, X.; Jones, M.; Rivers, M. Safeguarding China’s native trees—A review of integrated conservation practices between 2008 and 2020. Glob. Ecol. Conserv. 2022, 35, e02101. [Google Scholar] [CrossRef]
- Guo, K.; Werger, M.J.A. Effect of prevailing monsoons on the distribution of beeches in continental East Asia. For. Ecol Manag. 2010, 259, 2197–2203. [Google Scholar] [CrossRef]
- Hou, X. 1:1,000,000 Vegetation Atlas of China. Chinese Academy of Science, the Editorial Board of Vegetation Map of China; Science Press: Beijing, China, 2001. [Google Scholar]
- Liu, H.Y.; Yin, Y.; Hao, Q.; Liu, G. Sensitivity of temperate vegetation to Holocene development of East Asian. Quat. Sci. Rev. 2014, 98, 126–134. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J.; Yu, F.H. Spatial conservation prioritization for dominant tree species of Chinese forest communities under climate change. Clim. Chang. 2017, 144, 303–316. [Google Scholar] [CrossRef]
- Booth, T.H. Why understanding the pioneering and continuing contributions of Bioclim to species distribution modelling is important. Austral Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Holdridge, L.R. Determination of world plant formations from simple climatic data. Science 1947, 105, 367–368. [Google Scholar] [CrossRef]
- Kira, T. A New Classification of Climate in Eastern Asia as the Basis for Agricultural Geography; Horticultural Institute, Kyoto University: Kyoto, Japan, 1945. [Google Scholar]
- Li, G.Q.; Xu, G.H.; Guo, K.; Du, S. Mapping the global potential geographical distribution of black locust (Robinia pseudoacacia L.) using herbarium data and a maximum entropy model. Forests 2014, 5, 2773–2792. [Google Scholar] [CrossRef] [Green Version]
- Worldclim. Global Climate Data—Free Climate Data for Ecological Modeling and GIS. Available online: http://www.worldclim.org/ (accessed on 10 May 2019).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of difference of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Stockwell, D.; Peters, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettman, F.; Lehikoinen, A.; Lehmann, A.; et al. Novel methods improve prediction of species’ distribution from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Serra-Diaz, J.M.; Franklin, J.; Ninyerola, M.; Davis, F.W.; Syphard, A.D.; Regan, H.M.; Ikegami, M. Bioclimatic velocity: The pace of species exposure to climate change. Divers. Distrib. 2014, 20, 169–180. [Google Scholar] [CrossRef]
- Hamann, A.; Roberts, D.R.; Barber, Q.E.; Carroll, C.; Nielsen, S.E. Velocity of climate change algorithms for guiding conservation and management. Glob. Chang. Biol. 2015, 21, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Artstein, R.; Poesio, M. Inter-coder agreement for computational linguistics. Comput. Linguist. 2008, 34, 555–596. [Google Scholar] [CrossRef] [Green Version]
- Burrows, M.T.; Schoeman, D.S.; Richardson, A.J.; Molinos, J.G.; Hoffmann, A.; Buckley, L.B.; Moore, P.J.; Brown, C.J.; Bruno, J.F.; Duarte, C.M.; et al. Geographical limits to species-range shifts are suggested by climate velocity. Nature 2014, 507, 492–495. [Google Scholar] [CrossRef] [Green Version]
- Iverson, L.R.; Prasad, A.M.; Matthews, S.N.; Peters, M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. For. Ecol. Manag. 2008, 254, 390–406. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Liu, G.; Lu, Q.; Xiong, D.Y.; Li, G.Q.; Du, S. Understanding the limiting climatic factors on the suitable habitat of Chinese Alfalfa. Forests 2022, 13, 482. [Google Scholar] [CrossRef]
- Garcia, R.; Cabeza, M.; Rahbek, C.; Araujo, M.B. Multiple dimensions of climate change and their implication for biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef] [Green Version]
- Harsch, M.A.; HilleRisLambers, J. Climate warming and seasonal precipitation change interact to limit species distribution shifts across western North America. PLoS ONE 2016, 11, e0159184. [Google Scholar] [CrossRef] [Green Version]
- Booth, T.H. Assessing species climatic requirements beyond the realized niche: Some lessons mainly from tree species distribution modelling. Clim. Chang. 2017, 145, 259–271. [Google Scholar] [CrossRef]
- Li, G.Q.; Zhang, X.Q.; Huang, J.H.; Wen, Z.M.; Du, S. Afforestation and climatic niche dynamics of black locust (Robinia pseudoacacia). For. Ecol. Manag. 2018, 407, 184–190. [Google Scholar] [CrossRef]
- Frick, E.C.; Ordonez, A.; Rogers, H.S.; Svenning, J. The effects of defaunation on plant’s capacity to track climate change. Science 2022, 375, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver-Parry, P.E.; Carroll, C.J.W.; Martin, P.H.; Talluto, M.V. Multi-scale integration of tree recruitment and range dynamics in a changing climate. Glob. Ecol. Biogeogr. 2020, 29, 102–116. [Google Scholar] [CrossRef] [Green Version]
- Solarik, K.A.; Cazelles, K.; Messier, C.; Bergeron, Y.; Gravel, D. Priority effects will impact range shifts of temperate tree species into boreal forest. J. Ecol. 2019, 108, 1155–1173. [Google Scholar] [CrossRef]
- Lafleur, B.; Pare, D.; Munson, A.; Bergeron, Y. Response of northeastern North American forests to climate change: Will soil conditions constrain tree species migration. Environ. Rev. 2010, 18, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.M.; McGill, B.J. Land use and life history limit migration capacity of eastern tree species. Glob. Ecol. Biogeogr. 2017, 27, 57–67. [Google Scholar] [CrossRef]
- Brice, M.; Cazelles, K.; Legendre, P.; Fortin, M. Disturbances amplify tree community responses to climate change in a temperate-boreal ecotone. Glob. Ecol. Biogeogr. 2019, 28, 1668–1681. [Google Scholar] [CrossRef]
- Bryan, B.A.; Gao, L.; Ye, Y.Q.; Sun, X.F.; Connor, J.D.; Crossman, N.D.; Stafford-Smith, M.; Wu, J.G.; He, C.Y.; Yu, D.Y.; et al. China’s response to a national land-system sustainability emergency. Nature 2018, 559, 193–204. [Google Scholar] [CrossRef]
- Cui, W.; Liu, J.; Jia, J.; Wang, P. Terrestrial ecological restoration in China: Identifying advances and gaps. Environ. Sci. Eur. 2021, 33, 123. [Google Scholar] [CrossRef]
- Mascarelli, A.L. Climate zone will shift faster as world warms. Nature 2013. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and uncertainties of species’ range shifts under climate change. Glob. Chang. Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Franklin, J.; Dawson, T.P.; Pilesjo, P. Geospatial tools address emerging issues in spatial ecology: A review and commentary on the special issue. Int. J. Geogr. Inf. Sci. 2011, 25, 337–365. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. (Eds.) 8—Ecological Scales: Issues of Resolution and Extent from Part II—Data Acquisition, Sampling Design, and Spatial Scales. In Habitat Suitability and Distribution Models with Applications in R.; Cambridge University Press: Cambridge, UK, 2017; pp. 135–150. [Google Scholar]
- Seo, C.; Thorne, J.H.; Hannah, L.; Thuiller, W. Scale effects in species distribution models: Implications for conservation planning under climate change. Biol. Lett. 2008, 5, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Kim, E.; Lee, D.; Lee, M.; Jeon, S.W. The sensitivity of species distribution modeling to scale differences. Ecol. Model. 2013, 248, 113–118. [Google Scholar] [CrossRef]

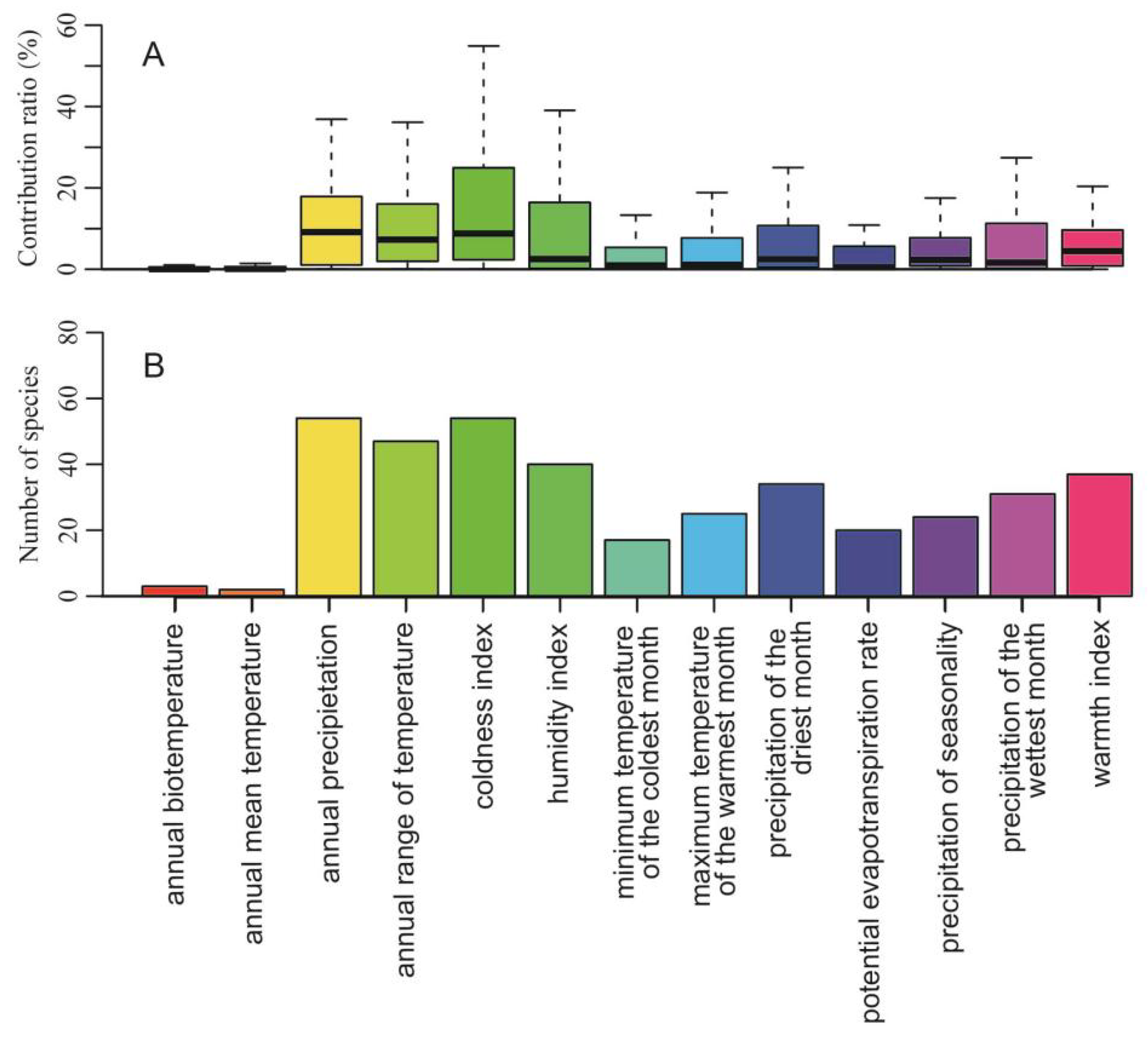



| ID | Variable | Abbreviation | Unit |
|---|---|---|---|
| 1 | Annual mean temperature | AMT | °C |
| 2 | Maximum temperature of the warmest month | MTWM | °C |
| 3 | Minimum temperature of the coldest month | MTCM | °C |
| 4 | Annual range of temperature | ART | °C |
| 5 | Annual precipitation | AP | mm |
| 6 | Precipitation of the wettest month | PWM | mm |
| 7 | Precipitation of the driest month | PDM | mm |
| 8 | Precipitation of seasonality | PSD | mm |
| 9 | Annual biotemperature | ABT | °C |
| 10 | Warmth index | WI | °C |
| 11 | Coldness index | CI | °C |
| 12 | Potential evapotranspiration rate | PER | - |
| 13 | Humidity index | HI | mm/°C |
| ID | Bearing | Abbreviation | Center Angle | Bearing Angle |
|---|---|---|---|---|
| 1 | North | N | 0 | 337.5–22.5 |
| 2 | Northeast | NE | 45 | 22.5–67.5 |
| 3 | East | E | 90 | 67.5–112.5 |
| 4 | Southeast | SE | 135 | 112.5–157.5 |
| 5 | South | S | 180 | 157.5–202.5 |
| 6 | Southwest | SW | 225 | 202.5–247.5 |
| 7 | West | W | 270 | 247.5–292.5 |
| 8 | Northwest | NW | 315 | 292.5–337.5 |
| Kappa | Agreement |
|---|---|
| <0.01 | No agreement |
| 0.01–0.20 | None to slight |
| 0.20–0.40 | Fair agreement |
| 0.40–0.60 | Moderate agreement |
| 0.60–0.80 | Substantial agreement |
| 0.80–1.00 | Almost perfect agreement |
| Direction | Number of Species | Speed of Shift (km/year) | ||||||
|---|---|---|---|---|---|---|---|---|
| RCP2.6 | RCP4.5 | RCP6.0 | RCP8.5 | RCP2.6 | RCP4.5 | RCP6.0 | RCP8.5 | |
| N | 16 | 13 | 13 | 14 | 0.86 ± 0.45 | 1.61 ± 0.77 | 1.53 ± 0.87 | 2.41 ± 1.58 |
| NE | 15 | 8 | 14 | 10 | 1.35 ± 0.88 | 1.63 ±1.37 | 1.79 ± 1.36 | 2.63 ± 2.47 |
| E | 6 | 10 | 7 | 6 | 2.34 ± 1.82 | 1.8 ± 1.96 | 2.3 ± 2.28 | 3.09 ± 2.49 |
| SE | 6 | 3 | 3 | 1 | 0.71 ± 0.53 | 1.52 ± 0.4 | 1.61 ± 0.43 | 2.27 |
| S | 0 | 1 | 1 | 3 | - | 0.79 | 0.80 | 1.13 ± 1.02 |
| SW | 3 | 5 | 7 | 8 | 2.59 ± 1.10 | 4.80 ± 3.24 | 3.31 ± 3.03 | 5.65 ± 5.14 |
| W | 20 | 23 | 26 | 30 | 2.00 ± 0.99 | 2.45 ± 1.27 | 2.46 ± 1.5 | 3.81 ± 2.42 |
| NW | 33 | 36 | 28 | 27 | 1.18 ± 0.75 | 1.84 ± 1.2 | 1.83 ± 1.04 | 3.21 ± 2.61 |
| Average shift speed across species and directions | 1.40 ± 0.99 | 2.06 ± 1.54 | 2.07 ± 1.53 | 3.34 ± 2.74 | ||||
| Region | Scenario | N | NE | E | SE | S | SW | W | NW |
|---|---|---|---|---|---|---|---|---|---|
| Monsoon region | RCP2.6 | 0.87 ± 0.47 | 1.47 ± 0.89 | 0.97 | 0.77 ± 0.77 | - | - | 2.21 ± 1.12 | 1.52 ± 0.87 |
| (14) | (10) | (1) | (3) | (6) | (15) | ||||
| RCP4.5 | 1.60 ± 0.86 | 1.88 ± 1.52 | 0.95 ± 0.87 | 1.60 | - | - | 3.40 ± 1.30 | 2.01 ± 1.29 | |
| (10) | (6) | (6) | (1) | (7) | (19) | ||||
| RCP6.0 | 1.53 ± 0.99 | 1.88 ± 1.44 | 1.13 ± 0.31 | 1.42 | - | 1.13 | 3.39 ± 1.85 | 2.09 ± 1.22 | |
| (10) | (11) | (3) | (1) | (1) | (7) | (16) | |||
| RCP8.5 | 2.42 ± 1.89 | 3.17 ± 2.49 | 1.35 ± 0.57 | 2.27 | 0.45 | 1.60 ± 0.13 | 6.23 ± 2.09 | 3.62 ± 3.16 | |
| (10) | (8) | (3) | (1) | (1) | (2) | (7) | (17) | ||
| Alpine region | RCP2.6 | - | - | - | - | - | - | 1.81 ± 0.64 | 1.01 ± 0.54 |
| (10) | (12) | ||||||||
| RCP4.5 | - | - | - | - | - | - | 2.00 ± 0.71 | 1.73 ± 0.87 | |
| (10) | (12) | ||||||||
| RCP6.0 | - | - | - | - | - | - | 2.37 ± 1.00 | 1.44 ± 0.48 | |
| (13) | (9) | ||||||||
| RCP8.5 | 2.21 | - | - | - | - | - | 3.02 ± 1.27 | 2.52 ± 1.05 | |
| (1) | (11) | (10) | |||||||
| Arid region | RCP2.6 | 0.79 ± 0.22 | 1.11 ± 0.90 | 2.61 ± 1.89 | 0.64 ± 0.33 | - | 2.59 ± 1.11 | 2.14 ± 1.63 | 0.66 ± 0.32 |
| (2) | (5) | (5) | (3) | (3) | (4) | (6) | |||
| RCP4.5 | 1.64 ± 0.50 | 0.85 ± 0.32 | 3.07 ± 2.58 | 1.48 ± 0.56 | 0.79 | 4.8 ± 3.24 | 2.08 ± 1.53 | 1.46 ± 1.63 | |
| (3) | (2) | (4) | (2) | (1) | (5) | (6) | (5) | ||
| RCP6.0 | 1.52 ± 0.31 | 1.48 ± 1.16 | 3.17 ± 2.81 | 1.70 ± 0.57 | 0.80 | 3.67 ± 3.15 | 1.57 ± 1.60 | 1.55 ± 1.06 | |
| (3) | (3) | (4) | (2) | (1) | (6) | (6) | (3) | ||
| RCP8.5 | 2.42 ± 0.15 | 0.49 ± 0.26 | 4.84 ± 2.44 | - | 1.47 ± 1.17 | 7.00 ± 5.31 | 3.11 ± 2.58 | - | |
| (3) | (2) | (3) | (2) | (6) | (12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Huang, J. Multi-Directional Rather Than Unidirectional Northward-Dominant Range Shifts Predicted under Climate Change for 99 Chinese Tree Species. Forests 2022, 13, 1619. https://doi.org/10.3390/f13101619
Li G, Huang J. Multi-Directional Rather Than Unidirectional Northward-Dominant Range Shifts Predicted under Climate Change for 99 Chinese Tree Species. Forests. 2022; 13(10):1619. https://doi.org/10.3390/f13101619
Chicago/Turabian StyleLi, Guoqing, and Jinghua Huang. 2022. "Multi-Directional Rather Than Unidirectional Northward-Dominant Range Shifts Predicted under Climate Change for 99 Chinese Tree Species" Forests 13, no. 10: 1619. https://doi.org/10.3390/f13101619





