Review on the Phase Change Materials in Wood for Thermal Regulative Wood-Based Products
Abstract
1. Introduction
2. Phase Change Material (PCM)
3. Classification of Phase Change Materials
4. Impregnation and Evaluation of Phase Change Material in Wood
5. Insight into Previous Works on Phase Change Materials Embedded in Wood
6. Challenges of PCM-Impregnated Wood in Real World Application
7. Opportunities for Future PCM-Based Wood Products
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Lou, X.; Feng, M.; Geng, Z.; Chen, L.; Ping, W.; Lu, G. Energy consumption analysis and saving of buildings based on static and dynamic input-output models. Energy 2022, 239, 122240. [Google Scholar] [CrossRef]
- Hassan, J.; Mohamad zin, R.; Abd Majid, M.Z.; Balubaid, S.; Hainin, M.R. Building Energy Consumption in Malaysia: An Overview. J. Teknol. 2014, 707, 2180–3722. [Google Scholar] [CrossRef]
- Temiz, A.; Gökhan, H.; Gaye, K.D.; Ahmet, S.; Mohd Hazim, M.A. Phase Change Material Impregnated Wood for Passive Thermal Management of Timber Buildings. Int. J. Energy Res. 2020, 44, 10495–10505. [Google Scholar] [CrossRef]
- Wróblewski, P.; Niekurzak, M. Assessment of the Possibility of Using Various Types of Renewable Energy Sources Installations in Single-Family Buildings as Part of Saving Final Energy Consumption in Polish Conditions. Energies 2022, 15, 1329. [Google Scholar] [CrossRef]
- Zhao, C.-S.; Niu, S.-W.; Zhang, X. Effects of Household Energy Consumption on Environment and its Influence Factors in Rural and Urban Areas. Energy Procedia 2012, 14, 805–811. [Google Scholar]
- Dincer, I.; Acar, C. A review on clean energy solutions for better sustainability. Int. J. Energy Res. 2015, 39, 585–606. [Google Scholar] [CrossRef]
- Aftab, W.; Usman, A.; Shi, J.; Yuan, K.; Qin, M.; Zou, R. Phase change material-integrated latent heat storage systems for sustainable energy solutions. Energy Environ. Sci. 2021, 14, 4268–4291. [Google Scholar] [CrossRef]
- Harvey, D. Reducing Energy Use in the Buildings Sector: Measures, Costs, and Examples. Energy Effic. 2009, 2, 139–163. [Google Scholar] [CrossRef]
- James, P.W. Use of Wood in Buildings and Bridges. In Wood Handbook—Wood as an Engineering Material, Centennial Edition ed.; Robert, J.R., Ed.; Forest Products Laboratory (FPL): Madison, WI, USA, 2010; 508p. [Google Scholar]
- Bak, M.; Molnár, F.; Rákosa, R.; Németh, Z.; Németh, R. Dimensional stabilization of wood by microporous silica aerogel using in-situ polymerization. Wood Sci. Technol. 2022, 56. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Pang, J.; Mei, C. Heat treatment induces chemical changes and silica sol penetration in wood for properties improvement: Hydrophobicity, thermal stability, and surface hardness. J. Wood Chem. Technol. 2022, 42, 104–113. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, Y.; Mei, C.; Cao, J. Polyethylene glycol and silica sol penetration improves hydrophobicity and dimensional stability of wood after a short-time treatment. Eur. J. Wood Wood Prod. 2021, 79, 1395–1404. [Google Scholar] [CrossRef]
- Shen, H.; Cao, J.; Jiang, J.; Xu, J. Antiweathering properties of a thermally treated wood surface by two-step treatment with titanium dioxide nanoparticle growth and polydimethylsiloxane coating. Prog. Org. Coat. 2018, 125, 1–7. [Google Scholar] [CrossRef]
- Michael, C.W. Characteristics and Availability of Commercially Important Woods. In Wood Handbook—Wood as an Engineering Material, Centennial Edition ed.; Robert, J.R., Ed.; Forest Products Laboratory (FPL): Madison, WI, USA, 2010; 508p. [Google Scholar]
- Zou, Y.; Yang, P.; Yang, L.; Li, N.; Duan, G.; Liu, X.; Li, Y. Boosting solar steam generation by photothermal enhanced polydopamine/wood composites. Polymer 2021, 217, 123464. [Google Scholar] [CrossRef]
- Tsoumis, G. Science and Technology of Wood: Structure, Properties, Utilization; Van Nostrand Reinhold: New York, NY, USA, 1991; Volume 115. [Google Scholar]
- Zhang, L.; Chen, Z.; Dong, H.; Fu, S.; Ma, L.; Yang, X. Wood plastic composites based wood wall’s structure and thermal insulation performance. J. Bioresour. Bioprod. 2021, 6, 65–74. [Google Scholar] [CrossRef]
- Robert, H.F. Wood as a Sustainable Building Material. In Wood Handbook—Wood as an Engineering Material, Centennial Edition ed.; Robert, J.R., Ed.; Forest Products Laboratory (FPL): Madison, WI, USA, 2010; Volume 1, 508p. [Google Scholar]
- Huang, C.; Chui, Y.; Gong, M.; Chana, F. Mechanical behaviour of wood compressed in radial direction: Part II. Influence of temperature and moisture content. J. Bioresour. Bioprod. 2020, 5, 266–275. [Google Scholar] [CrossRef]
- Ma, X.; Xiong, Y.; Liu, Y.; Han, J.; Duan, G.; Chen, Y.; He, S.; Mei, C.; Jiang, S.; Zhang, K. When MOFs meet wood: From opportunities toward applications. Chem 2022, 8, 2342–2361. [Google Scholar] [CrossRef]
- Demirbas, M.F. Thermal Energy Storage and Phase Change Materials: An Overview. Energy Sour. Part B Econ. Plan. Policy 2006, 1, 85–95. [Google Scholar] [CrossRef]
- Eanest Jebasingh, B.; Valan Arasu, A. A comprehensive review on latent heat and thermal conductivity of nanoparticle dispersed phase change material for low-temperature applications. Energy Storage Mater. 2020, 24, 52–74. [Google Scholar] [CrossRef]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Mohsen, M. (Ed.) Introductory Chapter: Phase Change Material. In Phase Change Materials and Their Applications; IntechOpen: London, UK, 2018; 174p. [Google Scholar]
- Li, Y.; Huang, X.; Lv, J.; Wang, F.; Jiang, S.; Wang, G. Enzymolysis-treated wood-derived hierarchical porous carbon for fluorescence-functionalized phase change materials. Compos. Part B Eng. 2022, 234, 109735. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Huang, X.; Jiang, S.; Wang, G. Anisotropy-functionalized cellulose-based phase change materials with reinforced solar-thermal energy conversion and storage capacity. Chem. Eng. J. 2021, 415, 129086. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.K.; Sharma, V.K. Application of phase change material for thermal energy storage: An overview of recent advances. Mater. Today Proc. 2021, 44, 368–375. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Zeynali Famileh, I.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Rathod, M.K. Thermal Stability of Phase Change Material. In Phase Change Materials and Their Applications; Mhadhbi, M., Ed.; IntechOpen: London, UK, 2018; 174p. [Google Scholar]
- Zari, N.; Raji, M.; El Mghari, H.; Bouhfid, R.; Qaiss, A.E.K. Chapter 3—Nanoclay and polymer-based nanocomposites: Materials for energy efficiency. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Jawaid, M., Khan, M.M., Eds.; Woodhead Publishing: Southen, UK, 2018; pp. 75–103. [Google Scholar]
- Bruno, F.; Belusko, M.; Liu, M.; Tay, N.H.S. Chapter 9—Solid-liquid phase change materials for thermal energy storage. In Advances in Thermal Energy Storage Systems, 2nd ed.; Cabeza, L.F., Ed.; Woodhead Publishing: Southen, UK, 2021; pp. 221–268. [Google Scholar]
- Almousa, N.H.; Alotaibi, M.R.; Alsohybani, M.; Radziszewski, D.; AlNoman, S.M.; Alotaibi, B.M.; Khayyat, M.M. Paraffin Wax [As a Phase Changing Material (PCM)] Based Composites Containing Multi-Walled Carbon Nanotubes for Thermal Energy Storage (TES) Development. Crystals 2021, 11, 951. [Google Scholar] [CrossRef]
- Vijayrakesh, K.; Muthuvel, S.; Gopinath, G.R.; Qarnain, S.S.; Bathrinath, S. Experimental investigation of the performance of paraffin wax-packed floor on a solar dryer. J. Energy Storage 2021, 43, 103163. [Google Scholar] [CrossRef]
- Gonzalez-Nino, D.; Boteler, L.M.; Ibitayo, D.; Jankowski, N.R.; Urciuoli, D.; Kierzewski, I.M.; Quintero, P.O. Experimental evaluation of metallic phase change materials for thermal transient mitigation. Int. J. Heat Mass Transf. 2018, 116, 512–519. [Google Scholar] [CrossRef]
- Sari, A.; Kaygusuz, K. Thermal performance of a eutectic mixture of lauric and stearic acids as PCM encapsulated in the annulus of two concentric pipes. Sol. Energy 2002, 72, 493–504. [Google Scholar] [CrossRef]
- Majó, M.; Sánchez, R.; Barcelona, P.; García, J.; Fernández, A.I.; Barreneche, C. Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization. Molecules 2021, 26, 982. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, N.; Hirschey, J.; Akamo, D.O.; Li, K.; Tugba, T.; Goswami, M.; Orlando, R.; LaClair, T.J.; Graham, S.; et al. Stable salt hydrate-based thermal energy storage materials. Compos. Part B Eng. 2022, 233, 109621. [Google Scholar] [CrossRef]
- Dhivya, S.; Hussain, S.I.; Jeya Sheela, S.; Kalaiselvam, S. Experimental study on microcapsules of Ag doped ZnO nanomaterials enhanced Oleic-Myristic acid eutectic PCM for thermal energy storage. Thermochim. Acta 2019, 671, 70–82. [Google Scholar] [CrossRef]
- Kateshia, J.; Lakhera, V. A comparative study of various fatty acids as phase change material to enhance the freshwater productivity of solar still. J. Energy Storage 2022, 48, 103947. [Google Scholar] [CrossRef]
- Zheng, M.; Peng, X.; Liu, J.; Zhang, S.; Zhang, X. Preparation and characterization of composite hydrate salt PCM of industrial grade disodium hydrogen phosphate with sodium carbonate. Int. J. Energy Res. 2021, 45, 7129–7144. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R.; Kuturu, V. Preparation and thermal performance of methyl palmitate and lauric acid eutectic mixture as phase change material (PCM). J. Energy Storage 2017, 13, 418–424. [Google Scholar] [CrossRef]
- Prasannaa, P.; Ramkumar, R.; Sunilkumar, K.; Rajasekar, R. Experimental study on a binary mixture ratio of fatty acid-based PCM integrated to PV panel for thermal regulation on a hot and cold month. Int. J. Sustain. Energy 2021, 40, 218–234. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, D. Thermal characteristics of hydrated salt blended with Tio2 for thermal energy storage. Heat Transf. 2022, 51, 5368–5385. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Appukuttan, S. Preparation and thermal properties of lauric acid/myristyl alcohol as a novel binary eutectic phase change material for indoor thermal comfort. Energy Storage 2019, 1, e80. [Google Scholar] [CrossRef]
- Lizcano-González, V.A.; Kafarov, V.V.; Mahkamov, K. Production of Fatty Esters from F Palm Oil By-Products for use as Phase Change Materials. Chem. Eng. Trans. 2022, 94, 331–336. [Google Scholar]
- Agrawal, R.; Singh, K.D.P.; Sharma, R.K. Experimental investigations on the phase change and thermal properties of nano enhanced binary eutectic phase change material of palmitic acid-stearic acid/CuO nanoparticles for thermal energy storage. Int. J. Energy Res. 2022, 46, 6562–6576. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Cho, H. Investigation on thermal properties enhancement of lauryl alcohol with multi-walled carbon nanotubes as phase change material for thermal energy storage. Case Stud. Therm. Eng. 2022, 31, 101826. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene–Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef]
- Zhao, P.-P.; Deng, C.; Zhao, Z.-Y.; Lu, P.; He, S.; Wang, Y.-Z. Hypophosphite tailored graphitized hierarchical porous biochar toward highly efficient solar thermal energy harvesting and stable Storage/Release. Chem. Eng. J. 2021, 420, 129942. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, W.; Yang, Y.; Zhao, J.; Liu, Y.; Guo, H. Transparent wood with phase change heat storage as novel green energy storage composites for building energy conservation. J. Clean. Prod. 2021, 296, 126598. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite phase change materials with good reversible thermochromic ability in delignified wood substrate for thermal energy storage. Appl. Energy 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, L. Delignified wood/capric acid-palmitic acid mixture stable-form phase change material for thermal storage. Sol. Energy Mater. Sol. Cells 2019, 194, 215–221. [Google Scholar] [CrossRef]
- Chen, X.; Guo, X.; Lin, X.; Fan, M.; Sun, W. pH-responsive wood-based phase change material for thermal energy storage building material application. J. Mater. Sci. 2022, 57, 13515–13526. [Google Scholar] [CrossRef]
- Chen, H.; Xuan, J.; Deng, Q.; Gao, Y. Wood/PCM Composite With Enhanced Energy Storage Density And Anisotropic Thermal Conductivity. Prog. Nat. Sci. Mater. Int. 2022, 32, 190–195. [Google Scholar] [CrossRef]
- Said, M.S.M.; Tohir, M.Z.M. The effect of ultraviolet coating on containment and fire hazards of phase change materials impregnated wood structure. J. Energy Storage 2020, 32, 101727. [Google Scholar] [CrossRef]
- Barreneche, C.; Vecstaudza, J.; Bajare, D.; Fernandez, A.I. PCM/wood composite to store thermal energy in passive building envelopes. IOP Conf. Ser. Mater. Sci. Eng. 2017, 251, 012111. [Google Scholar] [CrossRef]
- Lin, X.; Jia, S.; Liu, J.; Wang, W.; Cao, H.; Guo, X.; Sun, W. Fabrication of thermal energy storage wood based on graphene aerogel encapsulated polyethylene glycol as phase change material. Mater. Res. Express 2020, 7, 095503. [Google Scholar] [CrossRef]
- Wang, W.; Cao, H.; Liu, J.; Jia, S.; Ma, L.; Guo, X.; Sun, W. A thermal energy storage composite by incorporating microencapsulated phase change material into wood. RSC Adv. 2020, 10, 8097–8103. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Park, J. An Experimental Study on the Thermal Performance of Phase-Change Material and Wood-Plastic Composites for Building Roofs. Energies 2017, 10, 195. [Google Scholar] [CrossRef]
- Sarı, A.; Hekimoğlu, G.; Tyagi, V.V. Low cost and eco-friendly wood fiber-based composite phase change material: Development, characterization and lab-scale thermoregulation performance for thermal energy storage. Energy 2020, 195, 116983. [Google Scholar] [CrossRef]
- Yang, H.; Chao, W.; Di, X.; Yang, Z.; Yang, T.; Yu, Q.; Liu, F.; Li, J.; Li, G.; Wang, C. Multifunctional wood based composite phase change materials for magnetic-thermal and solar-thermal energy conversion and storage. Energy Convers. Manag. 2019, 200, 112029. [Google Scholar] [CrossRef]
- Saavedra, H.; García-Herrera, C.; Vasco, D.A.; Salinas-Lira, C. Characterization of mechanical performance of Pinus radiata wood impregnated with octadecane as phase change material. J. Build. Eng. 2021, 34, 101913. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Wang, X.; Chao, W.; Wang, N.; Ding, X.; Liu, F.; Yu, Q.; Yang, T.; Yang, Z.; et al. Wood-based composite phase change materials with self-cleaning superhydrophobic surface for thermal energy storage. Appl. Energy 2020, 261, 114481. [Google Scholar] [CrossRef]
- Liu, S.; Sheng, M.; Wu, H.; Shi, X.; Lu, X.; Qu, J. Biological porous carbon encapsulated polyethylene glycol-based phase change composites for integrated electromagnetic interference shielding and thermal management capabilities. J. Mater. Sci. Technol. 2022, 113, 147–157. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Xu, X.; Guo, X.; Cao, J. Processing solid wood into a composite phase change material for thermal energy storage by introducing silica-stabilized polyethylene glycol. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106098. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, J.; Zhao, Y.; Wang, G.; Gu, W.; Ji, G. Hierarchically porous wood-derived carbon scaffold embedded phase change materials for integrated thermal energy management, electromagnetic interference shielding and multifunctional application. Carbon 2021, 183, 515–524. [Google Scholar] [CrossRef]
- Cheng, L.; Feng, J. Form-stable phase change materials based on delignified wood flour for thermal management of buildings. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105690. [Google Scholar] [CrossRef]
- Shi, X.; Meng, Y.; Bi, R.; Wan, Z.; Zhu, Y.; Rojas, O.J. Enabling unidirectional thermal conduction of wood-supported phase change material for photo-to-thermal energy conversion and heat regulation. Compos. Part B Eng. 2022, 245, 110231. [Google Scholar] [CrossRef]
- Mohamad Amini, M.H.; Temiz, A.; Hekimoğlu, G.; Köse Demirel, G.; Sarı, A. Properties of Scots pine wood impregnated with capric acid for potential energy saving building material. Holzforschung 2022, 76, 744–753. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, H.; Wang, X.; Wang, X.; Cui, E.; Wang, L. Form-stable paraffin/rice straw/polyvinyl alcohol composite phase change material for thermal energy storage. Mater. Lett. 2021, 294, 129790. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, C.; Jin, T.; Dong, H. Water evaporation inspired biomass-based PCM from daisy stem and paraffin for building temperature regulation. Renew. Energy 2022, 194, 211–219. [Google Scholar] [CrossRef]
- Gencel, O.; Sarı, A.; Kaplan, G.; Ustaoglu, A.; Hekimoğlu, G.; Bayraktar, O.Y.; Ozbakkaloglu, T. Properties of eco-friendly foam concrete containing PCM impregnated rice husk ash for thermal management of buildings. J. Build. Eng. 2022, 58, 104961. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Pan, X.; Zhong, W.; Qiu, B.; Cai, Y.; Yuan, Y. Thermal properties of biomass-based form-stable phase change material for latent heat thermal energy storage. Int. J. Energy Res. 2021, 45, 20372–20383. [Google Scholar] [CrossRef]
- Wen, R.; Liu, Y.; Yang, C.; Zhu, X.; Huang, Z.; Zhang, X.; Gao, W. Enhanced thermal properties of stearic acid/carbonized maize straw composite phase change material for thermal energy storage in buildings. J. Energy Storage 2021, 36, 102420. [Google Scholar] [CrossRef]
- Feng, N.; Kang, Z.; Hu, D. Shape-stabilized and antibacterial composite phase change materials based on wood-based cellulose micro-framework, erythritol-urea or erythritol-thiourea for thermal energy storage. Sol. Energy 2021, 223, 19–32. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Du, Y.; Lu, X.; Qu, J. Shape-stable composite phase change materials encapsulated by bio-based balsa wood for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 230, 111187. [Google Scholar] [CrossRef]
- Liang, B.; Lu, X.; Li, R.; Tu, W.; Yang, Z.; Yuan, T. Solvent-free preparation of bio-based polyethylene glycol/wood flour composites as novel shape-stabilized phase change materials for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110037. [Google Scholar] [CrossRef]
- Fernández, V.; Valderrama-Ulloa, C.; Rouault, F.; Schmitt, C.; del Río, R.; Vasco, D. Thermal and Mechanical Analysis of Plywood Boards Thermally Enhanced with Phase Change Materials. IOP Conf. Ser. Earth Environ. Sci. 2020, 503, 012074. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Liu, Z.; Liang, D.; Liu, F.; Zhang, W.; Di, X.; Wang, C.; Ho, S.-H.; Chen, W.-H. Enhanced thermal conductivity of waste sawdust-based composite phase change materials with expanded graphite for thermal energy storage. Bioresour. Bioprocess. 2017, 4, 52. [Google Scholar] [CrossRef]
- Sarı, A.; Hekimoğlu, G.; Karabayır, Y.; Sharma, R.K.; Arslanoğlu, H.; Gencel, O.; Tyagi, V.V. Capric-stearic acid mixture impregnated carbonized waste sugar beet pulp as leak-resistive composite phase change material with effective thermal conductivity and thermal energy storage performance. Energy 2022, 247, 123501. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.; Olayiwola, S.; Lau, C.; Fan, M.; Ng, K.; Tan, G. Biomass-derived porous carbons support in phase change materials for building energy efficiency: A review. Mater. Today Energy 2022, 23, 100905. [Google Scholar] [CrossRef]
- Kalbasi, R. Usefulness of PCM in building applications focusing on envelope heat exchange—Energy saving considering two scenarios. Sustain. Energy Technol. Assess. 2022, 50, 101848. [Google Scholar] [CrossRef]
- Soleiman Dehkordi, B.; Afrand, M. Energy-saving owing to using PCM into buildings: Considering of hot and cold climate region. Sustain. Energy Technol. Assess. 2022, 52, 102112. [Google Scholar] [CrossRef]
- Casini, M. (Ed.) 5—Phase-change materials. In Smart Buildings; Woodhead Publishing: Southen, UK, 2016; pp. 179–218. [Google Scholar]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Thermal cycling test of few selected inorganic and organic phase change materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Tebaldi, M.L.; Belardi, R.M.; Montoro, S.R. Chapter 8—Polymers with Nano-Encapsulated Functional Polymers: Encapsulated Phase Change Materials. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Boston, MA, USA, 2016; pp. 155–169. [Google Scholar]
- Shah, K.W.; Huseien, G.F. Chapter 17—Nanostructures encapsulated phase-change materials for sustained thermal energy storage in concrete. In Green Nanomaterials for Industrial Applications; Shanker, U., Hussain, C.M., Rani, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 477–507. [Google Scholar]
- Roy, U.; Pant, H.K. Chapter 9—Current progress in heat exchangers with phase change materials (PCMs): A comprehensive investigation. In Advanced Analytic and Control Techniques for Thermal Systems with Heat Exchangers; Pekař, L., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 219–230. [Google Scholar]
- Safarian, J.; Tangstad, M. Chapter 4—Phase change materials for high-temperature operation. In Ultra-High Temperature Thermal Energy Storage, Transfer and Conversion; Datas, A., Ed.; Woodhead Publishing: Southen, UK, 2021; pp. 85–111. [Google Scholar]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Abu-Eishah, S.I. Correlations for the Thermal Conductivity of Metals as a Function of Temperature. Int. J. Thermophys. 2001, 22, 1855–1868. [Google Scholar] [CrossRef]
- Bauer, T.; Tamme, R.; Christ, M.; Öttinger, O. PCM-graphite composites for high temperature thermal energy storage. In Proceedings of the 10th International Conference on Thermal Energy Storage (ECOSTOCK 2006), Galloway, NJ, USA, 31 May–2 June 2006. [Google Scholar]
- Bugaje, I.M. Enhancing the thermal response of latent heat storage systems. Int. J. Energy Res. 1997, 21, 759–766. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Impregnation of porous material with phase change material for thermal energy storage. Mater. Chem. Phys. 2009, 115, 846–850. [Google Scholar] [CrossRef]
- Tauseef ur, R.; Ali, H.M.; Janjua, M.M.; Sajjad, U.; Yan, W.-M. A critical review on heat transfer augmentation of phase change materials embedded with porous materials/foams. Int. J. Heat Mass Transf. 2019, 135, 649–673. [Google Scholar] [CrossRef]
- Kong, I. Chapter 7—Polymers with Nano-Encapsulated Functional Polymers. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Boston, MA, USA, 2016; pp. 125–154. [Google Scholar]
- Rathore, P.K.S.; Shukla, S.K. Improvement in thermal properties of PCM/Expanded vermiculite/expanded graphite shape stabilized composite PCM for building energy applications. Renew. Energy 2021, 176, 295–304. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Yang, S.; Ok, Y.S.; Kim, S. Characterization of biocomposite using coconut oil impregnated biochar as latent heat storage insulation. Chemosphere 2019, 236, 124269. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-G.; Lee, J.-H.; Seo, J.; Kim, S. Thermal performance evaluation of Bio-based shape stabilized PCM with boron nitride for energy saving. Int. J. Heat Mass Transf. 2014, 71, 245–250. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Alam, M.; Zou, P.X.W.; Sanjayan, J.; Ramakrishnan, S. Energy saving performance assessment and lessons learned from the operation of an active phase change materials system in a multi-storey building in Melbourne. Appl. Energy 2019, 238, 1582–1595. [Google Scholar] [CrossRef]
- Hamidi, Y.; Aketouane, Z.; Malha, M.; Bruneau, D.; Bah, A.; Goiffon, R. Integrating PCM into hollow brick walls: Toward energy conservation in Mediterranean regions. Energy Build. 2021, 248, 111214. [Google Scholar] [CrossRef]
- Mahdaoui, M.; Hamdaoui, S.; Ait Msaad, A.; Kousksou, T.; El Rhafiki, T.; Jamil, A.; Ahachad, M. Building bricks with phase change material (PCM): Thermal performances. Constr. Build. Mater. 2021, 269, 121315. [Google Scholar] [CrossRef]
- Paranjothi, G.; Odukomaiya, A.; Cui, S.; Bulk, A. Evaluation of phase change plaster/paste composites for building envelopes. Energy Build. 2021, 253, 111372. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Nas, M.; Ouikhalfan, M.; Sarı, A.; Tyagi, V.V.; Sharma, R.K.; Kurbetci, Ş.; Saleh, T.A. Silica fume/capric acid-stearic acid PCM included-cementitious composite for thermal controlling of buildings: Thermal energy storage and mechanical properties. Energy 2021, 219, 119588. [Google Scholar] [CrossRef]
- Larwa, B.; Cesari, S.; Bottarelli, M. Study on thermal performance of a PCM enhanced hydronic radiant floor heating system. Energy 2021, 225, 120245. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, Y. A semi-analytical model for evaluating the thermal storage capacity and heat use efficiency of flexible thermal storage heating floor. Appl. Therm. Eng. 2021, 198, 117448. [Google Scholar] [CrossRef]
- Rida, M.; Hoffmann, S. The influence of macro-encapsulated PCM panel’s geometry on heat transfer in a ceiling application. Adv. Build. Energy Res. 2022, 16, 445–465. [Google Scholar] [CrossRef]
- Gallardo, A.; Berardi, U. Evaluation of the energy flexibility potential of radiant ceiling panels with thermal energy storage. Energy 2022, 254, 124447. [Google Scholar] [CrossRef]
- Boobalakrishnan, P.; Manoj Kumar, P.; Balaji, G.; Jenaris, D.S.; Kaarthik, S.; Jaya Prakash Babu, M.; Karthhik, K. Thermal management of metal roof building using phase change material (PCM). Mater. Today Proc. 2021, 47, 5052–5058. [Google Scholar] [CrossRef]
- Meng, E.; Yang, J.; Zhou, B.; Wang, C.; Li, J. Preparation and thermal performance of phase change material (PCM) foamed cement used for the roof. J. Build. Eng. 2022, 53, 104579. [Google Scholar] [CrossRef]


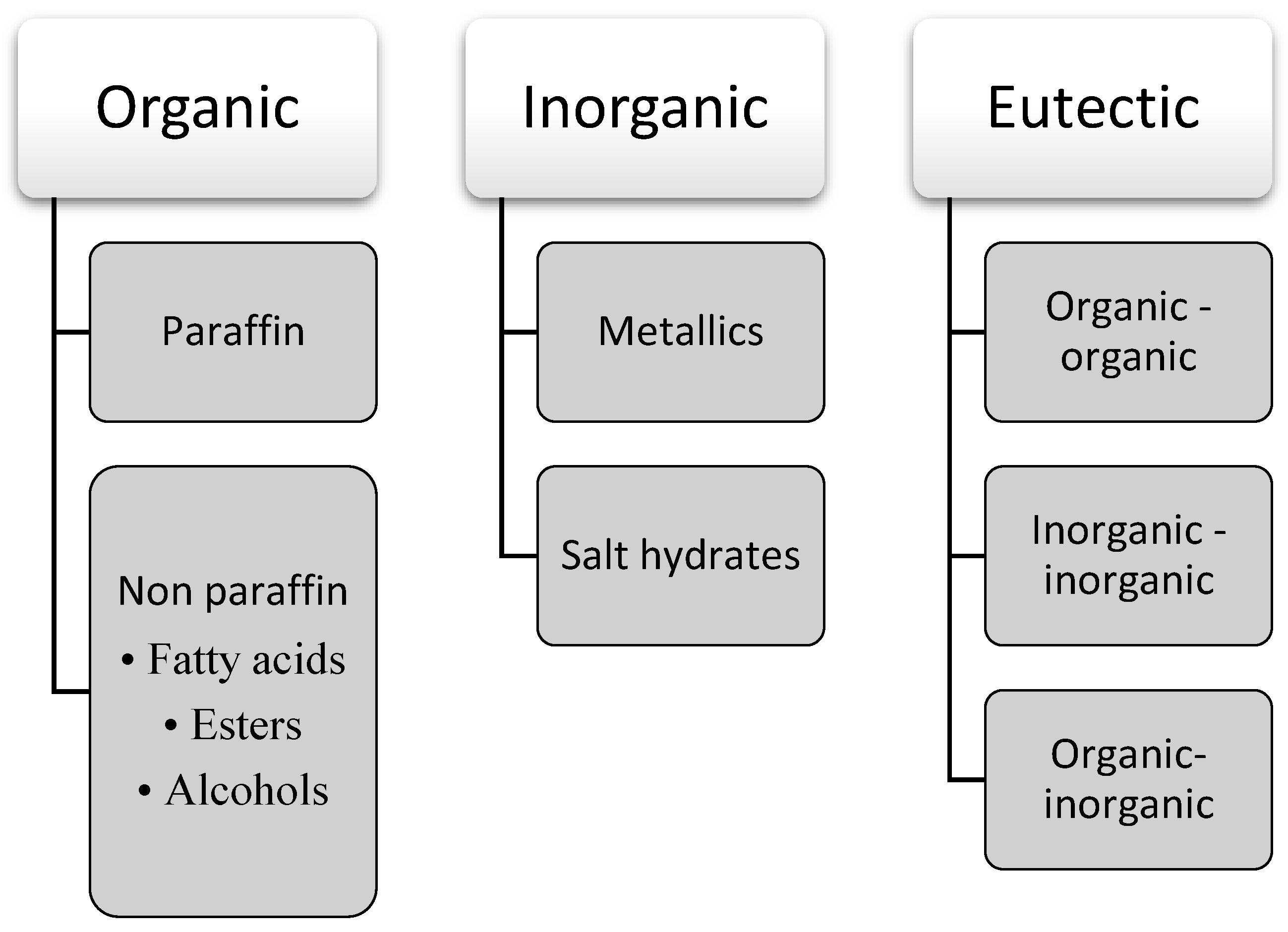
| Organics | Inorganics | Eutectics | |
|---|---|---|---|
| Advantages | High heat of fusion | High heat of fusion | Sharp melting temperature |
| Available in large temperature range | High thermal conductivity | High volumetric thermal storage density | |
| Minimal supercooling (self-nucleation) | Low vapor pressure in the melt state | ||
| Melt and freeze repeatedly without phase segregation | Sharp melting point | ||
| Congruent phase transition process | High volumetric latent heat storage capacity | ||
| High thermal stability | Low volume change | ||
| Non-corrosive, non-reactive | Non-corrosive, nonreactive and non-flammable | ||
| Recyclable | Low cost and easy availability | ||
| Chemically stable | Good compatibility with conventional construction materials | ||
| Drawbacks | Expensive | Slightly toxic in nature | Highly expensive |
| Low thermal conductivity | Supercooling (low degree of nucleation) | ||
| Low density | Corrosive with some metals | ||
| Least compatible with plastic containments | Dehydration occurs during the phase change process | ||
| Flammable (depending on the containment) | Compatibility with some building materials is limited | ||
| Low volumetric latent heat storage capacity | High volume change | ||
| Examples | Paraffin wax (Paraffin type) [32,33] | Fields’ metal (32.5Bi/51In/16.5Sn wt%) and 49Bi/18Pb/12Sn/21In wt% (Metallic type) [34] | Mixture of lauric and stearic acid [35] |
| Capric acid (Non-paraffin, Fatty acid type) [36] | Sodium sulfate decahydrate (SSD) salt hydrate (Salt hydrates) [37] | Oleic-Myristic acid eutectic PCM [38] | |
| Stearic acid (Non-paraffin, Fatty acid type) [39] | Na2HPO4·12H2O hydrated salt (Salt hydrates) [40] | Methyl palmitate and lauric acid eutectic mixture [41] | |
| Palmitic acid (Non-paraffin, Fatty acid type) [42] | NaNO3/KNO3 (Salt hydrates) [43] | Lauric acid/myristyl alcohol eutectic mixture [44] | |
| Hydrogenated palm stearin (Non-paraffin, Ester type) [45] | Palmitic acid-stearic acid/CuO nanoparticles [46] | ||
| Lauryl alcohol (Non-paraffin, alcohol type) [47] |
| Phase Change Material | Type of PCM | Additive | Application | Citation |
|---|---|---|---|---|
| Paraffin wax RT21 and propyl ester (80% stearic + 20% palmitate) | Organic and Eutectic | Ammonium polyphosphate (APP) as flame retardant | Impregnated into solid pine wood before UV curable coating | Said and Tohir [56] |
| Paraffin waxes (RT-21 and RT-27 from Rubitherm) | Organic | N/A | Impregnated into Black Alder solid wood prior to polystyrene coating | Barreneche et al. [57] |
| Polyethylene glycol (PEG) | Organic | N/A | Encapsulated and impregnated into wood | Lin et al. [58] |
| Polyethylene glycol (PEG) | Organic | Glucose as carbon quantum dots (CQDs) | Impregnated into enzymolysis treated Bass solid wood | Li, Huang, Lv, Wang, Jiang and Wang [25] |
| Polyethylene glycol-800 (PEG800) | Organic | N/A | Impregnated into solid Poplar wood together with graphene oxide | Lin, Jia, Liu, Wang, Cao, Guo and Sun [58] |
| MicroPCM emulsion (wall material of MicroPCM was formed by two N-alkyl acrylamide monomers) | N/A | N/A | Impregnated into Balsa sapwood | Wang et al. [59] |
| Capric acid (CA) + stearic acid (SA) | Eutectic | N/A | Impregnated into Scots pine solid wood | Temiz, Gökhan, Gaye, Ahmet and Mohd. Hazim [3] |
| Polyethylene glycol 6000 (PEG6000) | Organic | Boron nitride to improve thermal performance | Impregnated into solid Balsa wood | Chen, Xuan, Deng and Gao [55] |
| Paraffin wax (RT 44 and RT 25) | Organic | N/A | PCM packed in nylon plastic bag was placed inside the hollow part of wood plastic composite (WPC) | Chung and Park [60] |
| Polyethylene glycol (PEG) | Organic | N/A | Impregnated into transparent balsa wood | Xia, Zhang, Yang, Zhao, Liu and Guo [51] |
| Capric acid (CA) + stearic acid (SA) | Eutectic | N/A | Impregnated into wood fibre | Sarı et al. [61] |
| 1-tetradecanol (TD) | Organic | Fe3O4 nanoparticles to provide magnetic property | Magnetic wood (Balsa wood) based phase change materials | Yang et al. [62] |
| Octadecane | Organic | N/A | Impregnated into delignified solid Pinus radiata wood | Saavedra et al. [63] |
| 1-tetradecanol (TD) | Organic | Modified SiO2 and epoxy resin as superhydrophobic coating | Produce wood (Basswood) based PCM with self-cleaning superhydrophobic surface | Yang et al. [64] |
| Polyethylene glycol (PEG) | Organic | Fe3O4 magnetic particles as electromagnetic interference (EMI) shielding | Produces PCM based wood as thermal management and electromagnetic shielding | Liu et al. [65] |
| Capric acid and palmitic acid (CA-PA) | Eutectic | N/A | Impregnated into solid Cedar wood | Ma, Wang and Li [53] |
| Polyethylene glycol-800 (PEG800) | Organic | Silica as PCM stabilizer | Silica-stabilized polyethylene glycol (PEG) impregnated into pine (Pinus spp.) sapwood | Xu et al. [66] |
| Paraffin wax | Organic | N/A | Impregnated into Balsa wood blocks | Zhou et al. [67] |
| Myristyl alcohol | Organic | N/A | Impregnated into delignified Poplar wood flour prior to fabrication into board | Cheng and Feng [68] |
| 1-Tetradecanol | Organic | Thermochromic compound (bisphenol-A + 1-Tetradecanol + Crystal violet lactone) | Impregnated into delignified Poplar solid wood | Yang, Wang, Yu, Cao, Yang, Ke, Di, Liu, Zhang and Wang [52] |
| Polyethylene glycol (PEG) | Organic | Polyethylenimine and boron nitride (to improve thermal conductivity) Pyrrole (enhances light absorption and electrical conductivity) | Impregnated into delignified Balsa solid wood to produce PCM based wood with photo-to-thermal energy conversion | Shi et al. [69] |
| Polyethylene glycol 2000 (PEG2000) | Organic | Litmus as pH indicator | Impregnated into particleboard | Chen, Guo, Lin, Fan and Sun [54] |
| Capric acid | Organic | N/A | Impregnated into Scots pine solid wood | Mohamad Amini et al. [70] |
| Paraffin | Organic | N/A | Form-stable paraffin/rice straw/polyvinyl alcohol composite phase change material | Zhang et al. [71] |
| Paraffin | Organic | N/A | Paraffin-loaded daisy stem | Wang et al. [72] |
| Lauryl alcohol (LA) | Organic | N/A | Foam concrete containing PCM impregnated rice husk ash | Gencel et al. [73] |
| Lauric acid, myristic acid, palmitic acid and stearic acid | Organic | N/A | Impregnation of PCM into delignified platane wood | Sun et al. [74] |
| Stearic acid | Organic | N/A | Stearic acid/carbonized maize straw composite phase change material | Wen et al. [75] |
| Erythritol-urea and erythritol-thiourea | Organic | N/A | Erythritol-urea and erythritol-thiourea incorporated into the cellulose skeleton retention of paulownia chip | Feng et al. [76] |
| Myristic acid, paraffin, and polyethylene glycol | Organic | N/A | Lignin and hemicellulose extracted Balsa wood, impregnated with PCM | Liu et al. [77] |
| Polyethylene glycol | Organic | N/A | Bio-based polyethylene glycol/wood flour composites | Liang et al. [78] |
| Microencapsulated paraffin wax | Organic | N/A | Embedded into the adhesive of plywood | Fernández et al. [79] |
| Polyethylene glycol | Organic | Expanded graphite to increase thermal conductivity | Incorporating polyethylene glycol into poplar sawdust with 5% expanded graphite | Yang et al. [80] |
| Capric-stearic acid mixture | Eutectic | N/A | Carbonized waste sugar beet pulp as PCM supporting material | Sarı et al. [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, N.S.; Mohamad Amini, M.H. Review on the Phase Change Materials in Wood for Thermal Regulative Wood-Based Products. Forests 2022, 13, 1622. https://doi.org/10.3390/f13101622
Sulaiman NS, Mohamad Amini MH. Review on the Phase Change Materials in Wood for Thermal Regulative Wood-Based Products. Forests. 2022; 13(10):1622. https://doi.org/10.3390/f13101622
Chicago/Turabian StyleSulaiman, Nurul Syuhada, and Mohd Hazim Mohamad Amini. 2022. "Review on the Phase Change Materials in Wood for Thermal Regulative Wood-Based Products" Forests 13, no. 10: 1622. https://doi.org/10.3390/f13101622
APA StyleSulaiman, N. S., & Mohamad Amini, M. H. (2022). Review on the Phase Change Materials in Wood for Thermal Regulative Wood-Based Products. Forests, 13(10), 1622. https://doi.org/10.3390/f13101622






