Extreme Winter Storms Have Variable Effects on the Population Dynamics of Canopy Dominant Species in an Old-Growth Subtropical Forest
Abstract
:1. Introduction
- (1)
- How do the population structures of the four canopy dominant species change following the winter storm?
- (2)
- How does damage to the four canopy dominant species caused by the winter storm vary across habitat types?
- (3)
- What is the spatial distribution pattern of dead individuals for the four dominant species?
2. Materials and Methods
2.1. Study Site and Tree Inventory
2.2. Data Collection and Statistical Analysis
2.2.1. Importance Value of Dominant Tree Species
2.2.2. Recruitment, Upgrade, and Mortality
2.2.3. Diameter Structure Classes and Spatial Point Patterns
3. Results
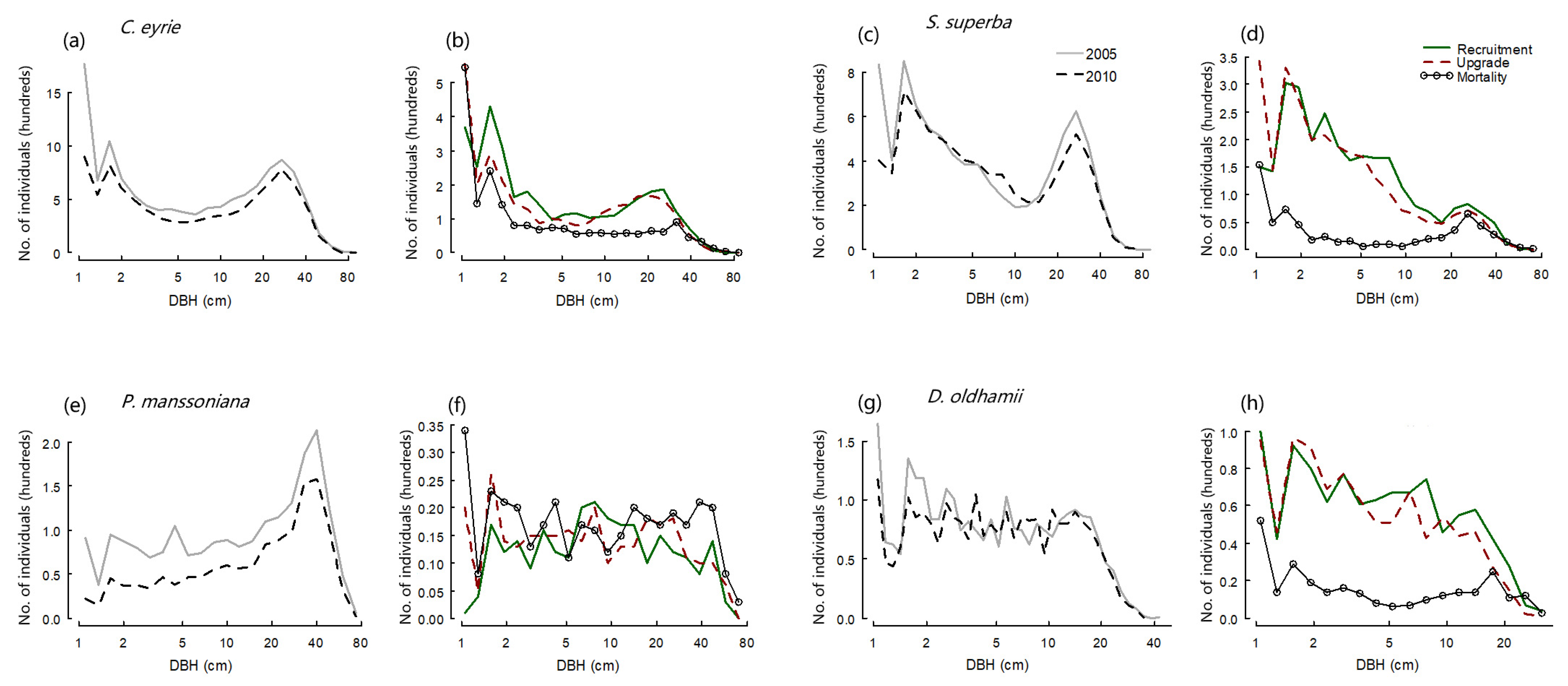
3.1. Changes in Population Characteristics of Canopy Dominant Species
3.2. Differences in the Distribution of Dead Trees in Different Habitats
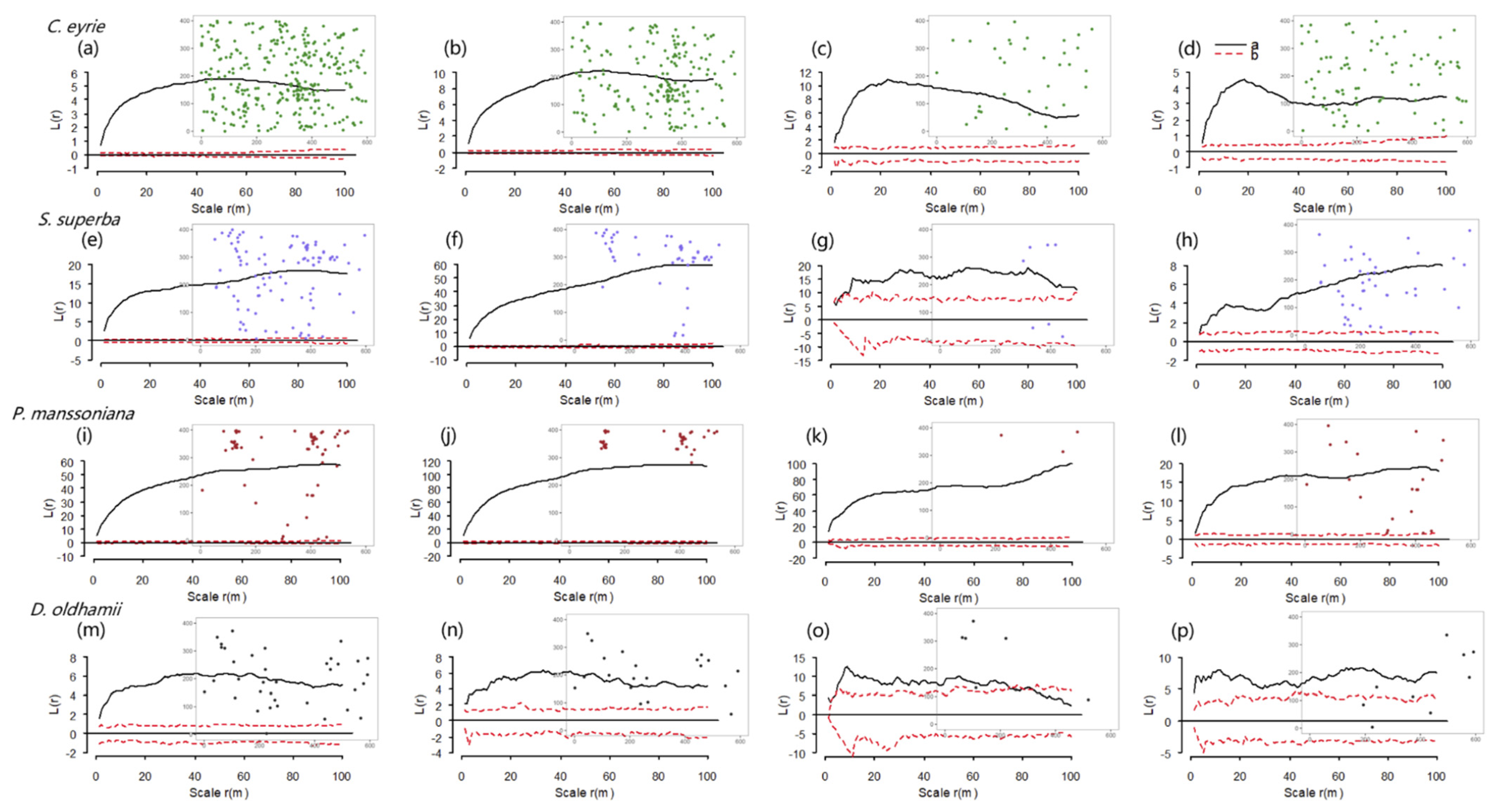
3.3. Spatial Point Patterns of Dead Trees across Life Stages
4. Discussion
4.1. Effects of Extreme Winter Storms on the Population Characteristics of Canopy Dominant Species
4.2. Effects of the Extreme Winter Storm on Mortality for Canopy Dominant Species in Different Habitats
4.3. Effects of the Winter Storm on the Spatial Point Pattern of Dead Trees
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, W.Q.; Chen, H.Q. Changes in the spatial-temporal characteristics of daily snowfall events over the Eurasian continent from 1980 to 2019. Int. J. Climatol. 2022, 42, 1841–1853. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Attribution of Extreme Weather Events in the Context of Climate Change; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Smith, M.D. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Aguirre-Gutierrez, J.; Oliveras, I.; Rifai, S.; Fauset, S.; Adu-Bredu, S.; Affum-Baffoe, K.; Baker, T.R.; Feldpausch, T.R.; Gvozdevaite, A.; Hubau, W.; et al. Drier tropical forests are susceptible to functional changes in response to a long-term drought. Ecol. Lett. 2019, 22, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Rudolf, V.H.W.; Ma, C.S. Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob. Chang. Biol. 2015, 21, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Cadotte, M.W.; Chen, J.H.; Mi, X.C.; Ren, H.B.; Liu, J.J.; Yu, M.J.; Zhang, J.T.; Ma, K.P. Neighborhood interactions on seedling survival were greatly altered following an extreme winter storm. For. Ecol. Manag. 2020, 461, 117940. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Gu, L.H.; Ding, Y.H.; Shao, L.; Wu, Z.M.; Yang, X.S.; Li, C.Z.; Li, Z.C.; Wang, X.M.; Cao, Y.H. The great 2008 Chinese ice storm: Its socioeconomic-ecological impact and sustainability lessons learned. Bull. Am. Meteorol. Soc. 2011, 92, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Man, X.X.; Mi, X.C.; Ma, K.P. Effects of an ice storm on community structure of an evergreen broad-leaved forest in Gutianshan National Nature Reserve, Zhejiang Province. Biodivers. Sci. 2011, 19, 197. [Google Scholar]
- Jin, Y.; Chen, J.H.; Mi, X.C.; Ren, H.B.; Ma, K.P.; Yu, M.J. Impacts of the 2008 ice storm on structure and composition of an evergreen broad-leaved forest community in eastern China. Biodivers. Sci. 2015, 23, 610. [Google Scholar] [CrossRef] [Green Version]
- Runkle, J.R. Canopy tree turnover in old-growth mesic forests of eastern North America. Ecology 2000, 81, 554–567. [Google Scholar] [CrossRef]
- van den Berg, E.; Chazdon, R.; Corrêa, B.S. Tree growth and death in a tropical gallery forest in Brazil: Understanding the relationships among size, growth, and survivorship for understory and canopy dominant species. Plant Ecol. 2012, 213, 1081–1092. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Curtis, P.S. Canopy structural changes following widespread mortality of canopy dominant trees. Forests 2013, 4, 537–552. [Google Scholar] [CrossRef]
- Ozanne, C.M.; Anhuf, D.; Boulter, S.L.; Keller, M.; Kitching, R.L.; Körner, C.; Meinzer, F.C.; Mitchell, A.; Nakashizuka, T.; Dias, P.S. Biodiversity meets the atmosphere: A global view of forest canopies. Science 2003, 301, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.L.; Wang, H.M.; Zhang, W.J.; Shao, Q.Q.; Yang, F.T.; Ma, Z.Q.; Wang, Y.D. Spatial response patterns of subtropical forests to a heavy ice storm: A case study in Poyang Lake Basin, southern China. Nat. Hazards 2013, 69, 2179–2196. [Google Scholar] [CrossRef]
- Duguay, S.M.; Arii, K.; Hooper, M.; Lechowicz, M.J. Ice storm damage and early recovery in an old-growth forest. Environ. Monit. Assess. 2001, 67, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Russo, S.E.; Yu, M. Effects of light and topography on regeneration and coexistence of evergreen and deciduous tree species in a Chinese subtropical forest. J. Ecol. 2018, 106, 1634–1645. [Google Scholar] [CrossRef]
- Jiang, C.C.; Fu, J.Q.; Wang, Y.Q.; Chai, P.T.; Yang, Y.D.; Mi, X.C.; Yu, M.J.; Ma, K.P.; Chen, J.H. The Habitat Type and Scale Dependences of Interspecific Associations in a Subtropical Evergreen Broad-Leaved Forest. Forests 2022, 13, 1334. [Google Scholar] [CrossRef]
- Dobbertin, M. Influence of stand structure and site factors on wind damage comparing the storms Vivian and Lothar. For. Snow Landsc. Res. 2002, 77, 187–205. [Google Scholar]
- Ge, J.L.; Xiong, G.M.; Wang, Z.X.; Zhang, M.; Zhao, C.M.; Shen, G.Z.; Xu, W.T.; Xie, Z.Q. Altered dynamics of broad-leaved tree species in a Chinese subtropical montane mixed forest: The role of an anomalous extreme 2008 ice storm episode. Ecol. Evol. 2015, 5, 1484–1493. [Google Scholar] [CrossRef]
- Li, X.F.; Jin, L.; Zhu, J.J.; Liu, L.M.; Zhang, J.X.; Wang, Y.; Zhu, C.Y. Response of species and stand types to snow/wind damage in a temperate secondary forest, Northeast China. J. For. Res. 2018, 29, 395–404. [Google Scholar] [CrossRef]
- Proulx, O.J.; Greene, D.F. The relationship between ice thickness and northern hardwood tree damage during ice storms. Can. J. For. Res. 2001, 31, 1758–1767. [Google Scholar] [CrossRef]
- Gadow, K.V.; Zhang, C.Y.; Wehenkel, C.; Pommerening, A.; Corral-Rivas, J.; Korol, M.; Myklush, S.; Hui, G.Y.; Kiviste, A.; Zhao, X.H. Forest Structure and Diversity. In Continuous Cover Forestry; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- He, C.M.; Jia, S.H.; Luo, Y.; Hao, Z.Q.; Yin, Q.L. Spatial Distribution and Species Association of Dominant Tree Species in Huangguan Plot of Qinling Mountains, China. Forests 2022, 13, 866. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Wei, B.L.; Chen, Y.; Jia, H.R.; Wei, Q.N.; Ye, Y.Z. How do similarities in spatial distributions and interspecific associations affect the coexistence of Quercus species in the Baotianman National Nature Reserve, Henan, China. Ecol. Evol. 2018, 8, 2580–2593. [Google Scholar] [CrossRef]
- Stone, R. Ecologists Report Huge Storm Losses in China’s Forests. Science 2008, 319, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Yu, M.J.; Ding, B.Y.; Fang, T.; Qian, H.Y.; Chen, Q.C. Types of evergreen broad-leaved forests and their species diversity in Gutian Mountain National Nature Reserve. Chin. J. Appl. Environ. Biol. 2003, 9, 341–345. [Google Scholar]
- Du, Y.J.; Mi, X.C.; Liu, X.J.; Chen, L.; Ma, K.P. Seed dispersal phenology and dispersal syndromes in a subtropical broad-leaved forest of China. For. Ecol. Manag. 2009, 258, 1147–1152. [Google Scholar] [CrossRef]
- Legendre, P.; Mi, X.C.; Ren, H.B.; Ma, K.P.; Yu, M.J.; Sun, I.F.; He, F.l. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer-Verlag: Berlin/Heidelberg, Germany; R. G. Landes Company: Georgetown, TX, USA, 1998. [Google Scholar]
- Chen, L.; Mi, X.C.; Comita, L.S.; Zhang, L.W.; Ren, H.B.; Ma, K.P. Community-level consequences of density dependence and habitat association in a subtropical broad-leaved forest. Ecol. Lett. 2010, 13, 695–704. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, G.F.; Zhang, L.W.; Shen, G.C.; Mi, X.C.; Ren, H.B.; Yu, M.J.; Chen, J.H.; Chen, S.W.; Fang, T. Community composition and structure of Gutianshan forest dynamic plot in a mid-subtropical evergreen broad-leaved forest, East China. Chin. J. Plant Ecol. 2008, 32, 262. [Google Scholar]
- Liu, Z.W.; Zhu, Y.; Wang, J.J.; Ma, W.; Meng, J.H. Species association of the dominant tree species in an old-growth forest and implications for enrichment planting for the restoration of natural degraded forest in subtropical China. Forests 2019, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Condit, R.; Ashton, P.S.; Manokaran, N.; LaFrankie, J.V.; Hubbell, S.P.; Foster, R.B. Dynamics of the forest communities at Pasoh and Barro Colorado: Comparing two 50–ha plots. Philos. Trans. R. Soc. B 1999, 354, 1739–1748. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Getzin, S.; Wiegand, T.; Ren, H.B.; Ma, K.P. The relative importance of Janzen-Connell effects in influencing the spatial patterns at the Gutianshan subtropical forest. PLoS ONE 2013, 8, e74560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripley, B.D. Modelling spatial patterns. J. R. Stat. Soc. B 1977, 39, 172–192. [Google Scholar] [CrossRef]
- Carlo, C.M. Markov chain monte carlo and gibbs sampling. Lect. Notes EEB 2004, 581, 540. [Google Scholar]
- Team, R.C.R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 November 2021).
- Højsgaard, S.; Halekoh, U. doBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities. R Package Version 4.6.11. 2021. Available online: https://cran.r-project.org/web/packages/doBy/index.html (accessed on 2 May 2021).
- Baddeley, A.; Turner, R.; Rubak, E. spatstat: Spatial Point Pattern Analysis, Model-Fitting, Simulation, Tests. R Package Version 2.3-4. 2022. Available online: https://cran.r-project.org/web/packages/spatstat/index.html (accessed on 1 April 2022).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. R Package Version 2.2.4. 2021. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 16 June 2021).
- Wang, Y.H.; Mi, X.C.; Chen, S.W.; Li, M.H.; Yu, M.J. Regeneration dynamics of major tree species during 2002–2007 in a subtropical evergreen broad-leaved forest in Gutianshan National Nature Reserve in East China. Biodivers. Sci. 2011, 19, 178. [Google Scholar]
- Takahashi, K.; Arii, K.; Lechowicz, M.J. Quantitative and qualitative effects of a severe ice storm on an old-growth beech–maple forest. Can. J. For. Res. 2007, 37, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C. Damage and recovery of tree species after two different tornadoes in the same old growth forest: A comparison of infrequent wind disturbances. For. Ecol. Manag. 2000, 135, 237–252. [Google Scholar] [CrossRef]
- Hopkin, A.; Williams, T.; Sajan, R.; Pedlar, J.; Nielsen, C. Ice storm damage to eastern Ontario forests: 1998–2001. For. Chron. 2003, 79, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Jalkanen, R.; Konocpka, B. Snow-packing as a potential harmful factor on Picea abies, Pinus sylvestris and Betula pubescens at high altitude in northern Finland. Eur. J. For. Pathol. 1998, 28, 373–382. [Google Scholar] [CrossRef]
- Yao, W.T.; Ma, Y.; Chen, F.; Xiao, Z.S.; Shu, Z.F.; Chen, L.J.; Xiao, W.H.; Liu, J.B.; Jiang, L.Y.; Zhang, S.Y. Analysis of Ice Storm Impact on and Post-Disaster Recovery of Typical Subtropical Forests in Southeast China. Remote Sens. 2020, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Zenner, E.K. Deriving restoration targets from diameter distributions of Dominant Biomass Strata development phases in an old-growth European beech forest. For. Ecol. Manag. 2021, 496, 119383. [Google Scholar] [CrossRef]
- Smolnik, M.; Hessl, A.; Colbert, J. Species-specific effects of a 1994 ice storm on radial tree growth in Delaware1. J. Torrey Bot. Soc. 2006, 133, 577–584. [Google Scholar] [CrossRef]
- Bistea, E.; Mahy, G. Vegetation and seed bank in a calcareous grassland restored from a Pinus forest. Appl. Veg. Sci. 2005, 8, 167–174. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Solantie, R. Effect of weather and climatological background on snow damage of forests in Southern Finland in November 1991. Silva Fenn. 1994, 28, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.C.; Härdtle, W.; Bruelheide, H.; Geißler, C.; Nadrowski, K.; Schuldt, A.; Yu, M.J.; von Oheimb, G. Tree morphology responds to neighbourhood competition and slope in species-rich forests of subtropical China. For. Ecol. Manag. 2010, 260, 1708–1715. [Google Scholar] [CrossRef]
- Storck, P.; Lettenmaier, D.P.; Bolton, S.M. Measurement of snow interception and canopy effects on snow accumulation and melt in a mountainous maritime climate, Oregon, United States. Water Resour. Res. 2002, 38, 5-1-5-16. [Google Scholar] [CrossRef]
- Chao, K.J.; Phillips, O.L.; Gloor, E.; Monteagudo, A.; Torres-Lezama, A.; Martínez, R.V. Growth and wood density predict tree mortality in Amazon forests. J. Ecol. 2008, 96, 281–292. [Google Scholar] [CrossRef]
- Chen, L.; Swenson, N.G.; Ji, N.N.; Mi, X.C.; Ren, H.B.; Guo, L.D.; Ma, K.P. Differential soil fungus accumulation and density dependence of trees in a subtropical forest. Science 2019, 366, 124–128. [Google Scholar] [CrossRef]
- Johnson, D.J.; Beaulieu, W.T.; Bever, J.D.; Clay, K. Conspecific negative density dependence and forest diversity. Science 2012, 336, 904–907. [Google Scholar] [CrossRef] [Green Version]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Russell, S.K.; Schupp, E.W. Effects of microhabitat patchiness on patterns of seed dispersal and seed predation of Cercocarpus ledifolius (Rosaceae). Oikos 1998, 117, 143–152. [Google Scholar] [CrossRef]
- He, F.L.; Duncan, R.P. Density-dependent effects on tree survival in an old-growth Douglas fir forest. J. Ecol. 2000, 88, 676–688. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.Q.; Sun, I.F.; Song, B.; Ye, J.; Li, B.H.; Wang, X.G. Density dependence on tree survival in an old-growth temperate forest in northeastern China. Ann. For. Sci. 2009, 66, 204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.B.; Ding, G.J.; Luo, X.M.; Chen, M.F. Bivariate distribution characteristics of spatial structure in five different Pinus massoniana forests. Dendrobiology 2017, 78, 75–84. [Google Scholar] [CrossRef]


| Species | Importance Value | DBH (cm) at Plot Level † | Basal Area (m2) at Plot Level † | |||||
|---|---|---|---|---|---|---|---|---|
| 2005 | 2010 | 2005 | 2010 | p-Value | 2005 | 2010 | p-Value | |
| Castanopsis eyrei | 0.152 | 0.149 | 14.495 (8.422) | 16.292 (9.126) | 0.001 | 0.504 (0.365) | 0.470 (0.353) | 0.07 |
| Schima superba | 0.091 | 0.093 | 20.593 (9.862) | 21.394 (10.253) | 0.506 | 0.283 (0.172) | 0.268 (0.269) | 0.04 |
| Pinus massoniana | 0.048 | 0.042 | 32.816 (13.741) | 34.033 (12.818) | 0.021 | 0.297 (0.218) | 0.272 (0.199) | 0.001 |
| Daphniphyllum oldhamii | 0.022 | 0.023 | 6.853 (4.272) | 7.201 (6.230) | 0.191 | 0.036 (0.045) | 0.034 (0.041) | 0.445 |
| Species | Individuals in 2005 | Individuals in 2010 | Survivors in 2005–2010 | Deaths in 2005–2010 | Recruits in 2010 | Mortality Rate | Recruitment Rate | Population Change Rate |
|---|---|---|---|---|---|---|---|---|
| Castanopsis eyrei | 12,333 | 9651 | 8722 | 3611 | 929 | 6.93% | 2.02% | −4.91% |
| Schima superba | 8470 | 7663 | 7285 | 1185 | 378 | 3.01% | 1.01% | −2.00% |
| Pinus massoniana | 2060 | 1318 | 1308 | 752 | 10 | 9.08% | 0.15% | −8.93% |
| Daphniphyllum oldhamii | 2711 | 2488 | 2210 | 501 | 278 | 4.09% | 2.37% | −1.72% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ji, Y.; Wang, Y.; Xie, J.; Jin, Y.; Mi, X.; Yu, M.; Ren, H.; Ma, K.; Chen, J. Extreme Winter Storms Have Variable Effects on the Population Dynamics of Canopy Dominant Species in an Old-Growth Subtropical Forest. Forests 2022, 13, 1634. https://doi.org/10.3390/f13101634
Yang Y, Ji Y, Wang Y, Xie J, Jin Y, Mi X, Yu M, Ren H, Ma K, Chen J. Extreme Winter Storms Have Variable Effects on the Population Dynamics of Canopy Dominant Species in an Old-Growth Subtropical Forest. Forests. 2022; 13(10):1634. https://doi.org/10.3390/f13101634
Chicago/Turabian StyleYang, Yidan, Yanli Ji, Yunquan Wang, Jiajie Xie, Yi Jin, Xiangcheng Mi, Mingjian Yu, Haibao Ren, Keping Ma, and Jianhua Chen. 2022. "Extreme Winter Storms Have Variable Effects on the Population Dynamics of Canopy Dominant Species in an Old-Growth Subtropical Forest" Forests 13, no. 10: 1634. https://doi.org/10.3390/f13101634





