Transcriptome Analysis of Apricot Kernel Pistils Reveals the Mechanisms Underlying ROS-Mediated Freezing Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. RNA-seq
2.3. Identification of DEGs and Enrichment Analyses
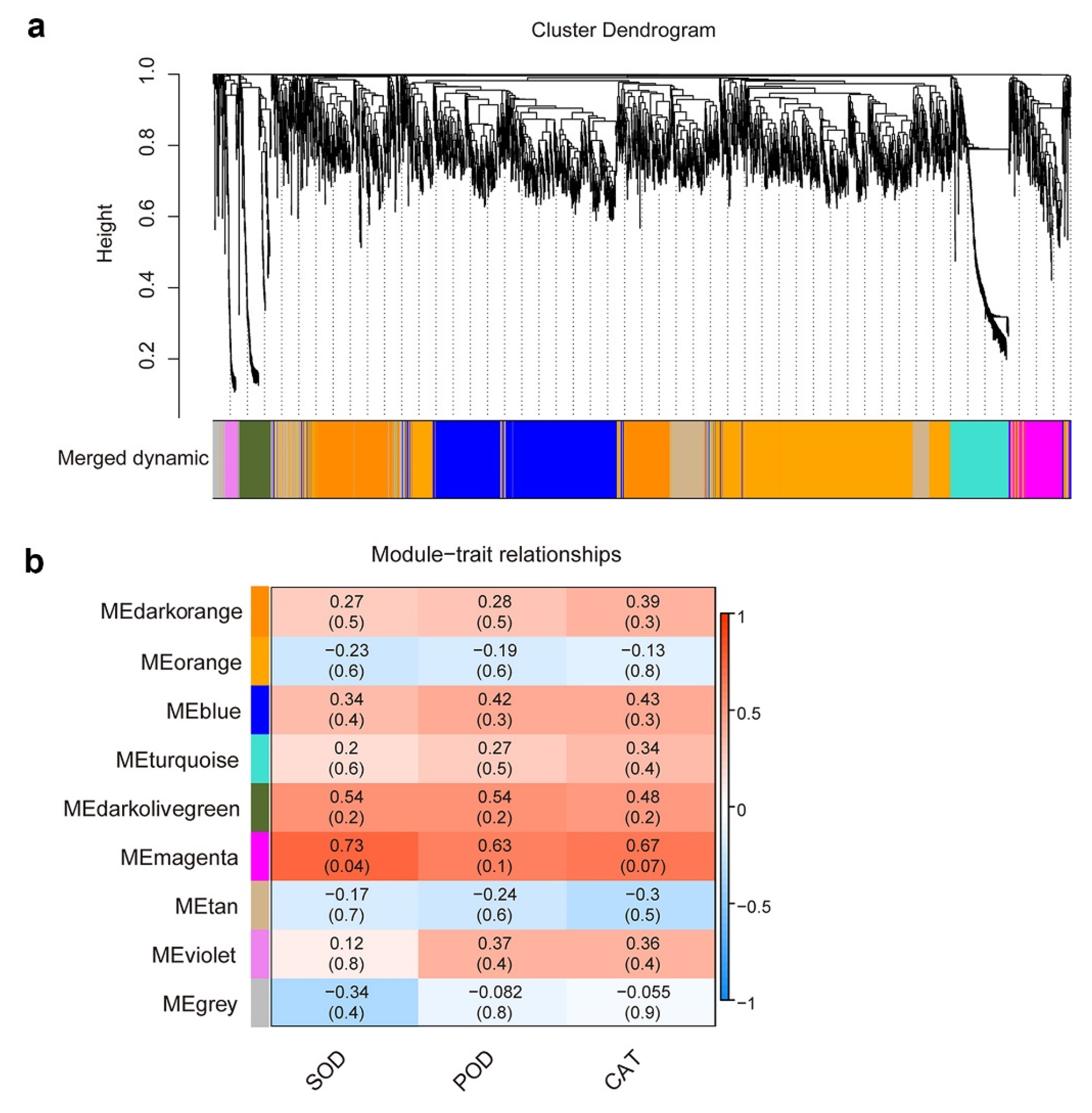
2.4. Weighted Gene Co-Expression Network and Hub Genes Analysis
2.5. Protein–Protein Interaction (PPI) Network Prediction
2.6. Verification of qRT-PCR Analysis
3. Results
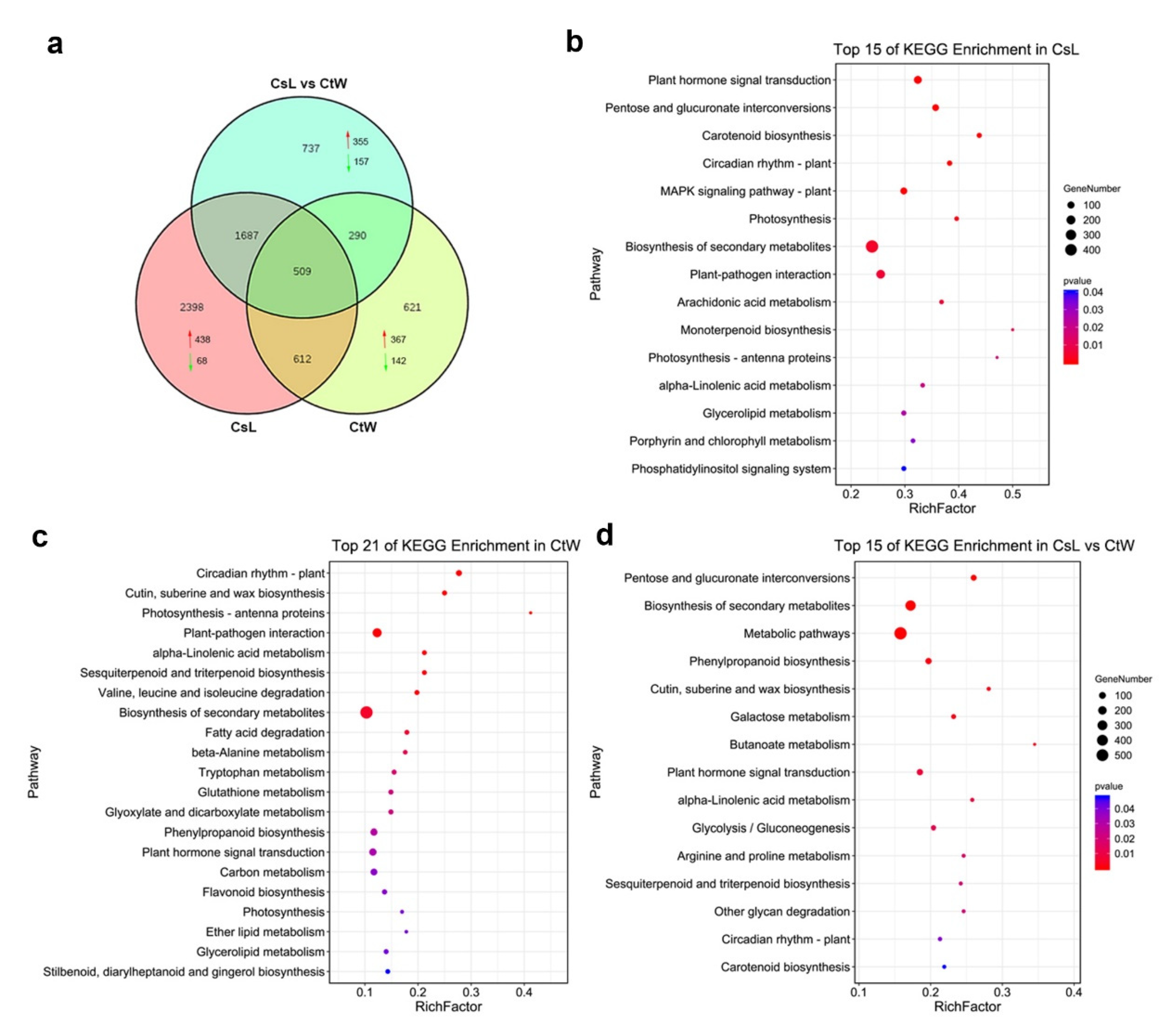
3.1. Transcriptome Analysis of Apricot Kernel Pistils under Freezing Stress
3.2. Identification and Functional Analysis of DEGs of Apricot Kernel Pistils under Freezing Stress
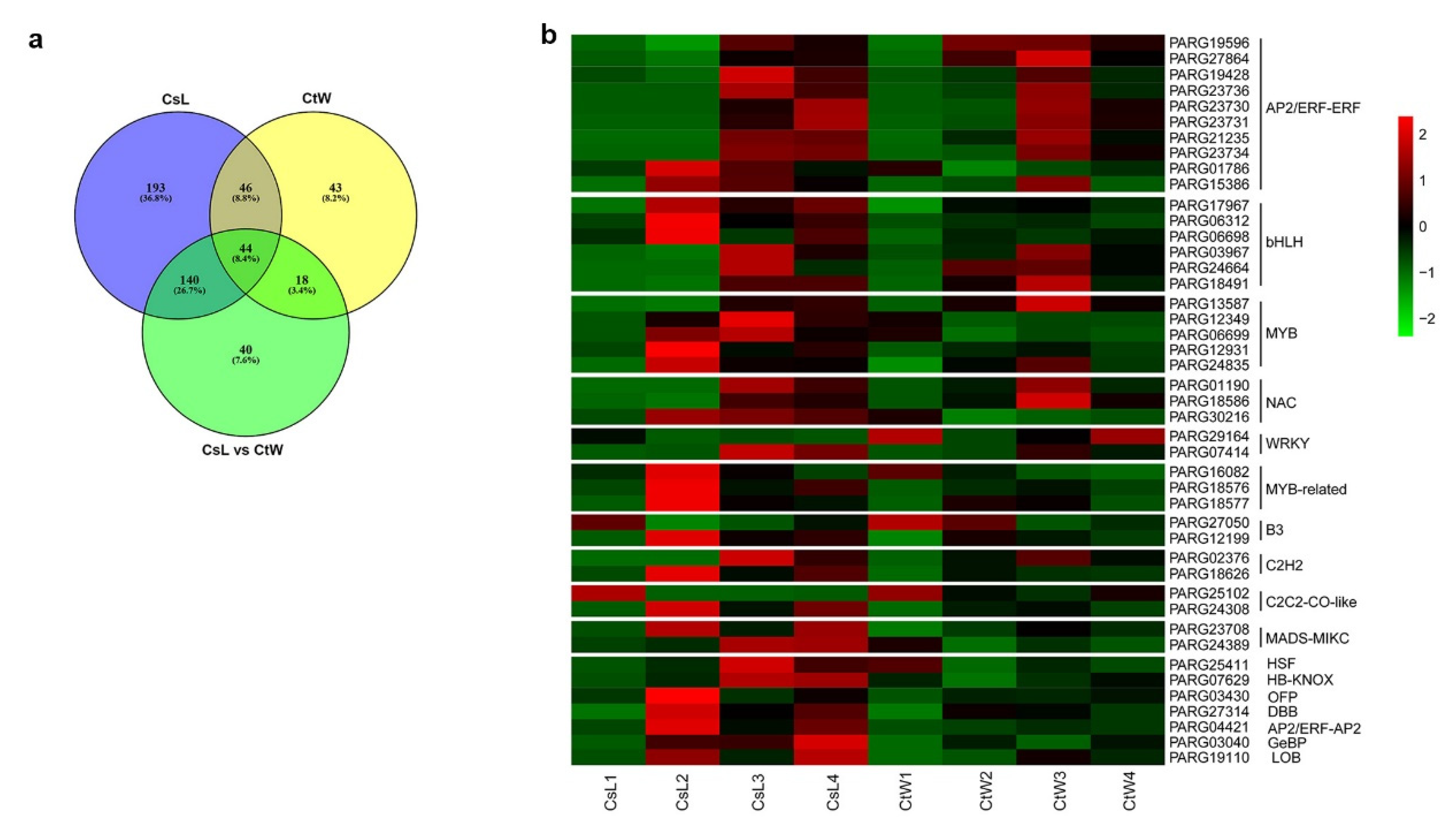
3.3. Differentially Expressed Transcription Factors of Apricot Kernel Pistils under Freezing Stress
3.4. The Co-Expression Network Analysis of DEGs Related to the Antioxidant Enzyme Activity
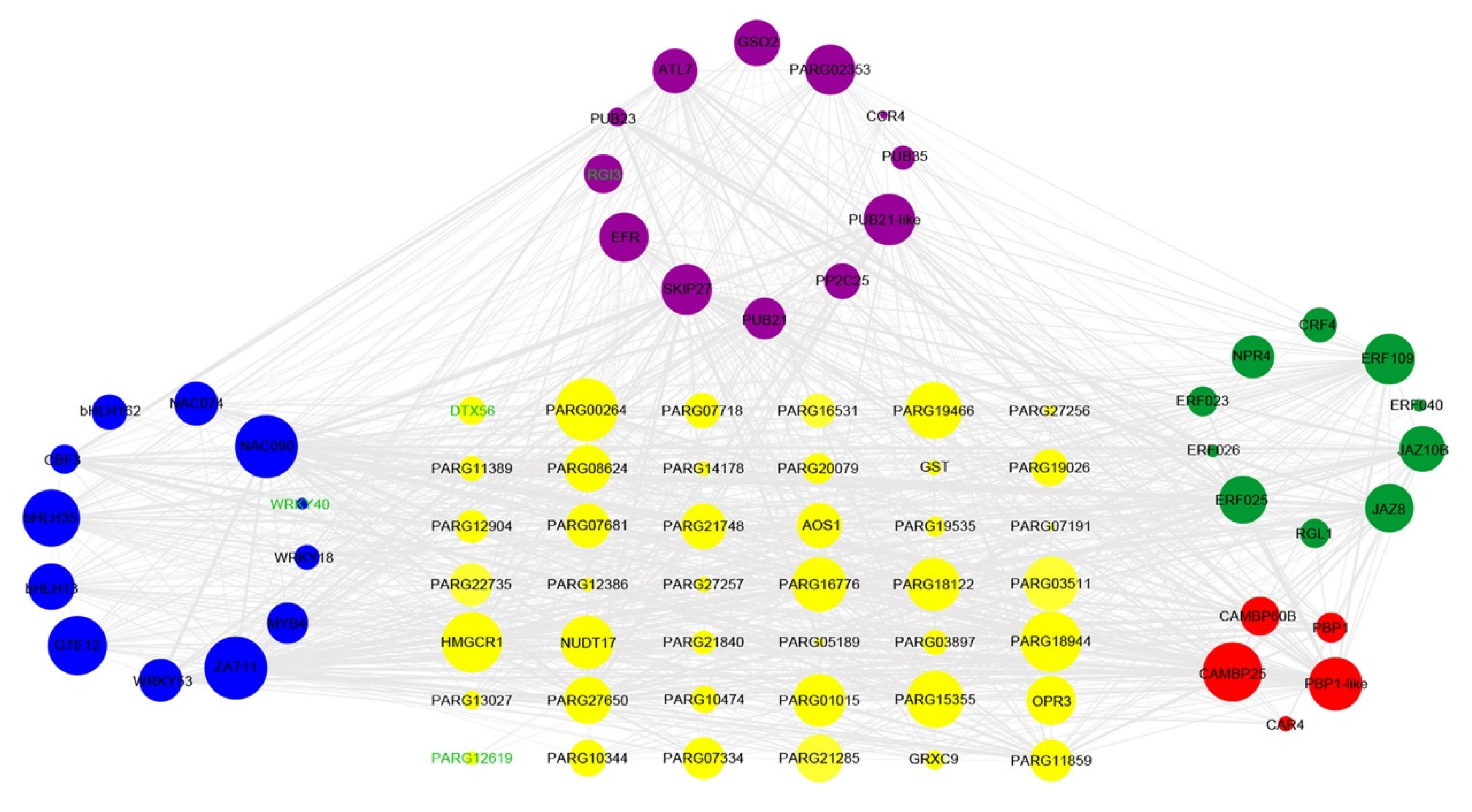
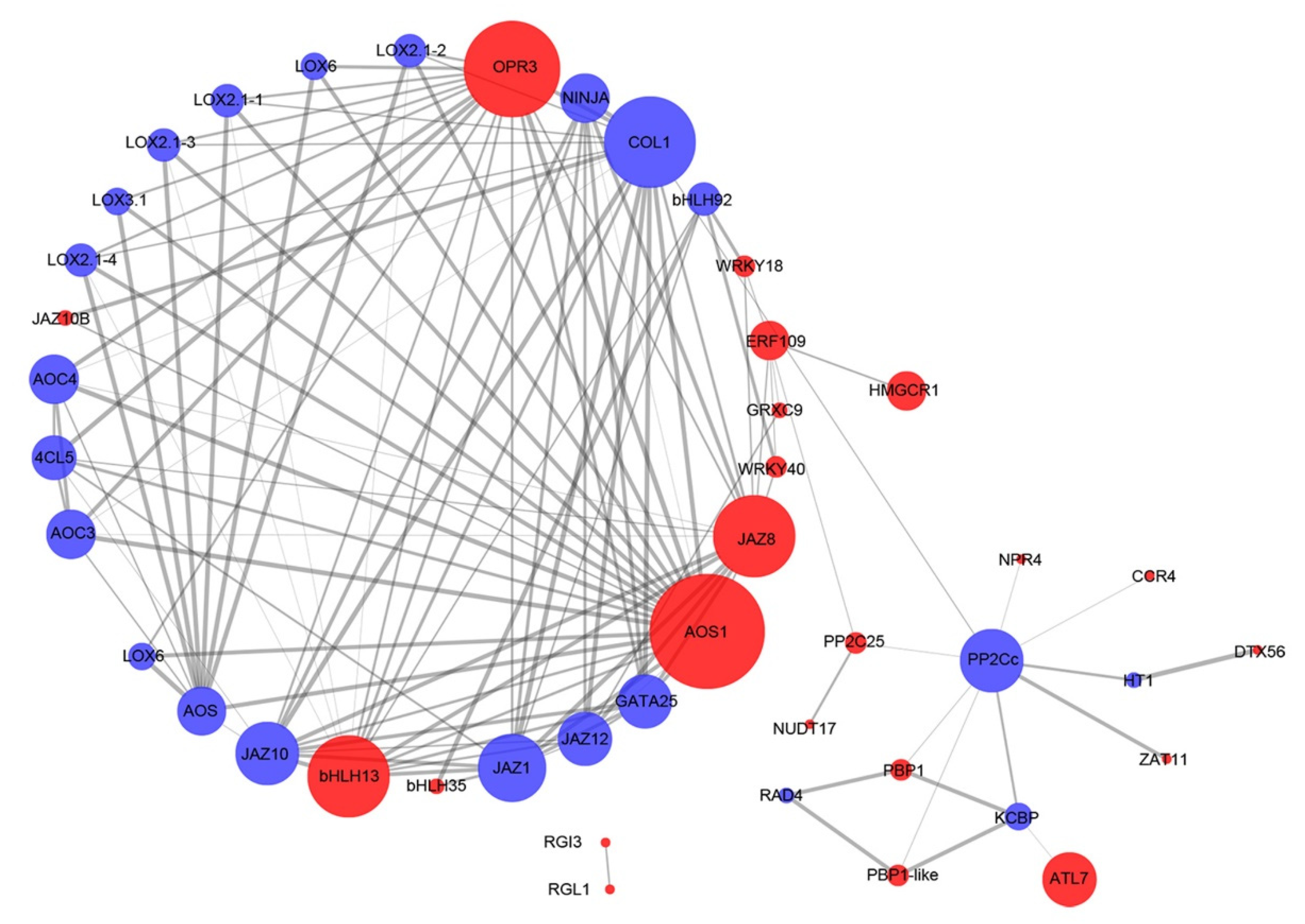
3.5. Interaction Network Analysis of Hub Genes Related to Antioxidant Enzyme Activity
3.6. qRT-PCR Validation of Key DEGs Involved in the Response of Apricot Kernels to Freezing Stress
4. Discussion
4.1. Transcriptional Regulation in Freezing Response of Apricot Kernels
4.2. Post-Translational Regulation in the Freezing Response of Apricot Kernels
4.3. Ca2+ and Hormone Signaling in Freezing Response of Apricot Kernels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitasse, Y.; Schneider, L.; Rixen, C.; Christen, D.; Rebetez, M. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric. For. Meteorol. 2018, 248, 60–69. [Google Scholar] [CrossRef]
- Yang, J.; Huo, Z.; Wang, P.; Wu, D.; Ma, Y.; Yao, S.; Dong, H. Process-based indicators for timely identification of apricot frost disaster on the warm temperate Zone, China. Theor. Appl. Climatol. 2021, 1463, 1143–1155. [Google Scholar] [CrossRef]
- Chen, L.J.; Xiang, H.Z.; Miao, Y.; Zhang, L.; Guo, Z.F.; Zhao, X.H.; Lin, J.W.; Li, T.L. An overview of cold resistance in plants. J. Agron. Crop. Sci. 2014, 200, 237–245. [Google Scholar] [CrossRef]
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Chen, W.Z.; Liu, W.S.; Liu, N.; Liu, S. Palynological study on the origin and systematic evolution of kernel-using apricots. Acta Hortic. Sin. 2010, 37, 1377–1387. [Google Scholar] [CrossRef]
- Ning, C.; Meng, Q.; Li, S.; Li, Y.; Yang, J. Comparative study of cold resistance in Kernel-apricot germplasm floral organs. J. Agric. Univ. Hebei 2010, 33, 37–41. [Google Scholar] [CrossRef]
- Yu, D.; Liu, X.; Cui, Y.; Bi, Q.; Zhao, Y.; Li, D.; Yu, H.; Wang, L. Comparative transcriptome combined with morpho-physiological analyses revealed candidate genes potentially for differential cold tolerance in two contrasting apricot Prunus armeniaca L. cultivars. Trees 2020, 34, 1205–1217. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cao, M.; Zhou, D.; Peng, W. Drought, salt and temperature stress-induced metabolic changes in plant. Biotechnol. Bull. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015, 561, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.H.; Hong, X.; Agarwal, M.; Zhu, J.K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol Plant. 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Castroverde, C.D.M.; Kalaji, H.M.; Albaqami, M.; Aftab, T. Molecular mechanisms of nitric oxide NO signaling and reactive oxygen species ROS homeostasis during abiotic stresses in plants. Int. J. Mol. Sci. 2021, 2217, 9656. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef]
- Tian, J.; Wei, A.; Liu, Y.; Wang, S.H.; Liu, Y. Research progress on cold resistance of kernel apricot. J. Nor. For. Univ. 2018, 331, 174–178. [Google Scholar] [CrossRef]
- Kaya, O.; Kose, C.; Esitken, A.; Gecim, T.; Donderalp, V.; Taskin, S.; Turan, M. Frost tolerance in apricot Prunus armeniaca L. receptacle and pistil organs: How is the relationship among amino acids, minerals, and cell death points? Int. J. Biometeorol. 2021, 65, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, F.; Song, Y.; Li, J.; Lü, Z. Principal component analysis on cold resistance of different apricot varieties in Shanxi. J. Shanxi Agric. Sci. 2020, 48, 1913–1915. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Ren, S.; Fu, Y.; Yang, F.; Yang, J. A new cold-resistant kernel apricot variety “Waixuan1”. Acta Hortic. Sin. 2010, 371, 155–156. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.; Wang, S.; Yang, L.; Luo, Y.; Gao, S.; Zhang, M.; Wu, S.; Hu, S.; Wang, Y. The apricot (Prunus armeniaca L.) genome elucidates Rosaceae evolution and beta-carotenoid synthesis. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef]
- Yu, D.; Liu, X.; Bi, Q.; Zhao, Y.; Ju, J.; Li, D.; Yu, H.; Wang, L. Response of pistils in two contrasting apricot Prunus armeniaca L. cultivars to low temperature stress and recovery. J. Nor. For. Univ. 2021, 491, 1–5. [Google Scholar] [CrossRef]
- Li, K.; Du, Y.; Miao, Y. Future challenges in understanding ROS in plant responses to abiotic stress. Sci. China Life Sci. 2016, 5912, 1343–1344. [Google Scholar] [CrossRef]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 711, 169. [Google Scholar] [CrossRef]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Maurya, N.K.; Goswami, A.K.; Singh, S.K.; Prakash, J.; Goswami, S.; Chinnusamy, V.; Talukdar, A.; Pradhan, S.; Kumari, A. Studies on expression of CBF1 and CBF2 genes and anti-oxidant enzyme activities in papaya genotypes exposed to low temperature stress. Sci. Horticult. 2020, 261, 108914. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Ngatia, J.N.; Wang, Y.R.; Khoso, M.A.; Farooq, U.; Chen, S. AP2/ERF, an important cold stress-related transcription factor family in plants: A review. Physiol. Mol. Biol. Plants 2021, 279, 1953–1968. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.H.; Fujii, H.; Zheng, X.; Zhu, J.K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Wei, W.; Song, Q.X.; Chen, H.W.; Zhang, Y.Q.; Wang, F.; Zou, H.F.; Gang, L.; Tian, A.G.; Zhang, W.K. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011, 682, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Yarra, R.; Wei, W. The NAC-type transcription factor GmNAC20 improves cold, salinity tolerance, and lateral root formation in transgenic rice plants. Funct. Integr. Genom. 2021, 213, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018, 60, 95–110. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, W.T. Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett. 2013, 587, 2584–2590. [Google Scholar] [CrossRef]
- Suh, J.Y.; Kim, W.T. Arabidopsis RING E3 ubiquitin ligase AtATL80 is negatively involved in phosphate mobilization and cold stress response in sufficient phosphate growth conditions. Biochem. Biophys. Res. Commun. 2015, 4634, 793–799. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.L.; Li, Z.Y.; Shi, Y.T.; Wang, J.L.; Hua, J.; Gong, Z.Z.; Zhou, J.M.; Yang, S.H. PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negative regulator MYB15. Dev. Cell 2019, 51, 222–235. [Google Scholar] [CrossRef]
- Fang, H.; Meng, Q.; Xu, J.; Tang, H.; Tang, S.; Zhang, H.; Huang, J. Knock-down of stress inducible OsSRFP1 encoding an E3 ubiquitin ligase with transcriptional activation activity confers abiotic stress tolerance through enhancing antioxidant protection in rice. Plant Mol. Biol. 2015, 874–875, 441–458. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.W.; Hu, Y.; Beyyoudh, L.; Yildiz Dasgan, H.; Kunert, K.; Beveridge, C.A.; Foyer, C.H. Strigolactones positively regulate chilling tolerance in pea and in Arabidopsis. Plant Cell Environ. 2018, 41, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dai, W.; Du, J.; Ming, R.; Dahro, B.; Liu, J.H. ERF109 of trifoliate orange Poncirus trifoliata L. Raf. contributes to cold tolerance by directly regulating expression of Prx1 involved in antioxidative process. Plant Biotechnol. J. 2019, 17, 1316–1332. [Google Scholar] [CrossRef] [PubMed]

| Variety | Treatment | Sample | Clean Reads | GC (%) | Q20 (%) | Q30 (%) | Read Mapped (%) | Unique Mapped (%) |
|---|---|---|---|---|---|---|---|---|
| ‘Longwangmao’ (CsL) | CsL1 | CsL11 | 60,245,518 | 45.92 | 97.21 | 92.15 | 94.14 | 91.47 |
| CsL12 | 48,251,936 | 45.93 | 97.01 | 91.73 | 93.41 | 90.81 | ||
| CsL13 | 48,950,240 | 46.25 | 97.06 | 91.86 | 93.94 | 91.31 | ||
| CsL2 | CsL21 | 47,482,406 | 45.86 | 97.66 | 93.19 | 94.74 | 92.12 | |
| CsL22 | 45,957,672 | 45.72 | 97.67 | 93.26 | 93.90 | 90.94 | ||
| CsL23 | 54,500,124 | 45.50 | 97.50 | 92.88 | 93.69 | 90.63 | ||
| CsL3 | CsL31 | 56,350,610 | 45.30 | 97.64 | 93.21 | 93.29 | 90.70 | |
| CsL32 | 56,506,172 | 45.78 | 97.65 | 93.25 | 92.27 | 89.72 | ||
| CsL33 | 55,646,854 | 45.85 | 97.67 | 93.31 | 92.45 | 89.86 | ||
| CsL4 | CsL41 | 54,666,004 | 45.78 | 97.66 | 93.34 | 87.55 | 84.85 | |
| CsL42 | 44,453,966 | 45.72 | 97.60 | 93.14 | 93.55 | 91.11 | ||
| CsL43 | 49,831,556 | 45.90 | 97.58 | 93.12 | 94.34 | 91.61 | ||
| ‘Weixuan 1’ (CtW) | CtW1 | CtW11 | 41,850,108 | 45.93 | 97.30 | 92.29 | 92.11 | 89.62 |
| CtW12 | 41,637,772 | 46.20 | 97.32 | 92.34 | 94.16 | 91.59 | ||
| CtW13 | 50,142,906 | 46.10 | 97.29 | 92.29 | 93.79 | 91.23 | ||
| CtW2 | CtW21 | 45,960,022 | 45.49 | 97.62 | 93.05 | 94.17 | 91.66 | |
| CtW22 | 55,251,738 | 45.38 | 97.75 | 93.40 | 94.46 | 91.98 | ||
| CtW23 | 51,158,772 | 45.88 | 97.61 | 93.14 | 94.37 | 91.71 | ||
| CtW3 | CtW31 | 43,518,372 | 45.87 | 97.72 | 93.45 | 93.96 | 91.45 | |
| CtW32 | 60,004,540 | 45.87 | 97.44 | 92.79 | 93.50 | 91.03 | ||
| CtW33 | 60,219,916 | 45.69 | 97.55 | 93.03 | 94.03 | 91.44 | ||
| CtW4 | CtW41 | 45,356,754 | 45.84 | 97.65 | 93.25 | 90.25 | 87.83 | |
| CtW42 | 46,029,912 | 45.81 | 97.83 | 93.67 | 94.45 | 91.78 | ||
| CtW43 | 43,832,166 | 45.84 | 97.70 | 93.37 | 93.16 | 90.58 |
| Module | No. of Genes | Module Membership vs. Gene Significance | |
|---|---|---|---|
| Correlation (r2) | p-Value | ||
| Orange | 997 | −0.0094 | 0.77 |
| Dark orange | 526 | −0.2 | 3.8 × 10−6 |
| Blue | 734 | −0.65 | 2.4 × 10−89 |
| Tan | 270 | −0.51 | 2.8 × 10−19 |
| Violet | 51 | −0.36 | 0.0095 |
| Dark olive green | 121 | 0.027 | 0.77 |
| Turquoise | 230 | 0.04 | 0.55 |
| Magenta | 173 | 0.58 | 6.2 × 10−17 |
| Grey | 121 | 0.036 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, Y.; Xu, H.; Yu, D.; Bi, Q.; Wang, L. Transcriptome Analysis of Apricot Kernel Pistils Reveals the Mechanisms Underlying ROS-Mediated Freezing Resistance. Forests 2022, 13, 1655. https://doi.org/10.3390/f13101655
Liu X, Yang Y, Xu H, Yu D, Bi Q, Wang L. Transcriptome Analysis of Apricot Kernel Pistils Reveals the Mechanisms Underlying ROS-Mediated Freezing Resistance. Forests. 2022; 13(10):1655. https://doi.org/10.3390/f13101655
Chicago/Turabian StyleLiu, Xiaojuan, Yingying Yang, Huihui Xu, Dan Yu, Quanxin Bi, and Libing Wang. 2022. "Transcriptome Analysis of Apricot Kernel Pistils Reveals the Mechanisms Underlying ROS-Mediated Freezing Resistance" Forests 13, no. 10: 1655. https://doi.org/10.3390/f13101655
APA StyleLiu, X., Yang, Y., Xu, H., Yu, D., Bi, Q., & Wang, L. (2022). Transcriptome Analysis of Apricot Kernel Pistils Reveals the Mechanisms Underlying ROS-Mediated Freezing Resistance. Forests, 13(10), 1655. https://doi.org/10.3390/f13101655




