Soil Organic Matter Fractions in Relation to Root Characteristics of Different Tree Species in Altitude Gradient of Temperate Forest in Carpathian Mountains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Laboratory Analysis
2.3. Statistical Analysis
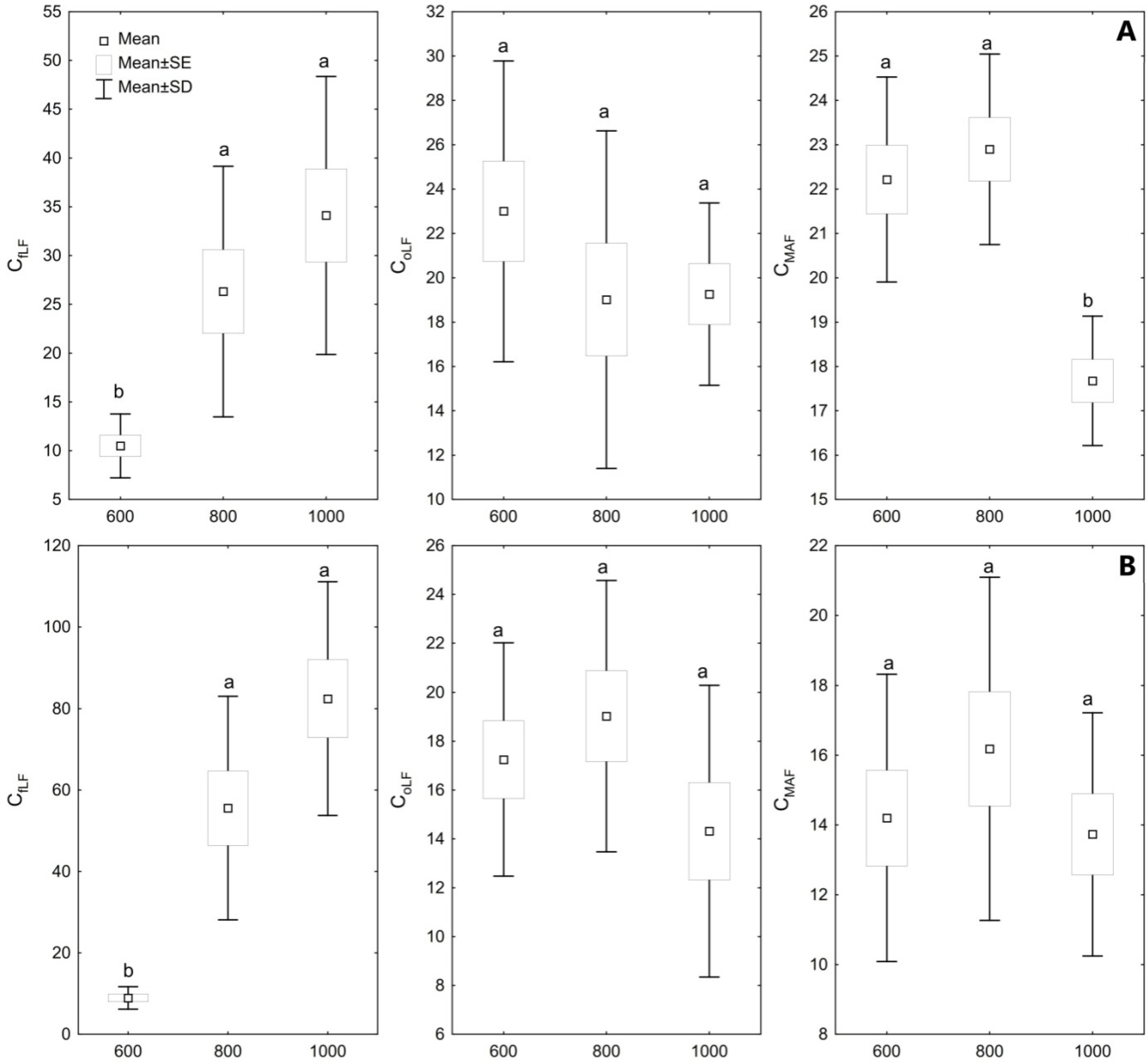
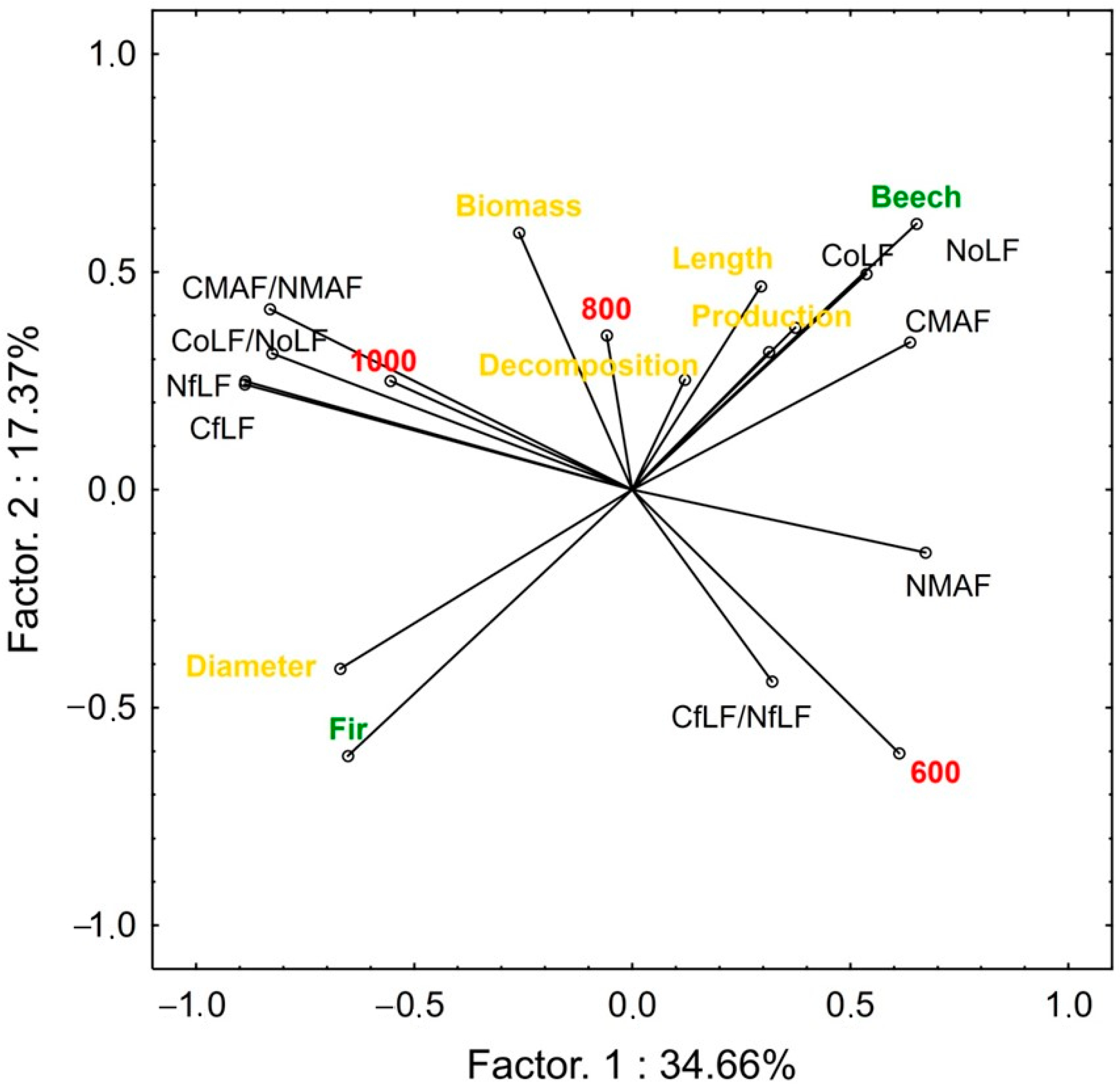
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prescott, C.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needles litter in forests of British Columbia: Influences of litter type, forest type and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hansen, K. Tree species is the major factor explaining C:N ratios in European forest soils. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; Von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S.; King, J.S.; Holmes, W.E. Elevated atmospheric CO2, fine roots and the response of soil microorganisms: A review and hypothesis. New Phytol. 2000, 147, 201–222. [Google Scholar] [CrossRef] [Green Version]
- Błońska, E.; Lasota, J.; Tullus, A.; Lutter, R.; Ostonen, I. Impact of deadwood decomposition on soil organic carbon sequestration in Estonian and Polish forests. Ann. For. Sci. 2019, 76, 102. [Google Scholar] [CrossRef] [Green Version]
- Lasota, J.; Błońska, E.; Łyszczarz, S.; Tibbett, M. Forest humus type governs heavy metal accumulation in specific organic matter fractions. Water Air Soil Pollut. 2020, 231, 80. [Google Scholar] [CrossRef] [Green Version]
- Grüneberg, E.; Schöning, I.; Hessenmöller, D.; Schulze, E.D.; Weisser, W.W. Organic layer and clay content control soil organic carbon stocks in density fractions of differently managed German beech forests. For. Ecol. Manag. 2013, 303, 1–10. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Trumbore, S.E.; Vittori Antisari, L.; Cocco, S.; Corti, G.; Agnelli, A. Small altitudinal change and rhizosphere affect the SOM light fractions but not the heavy fraction in European beech forest soil. Catena 2019, 181, 104091. [Google Scholar] [CrossRef]
- Cheng, W.; Kuzyakov, Y. Root effects on soil organic matter decomposition. In Roots and Soil Management: Interactions between Roots and the Soil. Agronomy Monograph; Wright, S., Zobel, R., Eds.; American Society of Agronomy: Madison, WI, USA, 2005; Volume 48, pp. 119–143. [Google Scholar]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesarz, S.; Fender, A.C.; Beyer, F.; Valtanen, K.; Pfeiffer, B.; Gansert, D.; Hertel, D.; Polle, A.; Daniel, R.; Leuschner, C.; et al. Roots from beech (Fagus sylvatica L.) and ash (Fraxinus excelsior L.) differentially affect soil microorganisms and carbon dynamics. Soil Biol. Biochem. 2013, 61, 23–32. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Gruba, P. Enzymatic activity and stabilization of organic matter in soil with different detritus inputs. J. Soil Sci. Plant Nutr. 2017, 63, 242–247. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization and the carbon saturation concept. Glob. Change Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [Green Version]
- Likulunga, E.; Pérez, C.A.R.; Schneider, D.; Daniel, R.; Polle, A. Tree species composition and soil properties in pure and mixed beech-conifer stands drive soil fungal communities. For. Ecol. Manag. 2021, 502, 119709. [Google Scholar] [CrossRef]
- Lu, J.Z.; Scheu, S. Response of soil microbial communities to mixed beech-conifer forests varies with site conditions. Soil Biol. Biochem. 2021, 155, 108155. [Google Scholar] [CrossRef]
- Salminen, H.; Jalkanen, R. Modelling the effect of temperature on height increment of Scots pine at high latitudes. Silva Fenn. 2005, 39, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Schenker, G.; Lenz, A.; Körner, C.; Hoch, G. Physiological minimum temperatures for root growth in seven common European broadleaved tree species. Tree Physiol. 2014, 34, 302–313. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soil and Creating Legends for Soil Maps; Update 2015, World Soil Resources Reports No. 106; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015; p. 190. [Google Scholar]
- Sohi, S.P.; Mahieu, N.; Arah, J.R.M.; Powlson, D.S.; Madari, B.; Gaunt, J.L. A procedure for isolating soil organic matter fractions suitable for modeling. Soil Sci. Soc. Am. J. 2001, 65, 1121–1128. [Google Scholar] [CrossRef]
- Böhm, W. Metody Badania Systemów Korzeniowych; PWRiL: Warsaw, Poland, 1985; p. 267. ISBN 83-09-00902-X. [Google Scholar]
- Bojko, O.; Kabała, C. Transformation of physicochemical soil properties along a mountain slope due to land management and climate changes—A case study from the Karkonosze Mountains, SW Poland. Catena 2016, 140, 43–54. [Google Scholar] [CrossRef]
- Bojko, O.; Kabała, C. Organic carbon pools in mountain soils—Sources of variability and predicted changes in relation to climate and land use changes. Catena 2017, 149, 209–220. [Google Scholar] [CrossRef]
- Fekete, I.; Berki, I.; Lajtha, K.; Trumbore, S.; Francioso, O.; Gioacchini, P.; Montecchio, D.; Várbíró, G.; Béni, Á.; Makádi, M.; et al. How will a drier climate change carbon sequestration in soils of the deciduous forests of Central Europe? Biogeochemistry 2020, 152, 13–32. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wang, X.; Jiang, S.L.; Zhou, X.H.; Liu, H.Y.; Xiao, J.J.; Hao, Z.G.; Wang, K.C. Climate and geochemistry interactions at different altitudes influence soil organic carbon turnover times in alpine grasslands. Agric. Ecosyst. Environ. 2021, 320, 107591. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Leuschner, C.; Tückmantel, T.; Meier, I.C. Temperature effects on root exudation in mature beech (Fagus sylvatica L.) forests along an elevational gradient. Plant Soil 2022. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Z.; Ryan, M.G.; You, Y.; Sun, O.J. Changes in soil organic carbon contents and fractionations of forests along a climatic gradient in China. For. Ecosyst. 2019, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Dai, Q.; Gao, R.; Gan, Y.; Yi, X. Effects of rainfall intensity on runoff and nutrient loss of gently sloping farmland in a karst area of SW China. PLoS ONE 2021, 16, e0246505. [Google Scholar] [CrossRef]
- Bu, X.; Ruan, H.; Wang, L.; Ma, W.; Ding, J.; Yu, X. Soil organic matter in density fractions as related to vegetation changes along an altitude gradient in the Wuyi Mountains, southeastern China. Appl. Soil Ecol. 2012, 52, 42–47. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, S.; Eitzinger, B.; Ferlian, O.; Bluhm, C.; Schröter, K.; Pena, R.; Maraun, M.; Scheu, S. Deprivation of root-derived resources affects microbial biomass but not community structure in litter and soil. PLoS ONE 2019, 14, e0214233. [Google Scholar] [CrossRef] [Green Version]
- Pierson, D.; Evans, L.; Kayhani, K.; Bowden, R.D.; Nadelhoffer, K.; Simpson, M.; Lajtha, K. Mineral stabilization of soil carbon is suppressed by live roots, outweighing influences from litter quality or quantity. Biogeochemistry 2021, 154, 433–449. [Google Scholar] [CrossRef]
- Meier, I.C.; Tückmantel, T.; Heitkötter, J.; Müller, K.; Preusser, S.; Thomas, J.; Wrobel, T.J.; Kandeler, E.; Marschner, B.; Leuschner, C. Root exudation of mature beech forests across a nutrient availability gradient: The role of root morphology and fungal activity. New Phytol. 2020, 226, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Białobok, S. Silver Fir Abies alba. State; Scientific Publishing House: Warsaw, Poland; Poznań, Poland, 1983. [Google Scholar]
- Vormstein, S.; Kaiser, M.; Piepho, H.-P.; Joergensen, R.G.; Ludwig, B. Effects of fine root characteristics of beech on carbonturnover in the topsoil and subsoil of a sandy Cambisol. Eur. J. Soil Sci. 2017, 68, 177–188. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D. Fine root biomass of temperate forests in relation to soil acidity and fertility, climate, age and species. Prog. Bot. 2003, 64, 405–438. [Google Scholar] [CrossRef]
- Sierra Cornejo, N.; Hertel, D.; Becker, J.N.; Hemp, A.; Leuschner, C. Biomass, Morphology, and Dynamics of the Fine Root System Across a 3,000-M Elevation Gradient on Mt. Kilimanjaro. Front. Plant Sci. 2020, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Hou, E.; Liu, Y.; Wen, D. Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci. Rep. 2016, 6, 24261. [Google Scholar] [CrossRef]

| Species | Altitude [m] | pH | Y | C | N | C/N | Ca | Mg | K | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | KCl | ||||||||||
| Beech | 600 | 4.84 ± 1.04 a | 4.18 ± 0.40 a | 2.4 ± 0.5 a | 5.23 ± 0.91 b | 0.37 ± 0.05 b | 14.1 ± 1.6 a | 142.46 ± 70.4 a | 17.47 ± 8.4 a | 11.35 ± 2.5 a | 1.13 ± 0.7 a |
| 800 | 4.09 ± 0.19 b | 3.45 ± 0.12 b | 4.9 ± 1.0 b | 6.72 ± 1.65 ab | 0.43 ± 0.09 ab | 15.7 ± 0.8 ab | 19.49 ± 7.6 b | 4.17 ± 0.9 b | 6.56 ± 1.6 b | 0.65 ± 0.1 b | |
| 1000 | 4.04 ± 0.25 b | 3.4 ± 0.19 b | 5.3 ± 0.9 b | 8.80 ± 2.02 a | 0.49 ± 0.09 a | 17.9 ± 1.8 b | 9.99 ± 4.1 b | 3.64 ± 0.6 a | 7.44 ± 2.5 b | 0.70 ± 0.1 b | |
| Fir | 600 | 4.91 ± 0.20 a | 3.91 ± 0.18 a | 2.6 ± 0.4 a | 4.51 ± 0.62 b | 0.32 ± 0.04 b | 14.0 ± 0.6 a | 93.76 ± 28.6 a | 12.89 ± 4.2 a | 11.63 ± 3.3 b | 0.83 ± 0.1 b |
| 800 | 3.94 ± 0.15 b | 3.27 ± 0.14 b | 5.8 ± 1.0 b | 10.44 ± 2.92 b | 0.55 ± 0.10 a | 18.7 ± 1.8 b | 14.57 ± 5.7 b | 4.18 ± 1.1 b | 7.15 ± 3.7 a | 0.93 ± 0.2 a | |
| 1000 | 3.65 ± 0.08 b | 3.02 ± 0.11 b | 8.6 ± 1.7 b | 14.81 ± 5.60 a | 0.75 ± 0.23 a | 19.6 ± 1.3 b | 17.71 ± 16.9 b | 5.60 ± 2.1 ab | 8.43 ± 4.3 b | 1.24 ± 0.3 ab | |
| Species | Altitude [m] | Diameter [mm] | Length [cm] | Biomass [g·dm−3] | Decomposition [%] | Production [g] |
|---|---|---|---|---|---|---|
| Beech | 600 | 0.59 ± 0.13 a | 6452.57 ± 5339.64 a | 2.14 ± 0.54 a | 24.37 ± 13.56 a | 0.11 ± 0.08 a |
| 800 | 0.59 ± 0.05 a | 4153.94 ± 1428.64 a | 2.17 ± 0.38 a | 29.24 ± 22.05 a | 0.14 ± 0.07 a | |
| 1000 | 0.57 ± 0.05 a | 3957.79 ± 1702.15 a | 2.34 ± 0.59 a | 23.67 ± 7.99 a | 0.10 ± 0.05 a | |
| Fir | 600 | 0.77 ± 0.21 a | 2269.63 ± 1760.69 a | 1.76 ± 0.33 b | 19.48 ± 6.89 a | 0.04 ± 0.04 b |
| 800 | 0.84 ± 0.14 a | 3378.09 ± 2218.55 a | 1.93 ± 0.44 b | 31.35 ± 13.69 a | 0.11 ± 0.05 a | |
| 1000 | 0.93 ± 0.13 a | 2475.61 ± 708.62 a | 2.56 ± 0.49 a | 19.60 ± 12.00 a | 0.04 ± 0.02 b |
| pH H2O | pH KCl | Y | N | C | Ca | K | Mg | Na | |
|---|---|---|---|---|---|---|---|---|---|
| CfLF | −0.597 * | −0.746 * | 0.821 * | 0.913 * | 0.927 * | −0.456 * | −0.012 | −0.368 * | 0.342 * |
| NfLF | −0.607 * | −0.756 * | 0.834 * | 0.893 * | 0.895 * | −0.481 * | −0.066 | −0.394 * | 0.312 * |
| CoLF | −0.065 | 0.238 | −0.251 | −0.241 | −0.245 | 0.231 | −0.057 | 0.231 | −0.004 |
| NoLF | 0.061 | 0.401 * | −0.334 * | −0.239 | −0.289 | 0.349 * | −0.011 | 0.309 * | 0.042 |
| CMAF | 0.376 * | 0.325 * | −0.309 * | −0.381 * | −0.417 * | 0.142 | −0.116 | 0.089 | −0.253 |
| NMAF | 0.444 * | 0.432 * | −0.491 * | −0.559 * | −0.581 * | 0.196 | 0.043 | 0.141 | −0.458 * |
| Diameter | Length | Biomass | Decomposition | Production | |
|---|---|---|---|---|---|
| CfLF | 0.489 * | −0.083 | 0.337 * | 0.381 * | −0.124 |
| NfLF | 0.510 * | −0.106 | 0.320 * | 0.393 * | −0.123 |
| CoLF | −0.240 | 0.369 * | 0.265 | −0.071 | −0.066 |
| NoLF | −0.434 * | 0.515 * | 0.234 | −0.079 | 0.218 |
| CMAF | −0.421 * | 0.136 | −0.202 | 0.030 | 0.310 * |
| NMAF | −0.326 * | 0.161 | −0.471 * | −0.165 | 0.121 |
| CfLF | NfLF | CoLF | NoLF | CMAF | NMAF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| Species | 26.5109 | 0.0000 | 28.6168 | 0.0000 | 4.8850 | 0.0318 | 20.9384 | 0.0000 | 48.067 | 0.0000 | 11.1602 | 0.0016 |
| Altitude | 33.3297 | 0.0000 | 36.9183 | 0.0000 | 1.4836 | 0.2370 | 3.1637 | 0.0512 | 6.273 | 0.0037 | 5.1995 | 0.0090 |
| Species * Altitude | 8.7124 | 0.0005 | 10.5703 | 0.0000 | 1.2513 | 0.2952 | 4.4425 | 0.0169 | 1.790 | 0.1778 | 1.0656 | 0.3525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszel, K.; Lasota, J.; Błońska, E. Soil Organic Matter Fractions in Relation to Root Characteristics of Different Tree Species in Altitude Gradient of Temperate Forest in Carpathian Mountains. Forests 2022, 13, 1656. https://doi.org/10.3390/f13101656
Staszel K, Lasota J, Błońska E. Soil Organic Matter Fractions in Relation to Root Characteristics of Different Tree Species in Altitude Gradient of Temperate Forest in Carpathian Mountains. Forests. 2022; 13(10):1656. https://doi.org/10.3390/f13101656
Chicago/Turabian StyleStaszel, Karolina, Jarosław Lasota, and Ewa Błońska. 2022. "Soil Organic Matter Fractions in Relation to Root Characteristics of Different Tree Species in Altitude Gradient of Temperate Forest in Carpathian Mountains" Forests 13, no. 10: 1656. https://doi.org/10.3390/f13101656





