Phosphorus Nutrition and Water Relations of European Beech (Fagus sylvatica L.) Saplings Are Determined by Plant Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Materials
2.2. Plant Tissue Analyses
2.2.1. Determination of Total C, Total N, and δ13C in Leaves and Fine Roots by IRMS
2.2.2. Pi analyses of Leaves and Fine Roots
2.2.3. Ascorbate Determination in Leaves and Fine Roots
2.3. Genetic Analyses
2.4. Soil Sampling and Soil Analyses
2.4.1. Soil Sampling
2.4.2. Determination of Available Mineral N in the Soil
2.4.3. Determination of Soil Pi Content
2.5. Statistics
3. Results
3.1. Growth Conditions in the Habitats of Sapling Origin
3.2. Biomass, Root/Shoot Ratio, and Tree Height
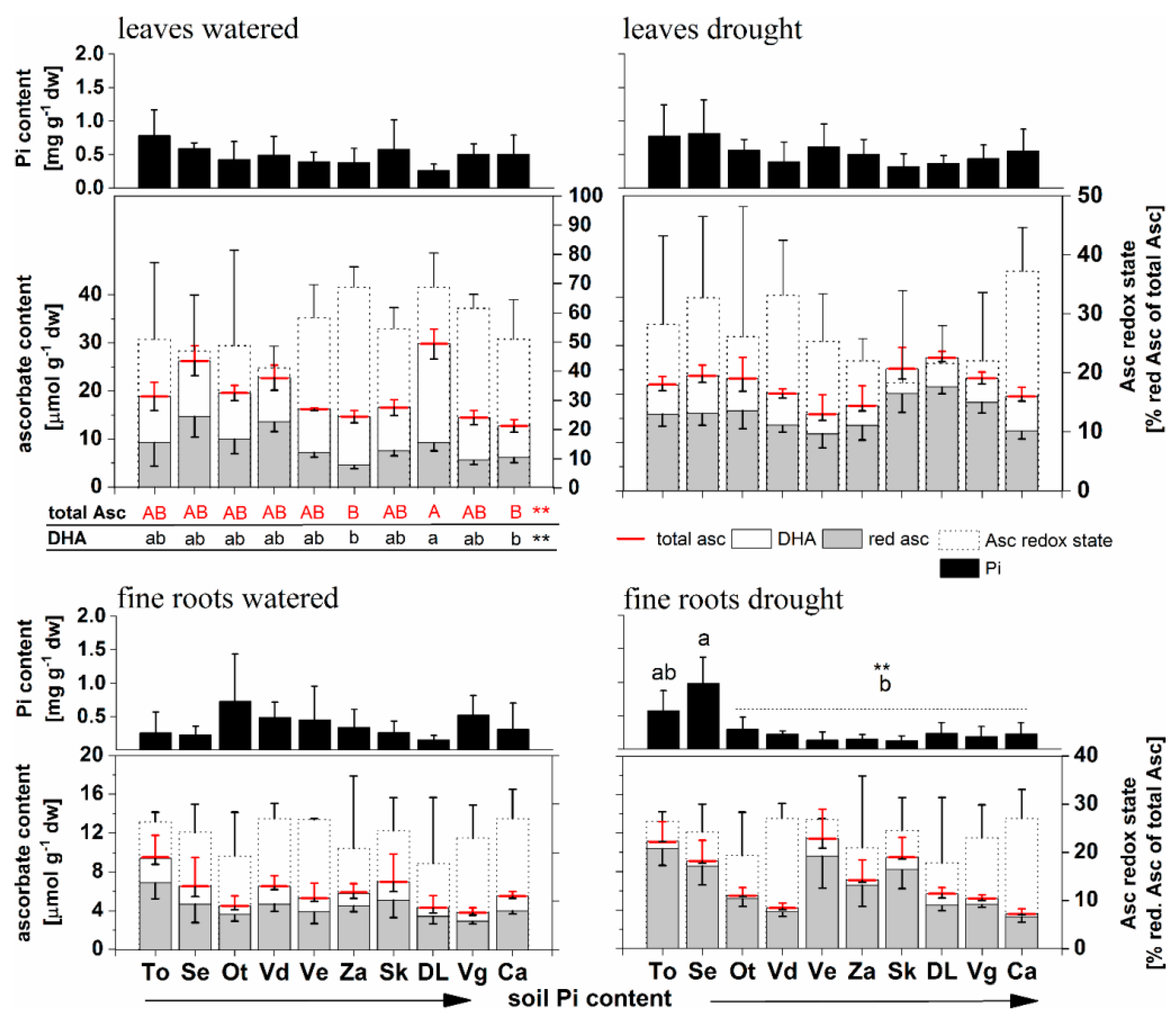
3.3. Physiological Parameters in Leaves and Fine Roots in the Well-Watered Treatment
3.4. Correlation Analyses for the Well-Watered Conditions
3.4.1. Pimin and Piorg in the Soil of the Habitat of Origin Correlated with Nutrient and Asc Contents in Well-Watered Saplings
3.4.2. Climatic Parameters in the Habitat of Origin Are Less Important for Nutrient and Asc Contents in Well-Watered Saplings
3.5. Physiological Parameters in Leaves and Fine Roots in the Drought Treatment
3.6. Correlation Analyses for the Drought-Stressed Conditions
3.6.1. Pimin and Piorg Control Nutrients and Asc in Drought-Treated Saplings
3.6.2. Climatic Parameters Hardly Explain Nutrient and Asc Contents in Drought-Treated Saplings
3.7. Significance of Drought for Physiological Parameters in Beech Saplings
3.7.1. Effects of Drought in Leaves: Redox State of Asc Most Affected
3.7.2. Effects of Drought on Fine Roots: The Redox State of Asc Most Affected
3.8. Pi Availability in the Soil in the Habitat of Origin Explains Physiological Responses to Drought
3.8.1. The Sensitivity of Ecotypes to Drought Is Driven by Pi in the Soil in the Habitat of Origin
3.8.2. The Strength of Physiological Responses of Ecotypes to Drought Is Dependent on Soil Pi in the Habitat of Origin
3.9. Do Genetic Relationships between Beech Ecotypes Explain the Dependencies of Physiological Parameters on Growth Conditions in the Habitats of Origin?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Durrant, T.H.; de Rigo, D.; Caudullo, G. Fagus sylvatica in Europe: Distributon, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; The Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen in Ökologischer, Dynamischer und Historischer Sicht, 5th ed.; Ulmer: Stuttgart, Germany, 1996. [Google Scholar]
- Dannenmann, M.; Bimüller, C.; Gschwendtner, S.; Leberecht, M.; Tejedor, J.; Bilela, S.; Gasche, R.; Hanewinkel, M.; Baltensweiler, A.; Kögel-Knabner, I.; et al. Climate Change Impairs Nitrogen Cycling in European Beech Forests. PLoS ONE 2016, 11, e0158823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilaš, I.; Medved, I.; Medak, J.; Tadić, M.P.; Medak, D. Ecological, Typological Properties and Photosynthetic Activity (FAPAR) of Common Beech (Fagus sylvatica L.) Ecosystems in Croatia. South-East Eur. For. 2017, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Gračan, J. Achievements in the breeding of common beech in Croatia. In Common Beech (Fagus sylvatica L.) in Croatia. Matić, Slavko (EIC); Academy of Forestry Sciences: Zagreb, Croatia, 2003; pp. 278–325. [Google Scholar]
- IPCC. Climate Change: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Glavač, V. Contribution to recognizing ecophysiological properties of beech in the light of recent forest damage. In Common Beech (Fagus sylvatica L.) Croatia; Matić, S., Ed.; Academy of Forestry Sciences: Zagreb, Croatia, 2003; pp. 170–2012. [Google Scholar]
- Ivanković, M.; Bogdan, S.; Božič, G. Varijabilnost visinskog rasta obične bukve (Fagus sylvatica L.) u testovima provenijencija u Hrvatskoj i Sloveniji. Šumarski List. 2008, 132, 529–541. [Google Scholar]
- Ivanković, M.; Popović, M.; Katičić, I.; Wuehlisch, G.V.; Bogdan, S. Quantitative genetic variation of European beech (Fagus sylvatica L.) provenances from the Southeastern Europe. Šumarski List. 2011, 135, 25–36. [Google Scholar]
- Stojnić, S.; Orlović, S.; Ballian, D.; Ivanković, M.; Šijačić-Nikolić, M.; Pilipović, A.; Bogdan, S.; Kvesić, S.; Mataruga, M.; Daničić, V.; et al. Provenance by site interaction and stability analysis of European beech (Fagus sylvatica L.) provenances grown in common garden experiments. Silvae Genet. 2015, 64, 133. [Google Scholar] [CrossRef] [Green Version]
- Ivanković, M.; Bogdan, S.; Littvay, T. Genetic variation of flushing and winter leaf retention in European Beech provenance test in Croatia. In Proceedings of the 8th IUFRO International Beech Symposium Organized by IUFRO Working Party 1.01.07 “Ecology and Silviculture of Beech”, Hokkaido, Japan, 8–13 September 2008; Kazuhiko, T., Palle, M., Sagheb-Talebi, K., Eds.; Nanae: Hokkaido, Japan, 2008; pp. 28–30. [Google Scholar]
- Mátyás, C.; Božić, G.; Gömöry, D.; Ivanković, M.; Rasztovits, E. Transfer analysis reveals macroclimatic adaptation of European beech (Fagus sylvatica L.). Acta Silv. Lignaria Hung. 2009, 5, 47–62. [Google Scholar]
- Mátyás, C.; Bozic, G.; Gömöry, D.; Ivankovic, M.; Rasztovits, E. Juvenile growth response of European beech (Fagus sylvatica L.) to sudden change of climatic environment in SE European trials. iForest-Biogeosci. For. 2009, 2, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Bogunović, S.; Bogdan, S.; Lanšćak, M.; Ćelepirović, N.; Ivanković, M. Use of a Common Garden Experiment in Selecting Adapted Beech Provenances for Artificial Stand Restoration. South-East Eur. For. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Gavranović, A.; Bogdan, S.; Lanšćak, M.; Čehulić, I.; Ivanković, M. Seed Yield and Morphological Variations of Beechnuts in Four European Beech (Fagus sylvatica L.) Populations in Croatia. South-East Eur. For. 2018, 9, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2006, 21, 1–11. [Google Scholar] [CrossRef]
- Rennenberg, H.; Seiler, H.; Matyssek, R.; Gessler, A.; Kreuzwieser, J. Die Buche (Fagus sylvatica L.)—Ein Waldbaum ohne Zukunft im südlichen Mitteleuropa? Allg. Forst Jagdztg. 2004, 10/11, 210–224. [Google Scholar]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagussylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M. Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Simon, J.; Dannenmann, M.; Pena, R.; Gessler, A.; Rennenberg, H. Nitrogen nutrition of beech forests in a changing climate: Importance of plant-soil-microbe water, carbon, and nitrogen interactions. Plant Soil 2017, 418, 89–114. [Google Scholar] [CrossRef]
- Netzer, F.; Schmid, C.; Herschbach, C.; Rennenberg, H. Phosphorus-nutrition of European beech (Fagus sylvatica L.) during annual growth depends on tree age and P-availability in the soil. Environ. Exp. Bot. 2017, 137, 194–207. [Google Scholar] [CrossRef]
- Netzer, F.; Herschbach, C.; Oikawa, A.; Okazaki, Y.; Dubbert, D.; Saito, K.; Rennenberg, H. Seasonal Alterations in Organic Phosphorus Metabolism Drive the Phosphorus Economy of Annual Growth in F. sylvatica Trees on P-Impoverished Soil. Front. Plant Sci. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Bauhus, J.; Frossard, E.; George, E.; Kaiser, K.; Kaupenjohann, M.; Krüger, J.; Matzner, E.; Polle, A.; Prietzel, J.; et al. Phosphorus in forest ecosystems: New insights from an ecosystem nutrition perspective. J. Plant Nutr. Soil Sci. 2016, 179, 129–135. [Google Scholar] [CrossRef]
- Lang, F.; Krüger, J.; Amelung, W.; Willbold, S.; Frossard, E.; Bünemann, E.K.; Bauhus, J.; Nitschke, R.; Kandeler, E.; Marhan, S.; et al. Soil phosphorus supply controls P nutrition strategies of beech forest ecosystems in Central Europe. Biogeochemistry 2017, 136, 5–29. [Google Scholar] [CrossRef] [Green Version]
- Netzer, F.; Thöm, C.; Celepirovic, N.; Ivankovic, M.; Alfarraj, S.; Dounavi, A.; Simon, J.; Herschbach, C.; Rennenberg, H. Drought effects on C, N, and P nutrition and the antioxidative system of beech seedlings depend on geographic origin. J. Plant Nutr. Soil Sci. 2016, 179, 135–150. [Google Scholar] [CrossRef]
- Peuke, A.D.; Rennenberg, H. Carbon, nitrogen, phosphorus, and sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): Phosphorus most affected by drought. Trees 2004, 18, 639–648. [Google Scholar] [CrossRef]
- Dounavi, A.; Netzer, F.; Celepirovic, N.; Ivanković, M.; Burger, J.; Figueroa, A.; Schön, S.; Simon, J.; Cremer, E.; Fussi, B.; et al. Genetic and physiological differences of European beech provenances (F. sylvatica L.) exposed to drought stress. For. Ecol. Manag. 2016, 361, 226–236. [Google Scholar] [CrossRef]
- Kempf, M.; Konnert, M. Distribution of genetic diversity in Fagus sylvatica at the north-eastern edge of the natural range. Silva Fenn. 2016, 50, 1663. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur. J. For. Res. 2009, 128, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Plassard, C.; Dell, B. Phosphorus nutrition of mycorrhizal trees. Tree Physiol. 2010, 30, 1129–1139. [Google Scholar] [CrossRef] [Green Version]
- Bieleski, R.L. Phosphate Pools, Phosphate Transport, and Phosphate Availability. Annu. Rev. Plant Physiol. 1973, 24, 225–252. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The Mineral Nutrition of Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Passioura, J.B. ‘Soil conditions and plant growth’. Plant Cell Environ. 2002, 25, 311–318. [Google Scholar] [CrossRef]
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, Ĺ.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) Populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocozza, C.; De Miguel, M.; Pšidová, E.; Ditmarová, L.; Marino, S.; Maiuro, L.; Alvino, A.; Czajkowski, T.; Bolte, A.; Tognetti, R. Variation in Ecophysiological Traits and Drought Tolerance of Beech (Fagus sylvatica L.) Seedlings from Different Populations. Front. Plant Sci. 2016, 7, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.; Dannenmann, M.; Gasche, R.; Holst, J.; Mayer, H.; Papen, H.; Rennenberg, H. Competition for nitrogen between adult European beech and its offspring is reduced by avoidance strategy. For. Ecol. Manag. 2011, 262, 105–114. [Google Scholar] [CrossRef]
- Loomis, W.; Battaile, J. Plant phenolic compounds and the isolation of plant enzymes. Phytochemistry 1966, 5, 423–438. [Google Scholar] [CrossRef]
- Murphy, J.A.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Okamura, M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin. Chim. Acta Int. J. Clin. Chem. 1980, 103, 259–268. [Google Scholar]
- Knörzer, O.C.; Burner, J.; Boger, P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plant. 1996, 97, 388–396. [Google Scholar] [CrossRef]
- Haberer, K.; Herbinger, K.; Alexou, M.; Rennenberg, H.; Tausz, M. Effects of drought and canopy ozone exposure on antioxidants in the fine roots of mature European beech (Fagus sylvatica). Tree Physiol. 2008, 28, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Ueno, S.; Taguchi, J.; Tomaru, N.; Tsumura, J. Development of EST-SSR markers from an inner bark cDNA library of Fagus crenata (Fagacaea). Conserv. Genet. 2009, 10, 1477–1485. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Schlotter, D.; Schack-Kirchner, H.; Hildebrand, E.E.; von Wilpert, K. Equivalence or complementarity of soil-solution extraction methods. J. Plant Nutr. Soil Sci. 2012, 175, 236–244. [Google Scholar] [CrossRef]
- Hofmann, K.; Heuck, C.; Spohn, M. Phosphorus resorption by young beech trees and soil phosphatase activity as dependent on phosphorus availability. Oecologia 2016, 181, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddou-Muratorio, S.; Davi, H. Simulating local adaptation to climate of forest trees with a Physio-Demo-Genetics model. Evol. Appl. 2014, 7, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Garciaplazaola, J.I.; Becerril, J. Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees 2000, 14, 485–490. [Google Scholar] [CrossRef]
- Peuke, A.D.; Schraml, C.; Hartung, W.; Rennenberg, H. Identification of drought-sensitive beech ecotypes by physiological parameters. New Phytol. 2002, 154, 373–387. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Spohn, M.; Zavišić, A.; Nassal, P.; Bergkemper, F.; Schulz, S.; Marhan, S.; Schloter, M.; Kandeler, E.; Polle, A. Temporal variations of phosphorus uptake by soil microbial biomass and young beech trees in two forest soils with contrasting phosphorus stocks. Soil Biol. Biochem. 2018, 117, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Zavišić, A.; Pena, R.; Polle, A. Phenology, photosynthesis, and phosphorus in European beech (Fagus sylvatica L.) in two forest soils with contrasting P contents. J. Plant Nutr. Soil Sci. 2016, 179, 151–158. [Google Scholar] [CrossRef]
- Zavišić, A.; Polle, A. Dynamics of phosphorus nutrition, allocation and growth of young beech (Fagus sylvatica L.) trees in P-rich and P-poor forest soil. Tree Physiol. 2017, 38, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, D.S.; Crous, K.Y.; Lambers, H.; Cooke, J. Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant, Cell Environ. 2015, 38, 1142–1156. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Rychter, A.; Rao, I. Role of Phosphorus in Photosynthetic Carbon Metabolism. In Handbook of Photosynthesis, 2nd ed.; Books in Soils, Plants, and the Environment; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Warren, C.R. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 2011, 31, 727–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.; Lovelock, C.; Plaxton, W.; Price, C.A.; Scheible, W.-R.; Shane, M.W.; White, P.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Hayes, P.E.; Laliberté, E.; Oliveira, R.S.; Turner, B.L. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavišić, A.; Nassal, P.; Yang, N.; Heuck, C.; Spohn, M.; Marhan, S.; Pena, R.; Kandeler, E.; Polle, A. Phosphorus availabilities in beech (Fagus sylvatica L.) forests impose habitat filtering on ectomycorrhizal communities and impact tree nutrition. Soil Biol. Biochem. 2016, 98, 127–137. [Google Scholar] [CrossRef]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.; Jaisi, D.; Lang, F.; Neal, A.; Stutter, M.I.; Almeida, D.; et al. Organic phosphorus in the terrestrial environment: A perspective on the state of the art and future priorities. Plant Soil 2017, 427, 191–208. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Phenes for Enhanced Soil Exploration and Phosphorus Acquisition: Tools for Future Crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, T.-J.; Lin, S.-I. Signaling Network in Sensing Phosphate Availability in Plants. Annu. Rev. Plant Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef]



| Forest Site | Abb. | Long. | Lat. | Elev (m) | MAP (mm) | MSP (mm) | MAT (°C) | MWMT (°C) | MCMT (°C) | CMD | Soil Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Čaglin | Ča | 45.287158 | 17.973376 | 320 | 817 | 395 | 10.3 | 20.3 | −0.5 | 212 | Eutric Cambisol |
| Donji Lapac | DL | 44.607089 | 15.937172 | 800 | 1157 | 421 | 8.3 | 18 | −1.5 | 127 | Rendzic Leptosol |
| Otočac | Ot | 44.59968 | 15.082561 | 705 | 1896 | 618 | 9.4 | 19 | 0 | 73 | Rendzic Leptosol |
| Senj | Se | 44.951944 | 15.063888 | 585.5 | 1212 | 478 | 10.1 | 19.7 | 0.7 | 106 | Chromic Cambisol |
| Skrad | Sk | 45.421944 | 14.912222 | 912 | 1782 | 669 | 8.1 | 17.8 | −1.1 | 21 | Rendzic Leptosol |
| Topusko | To | 45.232106 | 15.85488 | 237 | 1068 | 467 | 11.1 | 21.1 | 0.8 | 149 | Dystric Cambisol |
| Velika (dore) | Vd | 45.483695 | 17.668177 | 403.5 | 953 | 464 | 10 | 20.1 | −0.7 | 122 | Dystric Cambisol |
| Veliki Grđevac | Ve | 45.79263 | 17.131775 | 196.5 | 796 | 373 | 11.2 | 21.4 | 0.5 | 237 | Luvisol |
| Velika (gore) | Vg | 45.511783 | 17.645474 | 610 | 1165 | 539 | 8.9 | 18.8 | −1.6 | 37 | Dystric Cambisol |
| Zagreb | Za | 45.895401 | 15.945519 | 978.5 | 1317 | 616 | 6.5 | 16.6 | −3.7 | 11 | Dystric Cambisol |
| Site | Total Pi mg kg−1 | Mineral Soil (mg kg dw−1) | Organic Layer (mg kg dw−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOmin | p | NHmin | p | Pimin | p | NOorg | p | NHorg | p | Piorg | p | ||
| Ča | 383 (86) | 0.3 (0.1) | ns | 6 (4) | b | 151 (21) | a | 3 (1) | b | 71 (20) | ns | 232 (69) | a |
| Vg | 347 (39) | 2 (2) | 7 (3) | b | 165 (36) | ab | 5 (1) | b | 84 (12) | 182 (32) | a | ||
| DL | 314 (35) | 2 (2) | 7 (2) | b | 107 (50) | ab | 5 (2) | b | 47 (9) | 202 (64) | ab | ||
| Sk | 308 (65) | 4 (4) | 22 (6) | a | 152 (29) | ab | 5 (2) | b | 33 (7) | 162 (32) | a | ||
| Za | 277 (46) | 7 (8) | 14 (7) | ab | 128 (31) | ab | 31 (10) | a | 98 (13) | 149 (80) | ab | ||
| Ve | 277 (81) | 2 (2) | 6 (6) | b | 153 (24) | ab | 7 (3) | ab | 79 (23) | 124 (40) | ab | ||
| Vd | 257 (71) | 0.3 (0.3) | 8 (2) | b | 98 (53) | ab | 4 (2) | b | 46 (13) | 159 (63) | ab | ||
| Ot | 250 (54) | 4 (2) | 4 (2) | b | 119 (38) | ab | 20 (5) | ab | 44 (18) | 131 (27) | ab | ||
| Se | 169 (75) | 1 (1) | 4 (1) | b | 91 (54) | b | 11 (5) | ab | 35 (6) | 79 (33) | ab | ||
| To | 103 (23) | 4 (3) | 5 (3) | b | 46 (17) | b | 2 (0.2) | b | 42 (11) | 57 (24) | b | ||
| Biomass (g Dry Weight) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Well-Watered | Water Deprivation | ||||||||||||||||||
| Leaves | p | Stem | p | Fine Roots | p | Total | p | Root/ Shoot | p | Leaves | p | Stem | p | Fine Roots | p | Total | p | Root/ Shoot | p | |
| Ča | 0.3 (0.4) | ns | 2.2 (0.8) | ab | 2.7 (2.0) | ab | 5.2 (3.1) | ab | 1.0 (0.4) | ns | 0.3 (0.2) | ns | 1.5 (0.5) | ns | 2.2 (0.9) | ns | 4.0 (1.6) | ns | 1.2 (0.3) | ns |
| Vg | 0.1 (0.0) | 1.1 (0.3) | b | 1.6 (0.6) | b | 2.7 (0.9) | bc | 1.3 (0.3) | 0.4 (0.1) | 2.2 (0.4) | 3.3 (0.3) | 5.8 (0.2) | 1.3 (0.3) | |||||||
| DL | 0.5 (0.3) | 2.8 (1.6) | ab | 4.1 (1.4) | ab | 7.4 (3.1) | abc | 1.4 (0.4) | 0.3 (0.1) | 2.8 (0.7) | 3.2 (0.6) | 6.2 (1.0) | 1.1 (0.3) | |||||||
| Sk | 0.5 (0.4) | 4.6 (1.7) | a | 5.2 (2.0) | ab | 10.3 (4.1) | a | 1.0 (0.1) | 0.5 (0.2) | 3.3 (1.8) | 4.1 (2.4) | 7.9 (4.3) | 1.0 (0.3) | |||||||
| Za | 0.8 (0.6) | 3.1 (0.7) | ab | 4.1 (1.9) | ab | 8.0 (3.1) | abc | 1.0 (0.3) | 0.4 (0.3) | 2.0 (0.6) | 3.6 (1.5) | 6.1 (2.3) | 1.5 (0.4) | |||||||
| Ve | 0.3 (0.2) | 1.5 (0.4) | b | 2.1 (0.9) | ab | 3.8 (1.5) | abc | 1.2 (0.1) | 0.6 (0.2) | 2.6 (0.2) | 2.8 (1.0) | 5.9 (1.7) | 0.9 (0.2) | |||||||
| Vd | 0.6 (0.4) | 2.4 (1.0) | ab | 2.6 (1.0) | ab | 5.6 (2.1) | abc | 1.0 (0.4) | 0.4 (0.2) | 1.8 (0.6) | 2.9 (1.6) | 5.0 (2.2) | 1.3 (0.3) | |||||||
| Ot | 0.7 (0.4) | 2.9 (0.8) | ab | 5.9 (1.9) | a | 9.5 (3.0) | ab | 1.6 (0.1) | 0.5 (0.7) | 2.9 (2.1) | 3.9 (1.9) | 7.3 (4.6) | 1.3 (0.3) | |||||||
| Se | 0.2 (0.2) | 0.7 (0.2) | b | 1.4 (0.6) | b | 2.5 (1.5) | bc | 0.9 (0.1) | 0.3 (0.1) | 1.7 (0.7) | 2.2 (0.4) | 4.2 (1.1) | 1.2 (0.3) | |||||||
| To | 0.1 (0.0) | 1.3 (0.8) | b | 1.1 (0.3) | ab | 1.9 (0.6) | c | 1.4 (0.2) | 0.3 (0.1) | 1.5 (0.2) | 2.4 (0.5) | 4.2 (0.6) | 1.3 (0.3) | |||||||
| Tree Height and Growth (cm) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Well-Watered | Water Deprivation | |||||||||||||||
| Site | Height Start | p | Height End | p | Rel Inc | p | Abs Inc | p | Height Start | p | Height End | p | Rel Inc | p | Abs Inc | p |
| cm | cm | % | cm | cm | cm | % | cm | |||||||||
| Ča | 27.2 (3.3) | ns | 28.7 (4.0) | ns | 5.4 (4.0) | ns | 1.5 (1.2) | ns | 24.2 (6.1) | ns | 25.0 (6.4) | ns | 3.3 (0.6) | ns | 0.8 (0.3) | ns |
| Vg | 23.1 (3.0) | 24.0 (2.7) | 4.1 (2.3) | 0.9 (0.4) | 26.9 (1.1) | 28.1 (1.7) | 4.4 (2.7) | 1.2 (0.8) | ||||||||
| DL | 30.0 (3.1) | 31.1 (3.2) | 3.7 (0.6) | 1.1 (0.2) | 26.7 (6.4) | 28.0 (5.7) | 5.9 (6.6) | 1.3 (1.0) | ||||||||
| Sk | 32.8 (5.3) | 33.9 (5.4) | 3.4 (0.5) | 1.1 (0.2) | 31.3 (3.0) | 32.1 (3.1) | 2.5 (0.8) | 0.8 (0.3) | ||||||||
| Za | 27.9 (5.7) | 29.1 (5.8) | 4.4 (3.9) | 1.2 (1.1) | 22.5 (5.5) | 23.5 (5.2) | 4.9 (2.9) | 1.0 (0.5) | ||||||||
| Ve | 25.7 (7.2) | 26.5 (7.2) | 3.3 (2.2) | 0.8 (0.4) | 27.5 (3.1) | 28.6 (2.8) | 4.2 (2.6) | 1.1 (0.5) | ||||||||
| Vd | 21.0 (4.5) | 23.3 (3.2) | 12.7 (11.1) | 2.3 (1.8) | 21.8 (2.8) | 23.0 (2.6) | 5.6 (2.5) | 1.2 (0.4) | ||||||||
| Ot | 28.1 (6.6) | 28.9 (6.8) | 2.8 (1.3) | 0.8 (0.4) | 30.9 (3.8) | 31.8 (3.5) | 3.1 (2.6) | 0.9 (0.7) | ||||||||
| Se | 31.6 (4.0) | 32.5 (4.1) | 2.9 (2.0) | 0.9 (0.7) | 29.2 (4.8) | 30.1 (5.1) | 3.0 (1.8) | 0.9 (0.7) | ||||||||
| To | 25.5 (2.3) | 26.5 (2.5) | 3.9 (3.5) | 1.0 (0.9) | 27.1 (3.4) | 28.2 (3.1) | 4.2 (1.9) | 1.1 (0.4) | ||||||||
| Leaves | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | C (mg g−1 dw) | δ13C (‰) | N (mg g−1 dw) | C/N | ||||||||||||
| Watered | p | Dry | p | Watered | p | Dry | p | Watered | p | Dry | p | Watered | p | Dry | p | |
| Ča | 47.4 (0.4) | ns | 44.7 (0.3) | ab | −30.5 (0.9) | b | −29.3 (0.2) | b | 1.1 (0.3) | ns | 1.3 (0.3) | ns | 44.8 (12.4) | ns | 36.1 (8.5) | ns |
| Vg | 48.4 (3.1) | 48.6 (2.4) | a | −29.5 (0.4) | b | −29.3 (0.6) | ab | 1.1 (0.3) | 1.0 (0.2) | 44.9 (8.9) | 50.3 (7.8) | |||||
| DL | 51.6 (5.9) | 42.2 (1.3) | b | −29.7 (0.9) | b | −30.0 (0.7) | b | 0.9 (0.1) | 1.1 (0.2) | 58.9 (15.0) | 40.7 (7.1) | |||||
| Sk | 46.4 (0.8) | 44.9 (1.5) | ab | −30.3 (0.3) | b | −29.1 (0.9) | ab | 1.2 (0.3) | 1.2 (0.3) | 41.9 (9.7) | 37.6 (7.7) | |||||
| Za | 51.0 (4.0) | 46.1 (2.1) | ab | −29.9 (0.3) | b | −29.6 (0.3) | b | 1.0 (0.2) | 1.4 (0.5) | 49.7 (4.2) | 36.9 (13.3) | |||||
| Ve | 47.6 (1.6) | 45.8 (2.3) | ab | −30.7 (0.8) | b | −28.6 (0.7) | b | 1.1 (0.2) | 1.3 (0.2) | 43.9 (8.6) | 35.1 (6.3) | |||||
| Vd | 50.3 (0.6) | 47.3 (2.7) | ab | −29.1 (0.7) | b | −28.4 (0.6) | ab | 1.1 (0.2) | 1.0 (0.1) | 45.1 (7.5) | 48.2 (9.2) | |||||
| Ot | 47.2 (0.6) | 45.7 (2.2) | ab | −30.2 (0.6) | b | −29.3 (1.3) | b | 1.2 (0.2) | 1.2 (0.3) | 40.9 (7.2) | 39.1 (9.9) | |||||
| Se | 49.0 (1.7) | 49.6 (1.6) | a | −26.8 (0.7) | a | −27.4 (0.8) | a | 1.1 (0.1) | 1.2 (0.2) | 43.6 (2.8) | 43.1 (8.3) | |||||
| To | 49.5 (1.9) | 48.7 (3.1) | a | −28.7 (1.3) | ab | −28.6 (0.6) | ab | 1.1 (0.1) | 1.1 (0.1) | 45.3 (4.0) | 46.6 (5.3) | |||||
| Fine Roots | ||||||||||||||||
| Site | C (mg g−1 dw) | δ13C (‰) | N (mg g−1 dw) | C/N | ||||||||||||
| Watered | p | Dry | p | Watered | p | Dry | p | Watered | p | Dry | p | Watered | p | Dry | p | |
| Ča | 44.7 (3.7) | ns | 51.4 (1.2) | ns | −26.7 (0.7) | ns | −27.5 (0.4) | be | 0.6 (0.1) | ns | 0.6 (0.1) | ns | 84.1 (20.7) | ns | 86.7 (7.4) | ns |
| Vg | 49.0 (2.3) | 52.1 (1.6) | −26.8 (0.1) | −27.7 (0.2) | b | 0.7 (0.1) | 0.6 (0.0) | 69.8 (4.1) | 87.6 (6.7) | |||||||
| DL | 47.7 (1.5) | 49.4 (2.9) | −25.1 (0.5) | −25.8 (0.6) | ad | 0.5 (0.1) | 0.5 (0.0) | 102.6 (17.7) | 93.0 (11.5) | |||||||
| Sk | 48.4 (1.2) | 48.3 (0.9) | −26.1 (0.8) | −26.0 (0.8) | ae | 0.5 (0.1) | 0.7 (0.1) | 92.9 (11.9) | 75.0 (11.8) | |||||||
| Za | 46.5 (1.8) | 48.8 (3.2) | −25.8 (0.4) | −26.2 (0.4) | bcde | 0.5 (0.1) | 0.7 (0.1) | 94.7 (11.8) | 76.1 (14.7) | |||||||
| Ve | 47.9 (3.5) | 49.5 (1.1) | −26.5 (1.4) | −26.9 (0.4) | bde | 0.5 (0.1) | 0.6 (0.0) | 89.5 (9.7) | 77.4 (4.2) | |||||||
| Vd | 52.5 (5.0) | 50.7 (2.3) | −26.2 (0.1) | −26.9 (0.8) | bcde | 0.6 (0.1) | 0.5 (0.1) | 88.6 (12.9) | 110.0 (15.8) | |||||||
| Ot | 46.6 (1.2) | 49.3 (0.8) | −25.8 (1.3) | −25.2 (0.8) | ac | 0.6 (0.0) | 0.5 (0.1) | 80.2 (6.0) | 103.6 (16.3) | |||||||
| Se | 47.1 (1.0) | 49.8 (1.0) | −24.5 (1.3) | −25.0 (0.6) | a | 0.7 (0.2) | 0.5 (0.1) | 71.8 (19.5) | 95.5 (12.1) | |||||||
| To | 47.5 (4.2) | 49.1 (0.2) | −25.6 (0.8) | −25.6 (0.6) | ad | 0.7 (0.1) | 0.6 (0.0) | 72.3 (2.2) | 85.0 (5.1) | |||||||
| Site | Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Asc | Red Asc | DHA | Redox | Pi | N | C | C/N | δ13C | |
| Ča | 1.63 (0.36) | 2.29 (1.11) | 0.71 (1.49) | −0.97 (1.52) | 0.18 (1.85) | 1.15 (0.15) | −1.06 (0.01) * | −1.22 (0.16) | 1.04 (0.02) |
| Vg | 1.60 (0.47) * | 3.24 (1.45) * | −1.84 (0.86) * | −2.85 (1.34) * | −1.51 (0.55) | −0.63 (1.45) | 0.50 (1.06) | 0.64 (1.42) | 1.01 (0.01) |
| Sk | 0.82 (1.87) | 2.39 (1.23) | −0.46 (2.95) | −2.65 (1.84) * | −2.79 (2.03) | 0.11 (1.36) | −0.53 (1.04) | −0.64 (1.14) | 1.06 (0.02) * |
| DL | −0.07 (1.52) | 2.73 (1.59) * | −3.61 (1.56) * | −3.30 (1.00) * | 1.17 (1.58) | 0.72 (1.28) | −1.23 (0.14) * | −1.51 (0.38) * | −0.01 (1.18) |
| Ve | 0.01 (2.06) | 1.90 (2.64) | −3.55 (3.76) | −2.64 (1.02) * | 1.08 (2.27) | 1.15 (0.12) | −0.54 (1.03) | −1.20 (0.14) | 1.08 (0.01) * |
| Za | 0.23 (1.97) | 2.17 (2.36) | −4.60 (4.24) | −3.28 (0.68) * | 1.15 (2.81) | 0.91 (1.63) | −1.13 (0.12) | −1.10 (1.58) | 0.51 (1.01) |
| Vd | −0.12 (1.68) | 0.18 (1.87) | −1.55 (0.36) | −1.42 (0.30) * | 0.07 (3.14) | −0.70 (1.34) | −1.08 (0.05) * | 0.11 (1.53) | 1.03 (0.01) |
| Ot | −0.14 (3.24) | 1.00 (3.63) | −11.54 (44.59) | 1.26 (17.60) | 1.02 (1.70) | −0.04 (1.31) | −0.03 (1.21) | 0.01 (1.25) | 0.53 (1.04) |
| Se | −0.19 (1.76) | 0.60 (1.86) | −1.04 (1.80) | −0.36 (1.70) | −0.64 (3.35) | 0.61 (1.10) | 0.02 (1.21) | −0.59 (1.11) | −0.52 (1.04) |
| To | 0.69 (1.17) | 1.47 (1.93) | −0.91 (1.93) | −1.26 (2.35) | 0.24 (1.63) | −0.57 (1.06) | −0.53 (1.05) | 0.04 (1.28) | 0.00 (1.18) |
| Site | Fine Roots | ||||||||
| Total Asc | Red Asc | DHA | Redox | Pi | N | C | C/N | δ13C | |
| Ča | −1.62 (1.73) | −0.34 (0.66) | −6.77 (4.50) * | −4.85 (3.70) * | 0.79 (3.03) | 0.69 (1.15) | 1.15 (0.09) * | −0.03 (1.41) | −0.48 (0.99) |
| Vg | 0.38 (2.03) | 0.63 (1.78) | −1.27 (2.16) | −1.70 (2.10) | −2.24 (0.76) | −1.18 (0.07) * | 0.57 (1.07) | 1.25 (0.03) * | −0.96 (0.01) * |
| Sk | 1.16 (2.29) | 1.45 (2.32) | −0.39 (2.54) | −1.74 (0.43) | −1.98 (3.40) | 1.32 (0.20) | 0.00 (1.16) | −1.33 (0.20) | 0.51 (1.00) |
| DL | 0.67 (1.80) | 0.77 (1.74) | 1.23 (4.69) | 0.57 (4.43) | 1.55 (0.96) | 0.84 (1.34) | 0.54 (1.03) | −0.79 (1.41) | −0.47 (0.99) |
| Ve | 1.52 (2.20) | 1.63 (1.99) | −1.37 (5.64) | −3.82 (6.09) | −1.71 (4.64) | 0.65 (1.15) | 0.54 (1.05) | −0.60 (1.13) | −0.46 (1.00) |
| Za | −0.05 (2.08) | 0.86 (2.43) | −3.93 (2.41) | −3.85 (1.36) | −3.43 (1.99) | 1.39 (0.26) * | 0.55 (1.07) | −1.34 (0.29) | −0.49 (0.99) |
| Vd | −1.09 (1.71) | −0.26 (1.52) | −8.14 (4.57) * | −6.59 (5.56) * | −1.37 (1.69) | −1.23 (0.12) | −0.06 (1.27) | 1.17 (0.10) | 0.03 (1.13) |
| Ot | 0.98 (1.75) | 1.29 (1.78) | −4.28 (4.23) | −5.05 (3.61) * | −2.55 (2.54) | −0.12 (1.47) | 0.55 (1.04) | 0.68 (1.35) | 0.06 (1.18) |
| Se | 1.48 (2.55) | 2.06 (2.50) | −10.44 (13.22) | −10.11 (7.96) | 6.91 (7.89) * | −0.69 (1.47) | 1.09 (0.05) * | 0.82 (1.45) | 0.05 (1.14) |
| To | 0.70 (1.57) | 1.06 (1.50) | −5.18 (4.26) | −5.65 (3.66) * | 7.25 (7.58) | −0.59 (1.19) | 0.06 (1.25) | 1.14 (0.07) * | 0.02 (1.15) |
| c > 4 | 4 > c > 3 | 3 > c > 2 | 2 > c > 1 | 1 > c >0 | 0 > c > −1 | −1 > c > −2 | −2 > c > −3 | −3 > c > −4 | c > −4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćelepirović, N.; Bogunović, S.; Dounavi, A.; Netzer, F.; Eiblmeier, M.; Dannenmann, M.; Rehschuh, S.; Rennenberg, H.; Ivanković, M. Phosphorus Nutrition and Water Relations of European Beech (Fagus sylvatica L.) Saplings Are Determined by Plant Origin. Forests 2022, 13, 1683. https://doi.org/10.3390/f13101683
Ćelepirović N, Bogunović S, Dounavi A, Netzer F, Eiblmeier M, Dannenmann M, Rehschuh S, Rennenberg H, Ivanković M. Phosphorus Nutrition and Water Relations of European Beech (Fagus sylvatica L.) Saplings Are Determined by Plant Origin. Forests. 2022; 13(10):1683. https://doi.org/10.3390/f13101683
Chicago/Turabian StyleĆelepirović, Nevenka, Sanja Bogunović, Aikaterini Dounavi, Florian Netzer, Monika Eiblmeier, Michael Dannenmann, Stephanie Rehschuh, Heinz Rennenberg, and Mladen Ivanković. 2022. "Phosphorus Nutrition and Water Relations of European Beech (Fagus sylvatica L.) Saplings Are Determined by Plant Origin" Forests 13, no. 10: 1683. https://doi.org/10.3390/f13101683





