Net Carbon Balance between Priming and Replenishment of Soil Organic Carbon with Biochar Addition Regulated by N Addition Differing in Contrasting Forest Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and 13C-Labelled Biochar Production
2.2. Experiment Design and Soil Incubation
2.3. Calculation of CO2 and Priming Effect
2.4. Measurement of Soil Properties
2.5. Statistical Analysis
3. Results
3.1. Biochar Decomposition
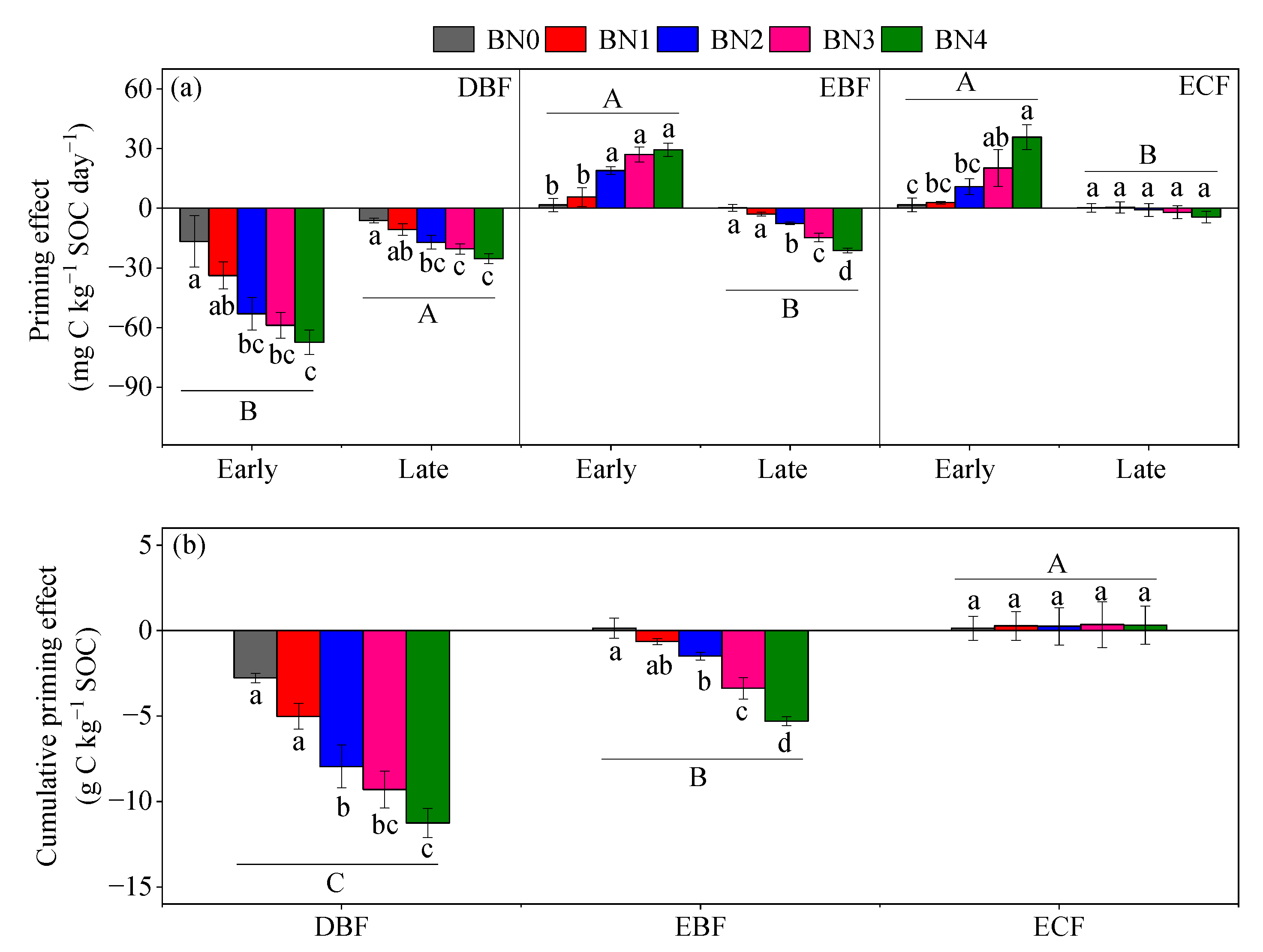
3.2. Priming of Native SOC Decomposition
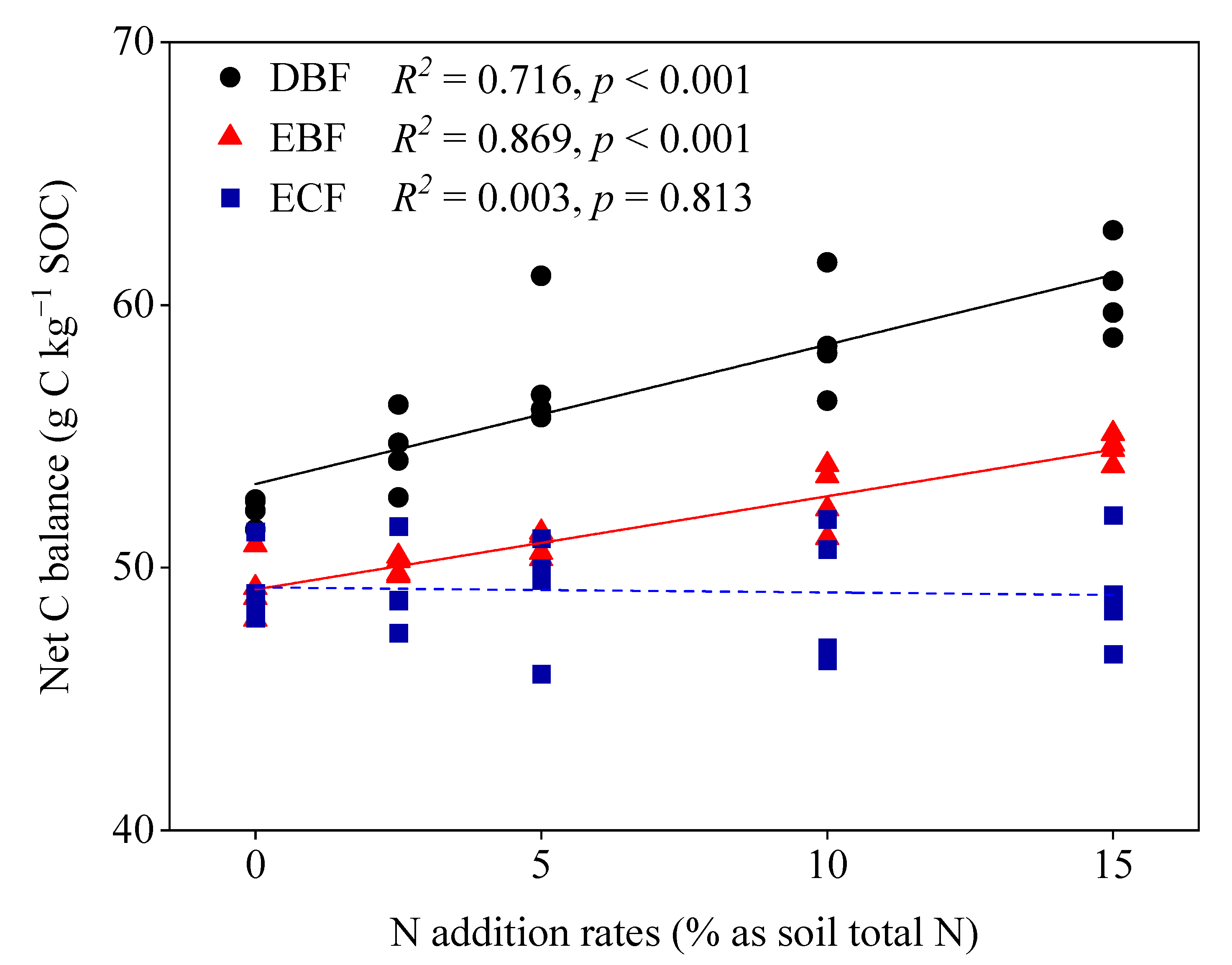
3.3. Carbon Balance
4. Discussion
4.1. Soil Priming Effect following Biochar and N Addition
4.2. Carbon Balance between Primed C Loss and Replenishment of Biochar
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmerman, A. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, K.; Han, L.F.; Chen, Y.L.; Liu, J.; Xing, B.S. Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol. Biochem. 2022, 169, 108657. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, H.W.; Bartocci, P.; Fantozzi, F.; Masek, O.; Agblevor, F.A.; Wei, Z.Y.; Yang, H.P.; Chen, H.P.; Lu, X.; et al. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 2021, 12, 1698. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; He, T.X.; Liu, L.; Wu, J.B. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- IPCC. 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar]
- Liu, Y.X.; Chen, Y.; Wang, Y.Y.; Lu, H.H.; He, L.L.; Yang, S.M. Negative priming effect of three kinds of biochar on the mineralization of native soil organic carbon. Land Degrad. Dev. 2018, 29, 3985–3994. [Google Scholar] [CrossRef]
- Whitman, T.; Enders, A.; Lehmann, J. Pyrogenic carbon additions to soil counteract positive priming of soil carbon mineralization by plants. Soil Biol. Biochem. 2014, 73, 33–41. [Google Scholar] [CrossRef]
- Bammingera, C.; Marschnera, B.; Juschke, E. An incubation study on the stability and biological effects of pyrogenic and hydrothermal biochar in two soils. Eur. J. Soil Sci. 2014, 65, 72–82. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Lu, W.W.; Ding, W.X.; Zhang, J.H.; Li, Y.; Luo, J.F.; Bolan, N.; Xie, Z.B. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.X.; Wang, Z.Y.; Xing, B.S. Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 610–611, 951–960. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Koide, R.T.; Dell, C.; Drohan, P.; Skinner, H.; Adler, P.R.; Nord, A. Turnover of soil carbon following addition of switchgrass-derived biochar to four soils. Soil Sci. Soc. Am. J. 2014, 78, 531–537. [Google Scholar] [CrossRef]
- Ding, F.; Zwieten, L.V.; Zhang, W.D.; Weng, Z.; Shi, S.W.; Wang, J.K.; Meng, J. A meta-analysis and critical evaluation of influencing factors on soil carbon priming following biochar amendment. J. Soil Sediment. 2018, 18, 1507–1517. [Google Scholar] [CrossRef]
- Sun, Z.L.; Liu, S.E.; Zhang, T.A.; Zhao, X.C.; Chen, S.; Wang, Q.K. Priming of soil organic carbon decomposition induced by exogenous organic carbon input: A meta-analysis. Plant Soil 2019, 443, 463–471. [Google Scholar] [CrossRef]
- Chen, R.R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.G.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Ackerman, D.; Millet, D.B.; Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Maestrini, B.; Herrmann, A.M.; Nannipieri, P.; Schmidt, M.W.I.; Abiven, S. Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol. Biochem. 2014, 69, 291–301. [Google Scholar] [CrossRef]
- Qiao, N.; Schaefer, D.; Blagodatskaya, E.; Zou, X.M.; Xu, X.L.; Kuzyakov, Y. Labile carbon retention compensates for CO2 released by priming in forest soils. Glob. Chang. Biol. 2014, 20, 1943–1954. [Google Scholar] [CrossRef]
- Liang, J.Y.; Zhou, Z.H.; Huo, C.F.; Shi, Z.; Cole, J.R.; Huang, L.; Konstantinidis, K.T.; Li, X.M.; Liu, B.; Luo, Z.K.; et al. More replenishment than priming loss of soil organic carbon with additional carbon input. Nat. Commun. 2018, 9, 3175. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lin, J.J.; Wang, P.; Zhang, S.; Liu, D.; Zhu, B. Resistant soil carbon is more vulnerable to priming effect than active soil carbon. Soil Biol. Biochem. 2022, 168, 108619. [Google Scholar] [CrossRef]
- Zheng, T.H.; Zhang, J.; Tang, C.J.; Liao, K.T.; Guo, L.P. Positive and negative priming effects in an Ultisol in relation to aggregate size class and biochar level. Soil Till. Res. 2021, 208, 104874. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S. Ecology of Mixed Plantation Forest; Science Press: Beijing, China, 2004; p. 3. [Google Scholar]
- Wang, Y.Z.; Xu, Z.H.; Zhou, Q.X. Impact of fire on soil gross nitrogen transformations in forest ecosystems. J. Soil. Sediment. 2014, 14, 1030–1040. [Google Scholar] [CrossRef]
- Borwn, A.A.; Davis, K.P. Forest Fire Control and Use, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1973. [Google Scholar]
- Chao, L.; Liu, Y.Y.; Freschet, G.T.; Zhang, W.D.; Yu, X.; Zheng, W.H.; Guan, X.; Yang, Q.P.; Chen, L.C.; Dijkstra, F.A.; et al. Litter carbon and nutrient chemistry control the magnitude of soil priming effect. Funct. Ecol. 2019, 33, 876–888. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods of Soil Analysis. Part 1; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–441. [Google Scholar]
- Mooshammer, M.; Wanek, W.; Haemmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Y.; Liu, L.; Mao, C.; Qin, S.Q.; Wang, J.; Liu, F.T.; Blagodatsky, S.; Yang, G.B.; Zhang, Q.W.; Zhang, D.Y.; et al. Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat. Commun. 2018, 9, 3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, B.; Schnecker, J.; Knoltsch, A.; Takriti, M.; Mooshammer, M.; Gentsch, N.; Mikutta, R.; Alves, R.J.E.; Gittel, A.; Lashchinskiy, N.; et al. Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Glob. Biogeochem. Cycles 2015, 29, 567–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 2014, 7, 577–590. [Google Scholar] [CrossRef]
- DeCiucies, S.; Whitman, T.; Woolf, D.; Enders, A.; Lehmann, J. Priming mechanisms with additions of pyrogenic organic matter to soil. Geochim. Cosmochim. Acta 2018, 238, 329–342. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Yang, Y.; Hunter, W.; Tao, S.; Crowley, D.; Gan, J. Effect of activated carbon on microbial bioavailability of phenanthrene in soils. Environ. Toxicol. Chem. 2009, 28, 2283–2288. [Google Scholar] [CrossRef]
- Lu, W.W.; Zhang, Y.R.; Yao, Y.X.; Wu, Y.Y.; Chen, H.Y.H.; Zhang, H.L.; Yu, J.; Shen, C.Q.; Liu, Q.; Ruan, H.H. Biochar-induced priming effects in young and old poplar plantation soils. Phyton—Int. J. Exp. Bot. 2020, 89, 13–26. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Singh, B.; Singh, B.P. Effect of temperature on biochar priming effects and its stability in soils. Soil Biol. Biochem. 2015, 80, 136–145. [Google Scholar] [CrossRef]
- Murray, J.; Keith, A.; Singh, B. The stability of low- and high-ash biochars in acidic soils of contrasting mineralogy. Soil Biol. Biochem. 2015, 89, 217–225. [Google Scholar] [CrossRef]
- Feng, J.G.; Zhu, B. Global patterns and associated drivers of priming effect in response to nutrient addition. Soil Biol. Biochem. 2021, 153, 108118. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Blagodatsky, S.A.; Anderson, T.H.; Kuzyakov, Y. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl. Soil Ecol. 2007, 37, 95–105. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Chang. Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Wang, S.L. Effects of NH4+ and NO3- on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China. Soil Biol. Biochem. 2012, 47, 116–122. [Google Scholar] [CrossRef]
- Criscuoli, I.; Ventura, M.; Wiedner, K.; Glaser, B.; Panzacchi, P.; Ceccon, C.; Loesch, M.; Raifer, B.; Tonon, G. Stability of woodchips biochar and impact on soil carbon stocks: Results from a two-year field experiment. Forests 2021, 12, 1350. [Google Scholar] [CrossRef]
- Rittl, T.F.; Novotny, E.H.; Balieiro, F.C.; Hoffland, E.; Alves, B.J.R.; Kuyper, T.W. Negative priming of native soil organic carbon mineralization by oilseed biochars of contrasting quality. Eur. J. Soil Sci. 2015, 66, 714–721. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2015, 8, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Rasul, M.; Cho, J.; Shin, H.S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Ventura, M.; Alberti, G.; Panzacchi, P.; Vedove, G.D.; Miglietta, F.; Tonon, G. Biochar mineralization and priming effect in a poplar short rotation coppice from a 3-year field experiment. Biol. Fertil. Soils 2018, 55, 67–78. [Google Scholar] [CrossRef]
- Yu, L.Q.; Tang, J.; Zhang, R.D.; Wu, Q.H.; Gong, M.M. Effects of biochar application on soil methane emission at different soil moisture levels. Biol. Fertil. Soils 2013, 49, 119–128. [Google Scholar] [CrossRef]
- Zheng, S.J.; Zhao, X.C.; Sun, Z.L.; Li, J.; Jing, Y.L.; Wang, Q.K. Carbon addition modified the response of heterotrophic respiration to soil sieving in ectomycorrhizal-dominated forests. Forests 2022, 13, 1263. [Google Scholar] [CrossRef]

| Variables | DBF | EBF | ECF |
|---|---|---|---|
| Dominant tree species | Castanopsis Fargesii Franch., Machilus pauhoi Kanehira | Quercus acutissima Carruth., Pinus massoniana Lamb. | Cunninghamia lanceolata (Lamb.) Hook. |
| MAT (°C) | 15.2 | 16.5 | 16.5 |
| Soil type | Haplic Calcisols | Alumic Acrisol | Alumic Acrisol |
| Soil texture | Silt clay loam | Clay loam | Clay loam |
| MAP (mm) | 1063 | 1200 | 1200 |
| Sand (%) | 29.5 a | 14.7 b | 11.1 c |
| Silt (%) | 37.1 b | 40.5 a | 37.7 b |
| Clay (%) | 33.4 c | 44.8 b | 51.2 a |
| Soil pH | 4.45 a | 3.73 c | 4.22 b |
| SOC (g kg−1) | 26.84 b | 39.53 a | 21.80 c |
| Total N (g kg−1) | 1.97 b | 3.45 a | 1.91 b |
| C/N ratio | 13.6 a | 11.5 b | 11.4 b |
| Total P (g kg−1) | 0.27 b | 0.65 a | 0.21 c |
| Mineral N (mg kg−1) | 7.17 c | 13.37 b | 14.93 a |
| Available P (mg kg−1) | 4.04 a | 1.89 b | 0.84 c |
| DOC (mg kg−1) | 188.2 a | 97.6 b | 61.4 c |
| MBC (mg kg−1) | 331.7 a | 358.6 a | 334.8 a |
| MBN (mg kg−1) | 48.62 b | 81.64 a | 80.20 a |
| C:N imbalance | 3.91 a | 1.78 b | 1.00 c |
| Treatments | BN0 | BN1 | BN2 | BN3 | BN4 |
|---|---|---|---|---|---|
| DBF | |||||
| Replenishment | 49.42 (0.03) a | 49.41 (0.03) a | 49.40 (0.03) ab | 49.34 (0.03) ab | 49.30 (0.03) b |
| Cumulative PE | −2.77 (0.27) a | −5.02 (0.75) a | −7.96 (1.25) b | −9.30 (1.09) bc | −11.25 (0.85) c |
| Net C balance | 52.19 (0.26) c | 54.42 (0.73) c | 57.35 (1.26) b | 58.64 (1.10) ab | 60.55 (0.88) a |
| EBF | |||||
| Replenishment | 49.38 (0.02) ab | 49.40 (0.03) a | 49.38 (0.02) ab | 49.33 (0.02) b | 49.24 (0.03) c |
| Cumulative PE | 0.14 (0.59) a | −0.64 (0.18) ab | −1.49 (0.23) b | −3.37 (0.62) c | −5.30 (0.26) d |
| Net C balance | 49.25 (0.59) d | 50.04 (0.18) cd | 50.87 (0.24) c | 52.70 (0.63) b | 54.54 (0.26) a |
| ECF | |||||
| Replenishment | 49.45 (0.02) a | 49.43 (0.02) a | 49.40 (0.03) ab | 49.34 (0.02) b | 49.33 (0.01) b |
| Cumulative PE | 0.14 (0.70) a | 0.28 (0.84) a | 0.25 (1.09) a | 0.35 (1.33) a | 0.32 (1.11) a |
| Net C balance | 49.31 (0.71) a | 49.15 (0.86) a | 49.14 (1.11) a | 48.99 (1.34) a | 49.01 (1.11) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, Q.; Zhu, Y. Net Carbon Balance between Priming and Replenishment of Soil Organic Carbon with Biochar Addition Regulated by N Addition Differing in Contrasting Forest Ecosystems. Forests 2022, 13, 1710. https://doi.org/10.3390/f13101710
Sun Z, Wang Q, Zhu Y. Net Carbon Balance between Priming and Replenishment of Soil Organic Carbon with Biochar Addition Regulated by N Addition Differing in Contrasting Forest Ecosystems. Forests. 2022; 13(10):1710. https://doi.org/10.3390/f13101710
Chicago/Turabian StyleSun, Zhaolin, Qingkui Wang, and Yifan Zhu. 2022. "Net Carbon Balance between Priming and Replenishment of Soil Organic Carbon with Biochar Addition Regulated by N Addition Differing in Contrasting Forest Ecosystems" Forests 13, no. 10: 1710. https://doi.org/10.3390/f13101710




