Estimating Carbon Sink Strength of Norway Spruce Forests Using Machine Learning
Abstract
:1. Introduction
- (1).
- How does the NEP of Norway spruce forests respond to variations of climate across Europe?
- (2).
- In what European regions do Norway spruce forests have the greatest CO2 sequestration potential?
- (3).
- How will the NEP of Norway spruce forests respond to a projected future climate?
2. Materials and Methods
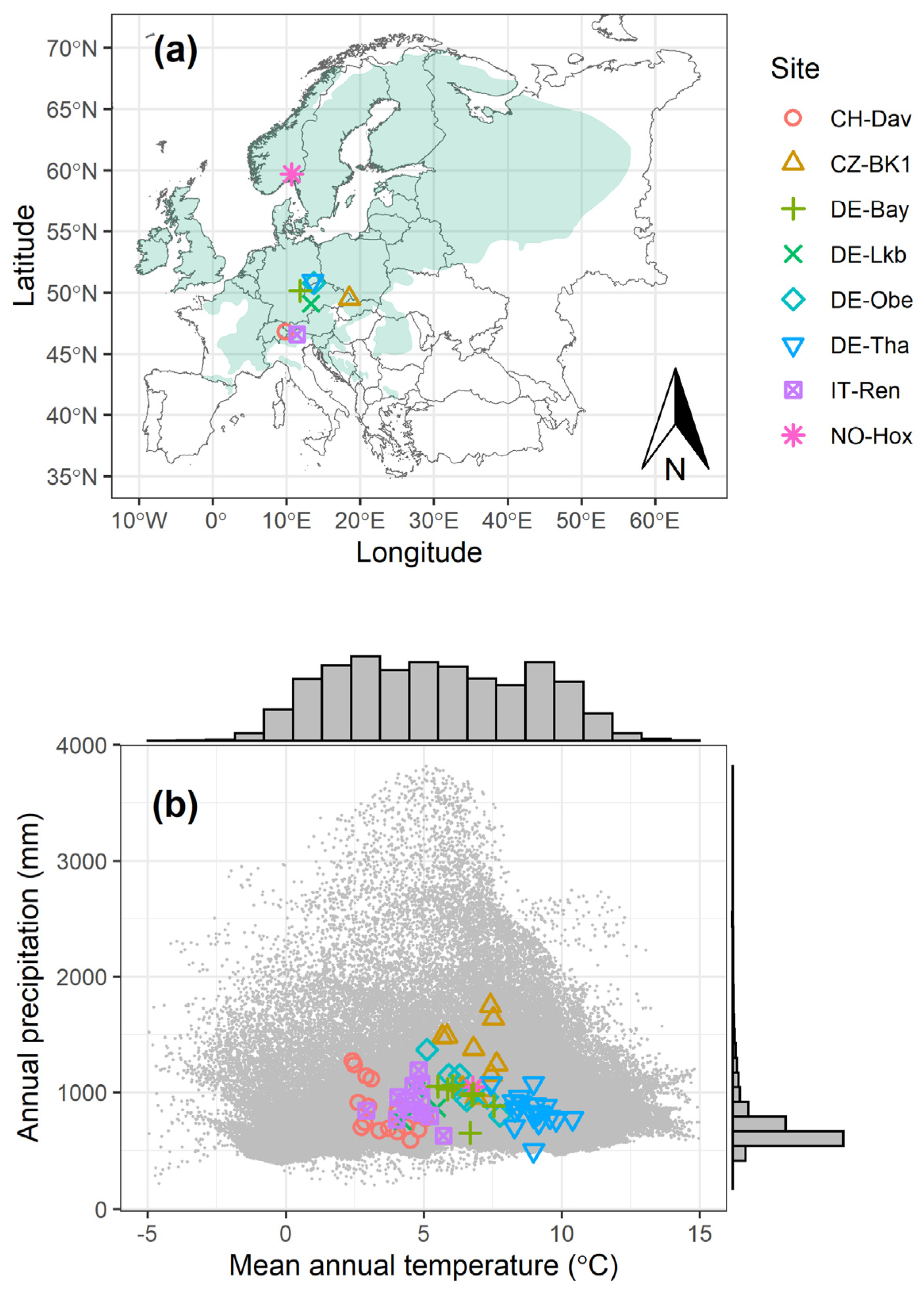
2.1. Flux Data from Different Sites
| Site Name | Country | Code | Latitude | Longitude | Elevation (m) | MAT | MAP | Years | LAI | Age (yr) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Davos | Switzerland | CH-Dav | 46.81533 | 9.85591 | 1639 | 3.55 | 850 | 1997–2014 | 3.9 | 240–257 * | [42] |
| Bily Kriz | Czech Republic | CZ-BK1 | 49.50208 | 18.53688 | 875 | 6.85 | 1292 | 2004–2014 | 10 | 23–33 | [43] |
| Lackenberg | Germany | DE-Lkb | 49.09962 | 13.30467 | 1308 | 4.82 | 879 | 2009–2013 | - | 0–4 | [44] |
| Oberbärenburg | Germany | DE-Obe | 50.78666 | 13.72129 | 734 | 6.49 | 1046 | 2008–2014 | 8 | 54–60 | - |
| Tharandt | Germany | DE-Tha | 50.96256 | 13.56515 | 385 | 8.91 | 844 | 1997–2014 | 7.6 | 111–128 | [45] |
| Renon | Italy | IT-Ren | 46.58686 | 11.43369 | 1735 | 4.58 | 904 | 1999–2013 | 5.1 | 180–194 * | [46] |
| Weidenbrunnen | Germany | DE-Bay | 50.14194 | 11.86694 | 775 | 6.46 | 947 | 2002–2014 | 4.8 | 49–61 | [39] |
| Hoxmark | Norway | NO-Hox | 59.66876 | 10.71749 | 91 | 6.79 | 1045 | 2019 | <1 | 8 | This study |
2.2. XGBoost Model and Effects on NEP
2.3. Norway Spruce Forest NEP in Europe and Its Future Trajectory
3. Results
3.1. Climate and Annual Carbon Budget of the Study Sites
3.2. XGBoost Model and Effects on NEP
3.3. NEP Predictions of Norway Spruce Forests in Europe
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ögren, E.; Evans, J.R. Photosynthetic Light-Response Curves 1. The Influence of CO2 Partial-Pressure and Leaf Inversion. Planta 1993, 189, 182–190. [Google Scholar] [CrossRef]
- Mahecha, M.D.; Reichstein, M.; Carvalhais, N.; Lasslop, G.; Lange, H.; Seneviratne, S.I.; Vargas, R.; Ammann, C.; Arain, M.A.; Cescatti, A. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 2010, 329, 838–840. [Google Scholar] [CrossRef] [Green Version]
- Sall, T.; Pettersson, P. A Model of Photosynthetic Acclimation as a Special Case of Reaction Norms. J. Theor. Biol. 1994, 166, 1–8. [Google Scholar] [CrossRef]
- Duffy, K.A.; Schwalm, C.R.; Arcus, V.L.; Koch, G.W.; Liang, L.Y.L.; Schipper, L.A. How close are we to the temperature tipping point of the terrestrial biosphere? Sci. Adv. 2021, 7, eaay1052. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; Arndt, S.K.; Bennett, L.T.; Knauer, J.; Beringer, J.; Griebel, A.; Hinko-Najera, N.; Liddell, M.J.; Metzen, D.; Pendall, E.; et al. Thermal optima of gross primary productivity are closely aligned with mean air temperatures across Australian wooded ecosystems. Glob. Change Biol. 2021, 27, 4727–4744. [Google Scholar] [CrossRef]
- Huang, M.T.; Piao, S.L.; Ciais, P.; Penuelas, J.; Wang, X.H.; Keenan, T.F.; Peng, S.S.; Berry, J.A.; Wang, K.; Mao, J.F.; et al. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol. 2019, 3, 772–779. [Google Scholar] [CrossRef]
- Niu, S.L.; Luo, Y.Q.; Fei, S.F.; Yuan, W.P.; Schimel, D.; Law, B.E.; Ammann, C.; Arain, M.A.; Arneth, A.; Aubinet, M.; et al. Thermal optimality of net ecosystem exchange of carbon dioxide and underlying mechanisms. New Phytol. 2012, 194, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Hartmann, H.; Trumbore, S.; Ziegler, W.; Zhang, Y. High temperature causes negative whole-plant carbon balance under mild drought. New Phytol. 2013, 200, 330–339. [Google Scholar] [CrossRef]
- Zheng, P.F.; Wang, D.D.; Yu, X.X.; Jia, G.D.; Liu, Z.Q.; Wang, Y.S.; Zhang, Y.G. Effects of drought and rainfall events on soil autotrophic respiration and heterotrophic respiration. Agr. Ecosyst. Environ. 2021, 308, 107267. [Google Scholar] [CrossRef]
- Mensah, C.; Sigut, L.; Fischer, M.; Foltynova, L.; Jocher, G.; Acosta, M.; Kowalska, N.; Kokrda, L.; Pavelka, M.; Marshall, J.D.; et al. Assessing the Contrasting Effects of the Exceptional 2015 Drought on the Carbon Dynamics in Two Norway Spruce Forest Ecosystems. Atmosphere 2021, 12, 988. [Google Scholar] [CrossRef]
- von Buttlar, J.; Zscheischler, J.; Rammig, A.; Sippel, S.; Reichstein, M.; Knohl, A.; Jung, M.; Menzer, O.; Arain, M.A.; Buchmann, N.; et al. Impacts of droughts and extreme-temperature events on gross primary production and ecosystem respiration: A systematic assessment across ecosystems and climate zones. Biogeosciences 2018, 15, 1293–1318. [Google Scholar] [CrossRef] [Green Version]
- Litvak, M.; Miller, S.; Wofsy, S.C.; Goulden, M. Effect of stand age on whole ecosystem CO2 exchange in the Canadian boreal forest. J. Geophys. Res.-Atmos. 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Musavi, T.; Migliavacca, M.; Reichstein, M.; Kattge, J.; Wirth, C.; Black, T.A.; Janssens, I.; Knohl, A.; Loustau, D.; Roupsard, O.; et al. Stand age and species richness dampen interannual variation of ecosystem-level photosynthetic capacity. Nat. Ecol. Evol. 2017, 1, 48. [Google Scholar] [CrossRef] [Green Version]
- He, L.M.; Chen, J.M.; Pan, Y.D.; Birdsey, R.; Kattge, J. Relationships between net primary productivity and forest stand age in U.S. forests. Glob. Biogeochem. Cycles 2012, 26, GB3009. [Google Scholar] [CrossRef]
- West, P.W. Do increasing respiratory costs explain the decline with age of forest growth rate? J. For. Res. 2020, 31, 693–712. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.L.; Luyssaert, S.; Ciais, P.; Janssens, I.A.; Chen, A.P.; Cao, C.; Fang, J.Y.; Friedlingstein, P.; Luo, Y.Q.; Wang, S.P. Forest annual carbon cost: A global-scale analysis of autotrophic respiration. Ecology 2010, 91, 652–661. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef] [Green Version]
- Drake, J.E.; Davis, S.C.; Raetz, L.M.; DeLucia, E.H. Mechanisms of age-related changes in forest production: The influence of physiological and successional changes. Glob. Chang. Biol. 2011, 17, 1522–1535. [Google Scholar] [CrossRef]
- McMillan, A.M.S.; Winston, G.C.; Goulden, M.L. Age-dependent response of boreal forest to temperature and rainfall variability. Glob. Chang. Biol. 2008, 14, 1904–1916. [Google Scholar] [CrossRef]
- Song, C.H.; Woodcock, C.E. A regional forest ecosystem carbon budget model: Impacts of forest age structure and landuse history. Ecol. Model 2003, 164, 33–47. [Google Scholar] [CrossRef]
- Tang, J.W.; Luyssaert, S.; Richardson, A.D.; Kutsch, W.; Janssens, I.A. Steeper declines in forest photosynthesis than respiration explain age-driven decreases in forest growth. Proc. Natl. Acad. Sci. USA 2014, 111, 8856–8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Shi, P.J.; Jia, G.S.; Dai, Y.J.; Zhao, X.; Wei, S.G.; Du, L.; Wu, H.; Luo, Y.Q. Age-dependent forest carbon sink: Estimation via inverse modeling. J. Geophys. Res.-Biogeosci. 2015, 120, 2473–2492. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.F.; Liu, D.S.; Cao, Y.; Zhang, L.J.; Peng, H.W.; Wang, K.L.; Xie, H.F.; Wang, C.Z. An integrated remote sensing and model approach for assessing forest carbon fluxes in China. Sci. Total Environ. 2022, 811, 152480. [Google Scholar] [CrossRef] [PubMed]
- Verbeeck, H.; Samson, R.; Verdonck, F.; Lemeur, R. Parameter sensitivity and uncertainty of the forest carbon flux model FORUG: A Monte Carlo analysis. Tree Physiol. 2006, 26, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Sykes, M.T.; Prentice, I.C.; Smith, P.; Smith, B.; Bugmann, H.; Zierl, B.; Friedlingstein, P.; Viovy, N.; Sabate, S.; et al. Comparing and evaluating process-based ecosystem model predictions of carbon and water fluxes in major European forest biomes. Glob. Chang. Biol. 2005, 11, 2211–2233. [Google Scholar] [CrossRef] [PubMed]
- Melesse, A.M.; Hanley, R.S. Artificial neural network application for multi-ecosystem carbon flux simulation. Ecol. Model 2005, 189, 305–314. [Google Scholar] [CrossRef]
- Dou, X.M.; Yang, Y.G. Estimating forest carbon fluxes using four different data-driven techniques based on long-term eddy covariance measurements: Model comparison and evaluation. Sci. Total. Environ. 2018, 627, 78–94. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. xgboost: Extreme Gradient Boosting. R Package Version 1.1.1.1. 2020. Available online: https://CRAN.R-project.org/package=xgboost (accessed on 17 September 2020).
- Joharestani, M.Z.; Cao, C.X.; Ni, X.L.; Bashir, B.; Talebiesfandarani, S. PM2.5 Prediction Based on Random Forest, XGBoost, and Deep Learning Using Multisource Remote Sensing Data. Atmosphere 2019, 10, 373. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yin, Y.B.; Quan, X.W.; Zhang, H. Gene Expression Value Prediction Based on XGBoost Algorithm. Front. Genet. 2019, 10, 1077. [Google Scholar] [CrossRef]
- Liu, J.L.; Wu, J.F.; Liu, S.R.; Li, M.D.; Hu, K.C.; Li, K. Predicting mortality of patients with acute kidney injury in the ICU using XGBoost model. PLoS ONE 2021, 16, e0246306. [Google Scholar] [CrossRef] [PubMed]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Niinimaki, S.; Tahvonen, O.; Makela, A.; Linkosalo, T. On the economics of Norway spruce stands and carbon storage. Can. J. For. Res. 2013, 43, 637–648. [Google Scholar] [CrossRef]
- Sabbatini, S.; Mammarella, I.; Arriga, N.; Fratini, G.; Graf, A.; Hortriagl, L.; Ibrom, A.; Longdoz, B.; Mauder, M.; Merbold, L.; et al. Eddy covariance raw data processing for CO2 and energy fluxes calculation at ICOS ecosystem stations. Int. Agrophys. 2018, 32, 495–515. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Sigut, L.; Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef] [Green Version]
- Foken, T. Energy and Matter Fluxes of a Spruce Forest Ecosystem; Springer International Publishing: Midtown Manhattan, NY, USA, 2017. [Google Scholar]
- Baldocchi, D.; Falge, E.; Gu, L.; Olson, R.; Hollinger, D.; Running, S.; Anthoni, P.; Bernhofer, C.; Davis, K.; Evans, R. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Meteorol. Soc. 2001, 82, 2415–2434. [Google Scholar] [CrossRef]
- Pastorello, G.; Trotta, C.; Canfora, E.; Chu, H.S.; Christianson, D.; Cheah, Y.W.; Poindexter, C.; Chen, J.Q.; Elbashandy, A.; Humphrey, M.; et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 2020, 7, 225. [Google Scholar] [CrossRef]
- Zielis, S.; Etzold, S.; Zweifel, R.; Eugster, W.; Haeni, M.; Buchmann, N. NEP of a Swiss subalpine forest is significantly driven not only by current but also by previous year’s weather. Biogeosciences 2014, 11, 1627–1635. [Google Scholar] [CrossRef]
- Krupkova, L.; Markova, I.; Havrankova, K.; Pokorny, R.; Urban, O.; Sigut, L.; Pavelka, M.; Cienciala, E.; Marek, M.V. Comparison of different approaches of radiation use efficiency of biomass formation estimation in Mountain Norway spruce. Trees-Struct. Funct. 2017, 31, 325–337. [Google Scholar] [CrossRef]
- Lindauer, M.; Schmid, H.P.; Grote, R.; Mauder, M.; Steinbrecher, R.; Wolpert, B. Net ecosystem exchange over a non-cleared wind-throw-disturbed upland spruce forest-Measurements and simulations. Agric. For. Meteorol. 2014, 197, 219–234. [Google Scholar] [CrossRef]
- Grunwald, T.; Bernhofer, C. A decade of carbon, water and energy flux measurements of an old spruce forest at the Anchor Station Tharandt. Tellus B 2007, 59, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Montagnani, L.; Manca, G.; Canepa, E.; Georgieva, E.; Acosta, M.; Feigenwinter, C.; Janous, D.; Kerschbaumer, G.; Lindroth, A.; Minach, L.; et al. A new mass conservation approach to the study of CO2 advection in an alpine forest. J. Geophys. Res.-Atmos. 2009, 114, D07306. [Google Scholar] [CrossRef] [Green Version]
- Irvin, J.; Zhou, S.R.; McNicol, G.; Lu, F.; Liu, V.; Fluet-Chouinard, E.; Ouyang, Z.T.; Knox, S.H.; Lucas-Moffat, A.; Trotta, C.; et al. Gap-filling eddy covariance methane fluxes: Comparison of machine learning model predictions and uncertainties at FLUXNET-CH4 wetlands. Agric. For. Meteorol. 2021, 308, 108528. [Google Scholar] [CrossRef]
- Greenwell, B.M. pdp: An R Package for Constructing Partial Dependence Plots. R J. 2017, 9, 421–436. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.; Kapelner, A.; Bleich, J.; Pitkin, E. Peeking Inside the Black Box: Visualizing Statistical Learning With Plots of Individual Conditional Expectation. J. Comput. Graph. Stat 2015, 24, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations–the CRU TS3.10 Dataset. Int. J. Clim. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 17 September 2020).
- Sun, J.F.; Peng, C.H.; McCaughey, H.; Zhou, X.L.; Thomas, V.; Berninger, F.; St-Onge, B.; Hua, D. Simulating carbon exchange of Canadian boreal forests II. Comparing the carbon budgets of a boreal mixedwood stand to a black spruce forest stand. Ecol. Model 2008, 219, 276–286. [Google Scholar] [CrossRef]
- Chi, J.S.; Nilsson, M.B.; Laudon, H.; Lindroth, A.; Wallerman, J.; Fransson, J.E.S.; Kljun, N.; Lundmark, T.; Lofvenius, M.O.; Peichl, M. The Net Landscape Carbon Balance-Integrating terrestrial and aquatic carbon fluxes in a managed boreal forest landscape in Sweden. Glob. Chang. Biol. 2020, 26, 2353–2367. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, M.; Harazono, Y.; Ohtaki, E.; Miyata, A. Controlling factors on the interannual CO2 budget at a subarctic black spruce forest in interior Alaska. Tellus B 2006, 58, 491–501. [Google Scholar] [CrossRef]
- Juran, S.; Edwards-Jonasova, M.; Cudlin, P.; Zapletal, M.; Sigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. Iforest 2018, 11, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Hlasny, T.; Konig, L.; Krokene, P.; Lindner, M.; Montagne-Huck, C.; Muller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.J.; Svoboda, M.; et al. Bark Beetle Outbreaks in Europe: State of Knowledge and Ways Forward for Management. Curr. Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- Jaime, L.; Batllori, E.; Ferretti, M.; Lloret, F. Climatic and stand drivers of forest resistance to recent bark beetle disturbance in European coniferous forests. Glob. Chang. Biol. 2022, 28, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Link, R.M.; Patthey, R.; Hocha, G.; Schuldt, B.; Kahmen, A. Rapid hydraulic collapse as cause of drought-induced mortality in conifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025251118. [Google Scholar] [CrossRef]
- Krejza, J.; Cienciala, E.; Svetlik, J.; Bellan, M.; Noyer, E.; Horacek, P.; Stepanek, P.; Marek, M.V. Evidence of climate-induced stress of Norway spruce along elevation gradient preceding the current dieback in Central Europe. Trees-Struct. Funct. 2021, 35, 103–119. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Stokland, J.N. Volume increment and carbon dynamics in boreal forest when extending the rotation length towards biologically old stands. For. Ecol. Manag. 2021, 488, 119017. [Google Scholar] [CrossRef]
- Raim, O.; Kaurilind, E.; Hallik, L.; Merilo, E. Why does needle photosynthesis decline with tree height in Norway spruce? Plant Biol. 2012, 14, 306–314. [Google Scholar] [CrossRef]
- Gundersen, P.; Thybring, E.E.; Nord-Larsen, T.; Vesterdal, L.; Nadelhoffer, K.J.; Johannsen, V.K. Old-growth forest carbon sinks overestimated. Nature 2021, 591, E21–E23. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Knohl, A.; Law, B.E.; Ciais, P.; Grace, J. Reply to: Old-growth forest carbon sinks overestimated. Nature 2021, 591, E24–E25. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, T.; Zhao, X.; Wu, D.H.; Li, Z.; Wu, H.; Du, L.; Luo, H. Age and climate contribution to observed forest carbon sinks in East Asia. Environ. Res. Lett. 2016, 11, 034021. [Google Scholar] [CrossRef]
- Reitz, O.; Graf, A.; Schmidt, M.; Ketzler, G.; Leuchner, M. Upscaling Net Ecosystem Exchange Over Heterogeneous Landscapes With Machine Learning. J. Geophys. Res.-Biogeosci. 2021, 126. [Google Scholar] [CrossRef]
- Curtis, P.S.; Gough, C.M. Forest aging, disturbance and the carbon cycle. New Phytol. 2018, 219, 1188–1193. [Google Scholar] [CrossRef]



| Parameter | Optimal Value | Flux Range | Train RMSE | Test RMSE | Variable Importance |
|---|---|---|---|---|---|
| 0.1 | [−108, 201] | 28.74 (9%) | 44.84 (15%) | Ta (0.35) | |
| max_depth | 3 | Rg (0.29) | |||
| min_child_weight | 0 | Age (0.17) | |||
| 0 | CO2 (0.08) | ||||
| subsample | 0.1 | WS (0.06) | |||
| colsample_bytree | 0.9 | PPT (0.03) | |||
| nrounds | 22 | Sine seasonality (0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Lange, H.; Meissner, H. Estimating Carbon Sink Strength of Norway Spruce Forests Using Machine Learning. Forests 2022, 13, 1721. https://doi.org/10.3390/f13101721
Zhao J, Lange H, Meissner H. Estimating Carbon Sink Strength of Norway Spruce Forests Using Machine Learning. Forests. 2022; 13(10):1721. https://doi.org/10.3390/f13101721
Chicago/Turabian StyleZhao, Junbin, Holger Lange, and Helge Meissner. 2022. "Estimating Carbon Sink Strength of Norway Spruce Forests Using Machine Learning" Forests 13, no. 10: 1721. https://doi.org/10.3390/f13101721
APA StyleZhao, J., Lange, H., & Meissner, H. (2022). Estimating Carbon Sink Strength of Norway Spruce Forests Using Machine Learning. Forests, 13(10), 1721. https://doi.org/10.3390/f13101721






