Lower Light Intensities Increase Shoot Germination with Improved Leaf Biosynthesis in Ma Bamboo (Dendrocalamus latiflorus Munro)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Measurement Indicators and Methods
2.3.1. Investigation of the Germination Number for Bamboo Shoots
2.3.2. Determination of Leaf Photosynthetic Characteristics
Photosynthetic Pigments
Gas Exchange Parameters
2.3.3. Determination of Carbon (C), Nitrogen (N), and Non-Structural Carbohydrates (NSCs) Contents in Leaves
2.3.4. Determination of Endogenous Hormone Contents in Leaves
2.4. Data Analysis
3. Result
3.1. Effects of Light Intensity on the Number of Germinated Shoots for Ma Bamboo
3.2. Effects of Light Intensity on Leaf Photosynthetic Properties
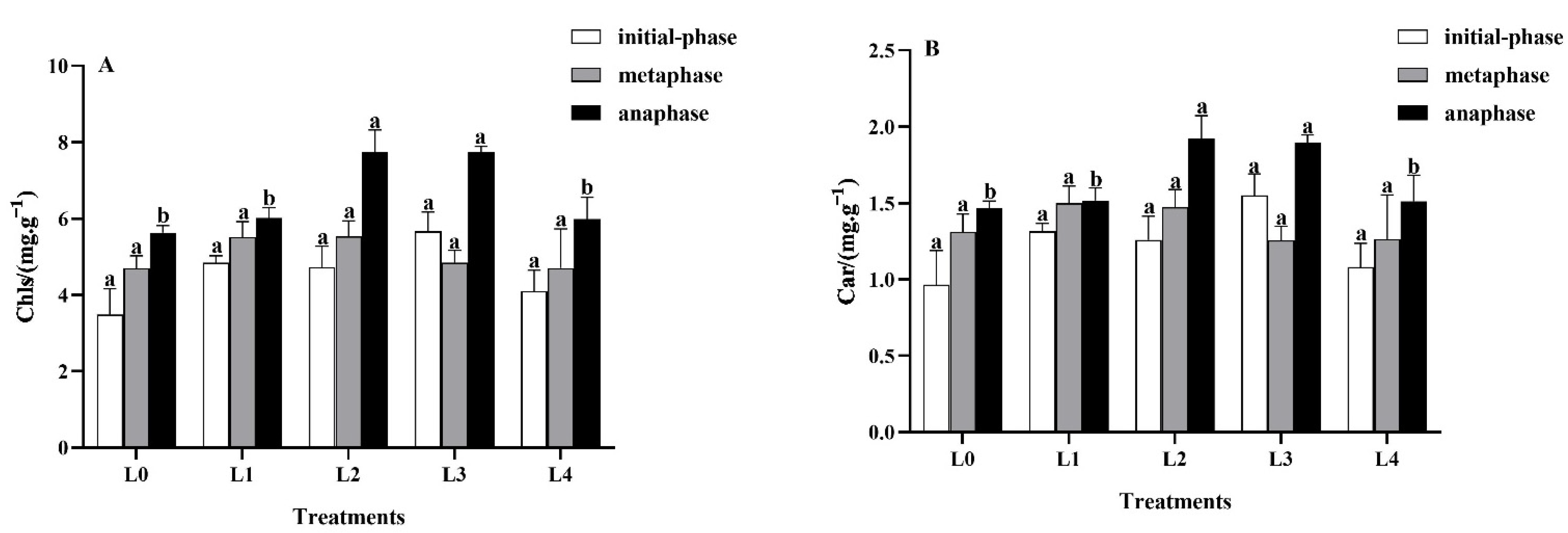
3.2.1. Effects of Light Intensity on Leaf Photosynthetic Pigments
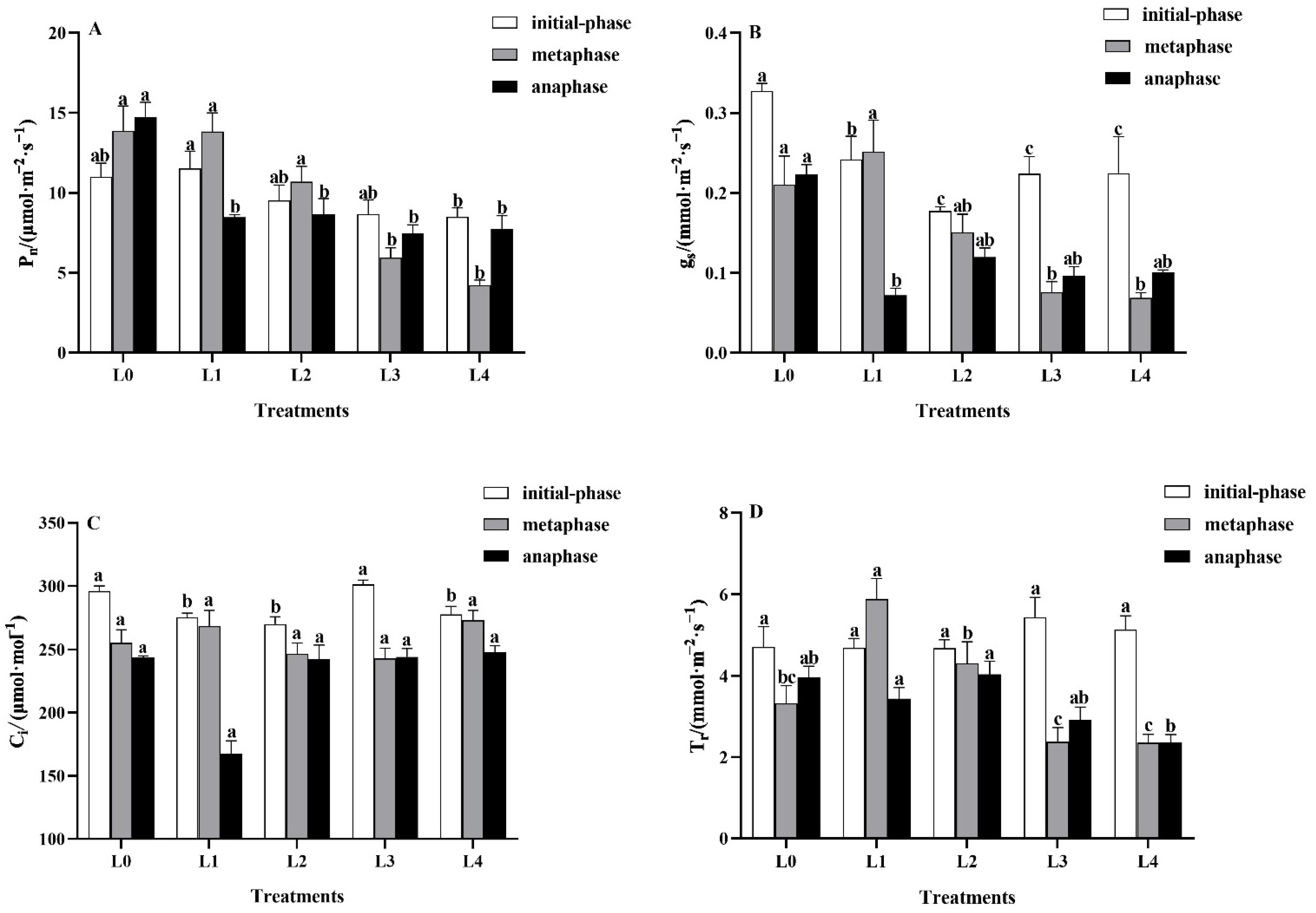
3.2.2. Effects of Light Intensity on Leaf Gas Exchange Indicators
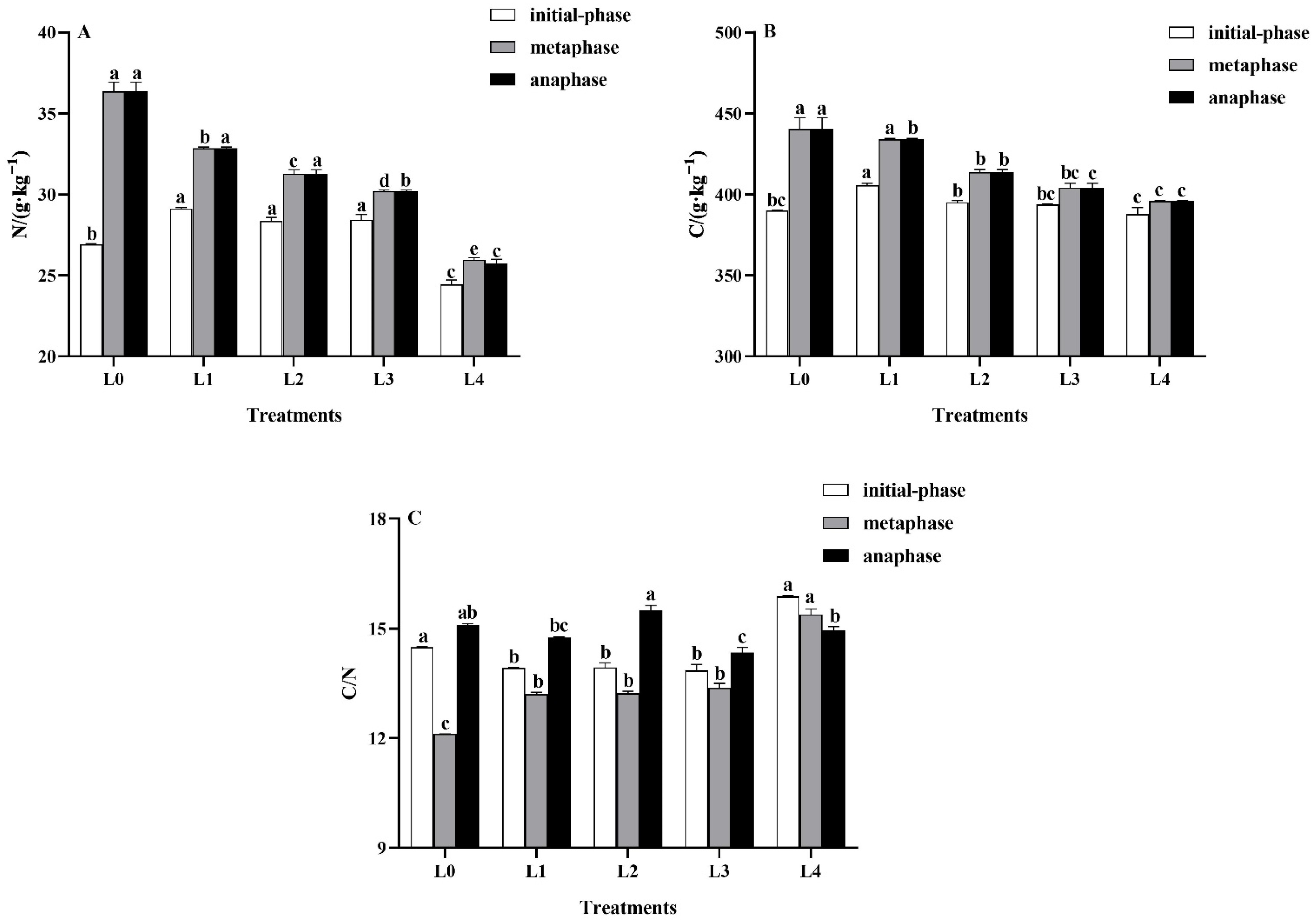
3.3. Effects of Light Intensity on Leaf C and N Properties
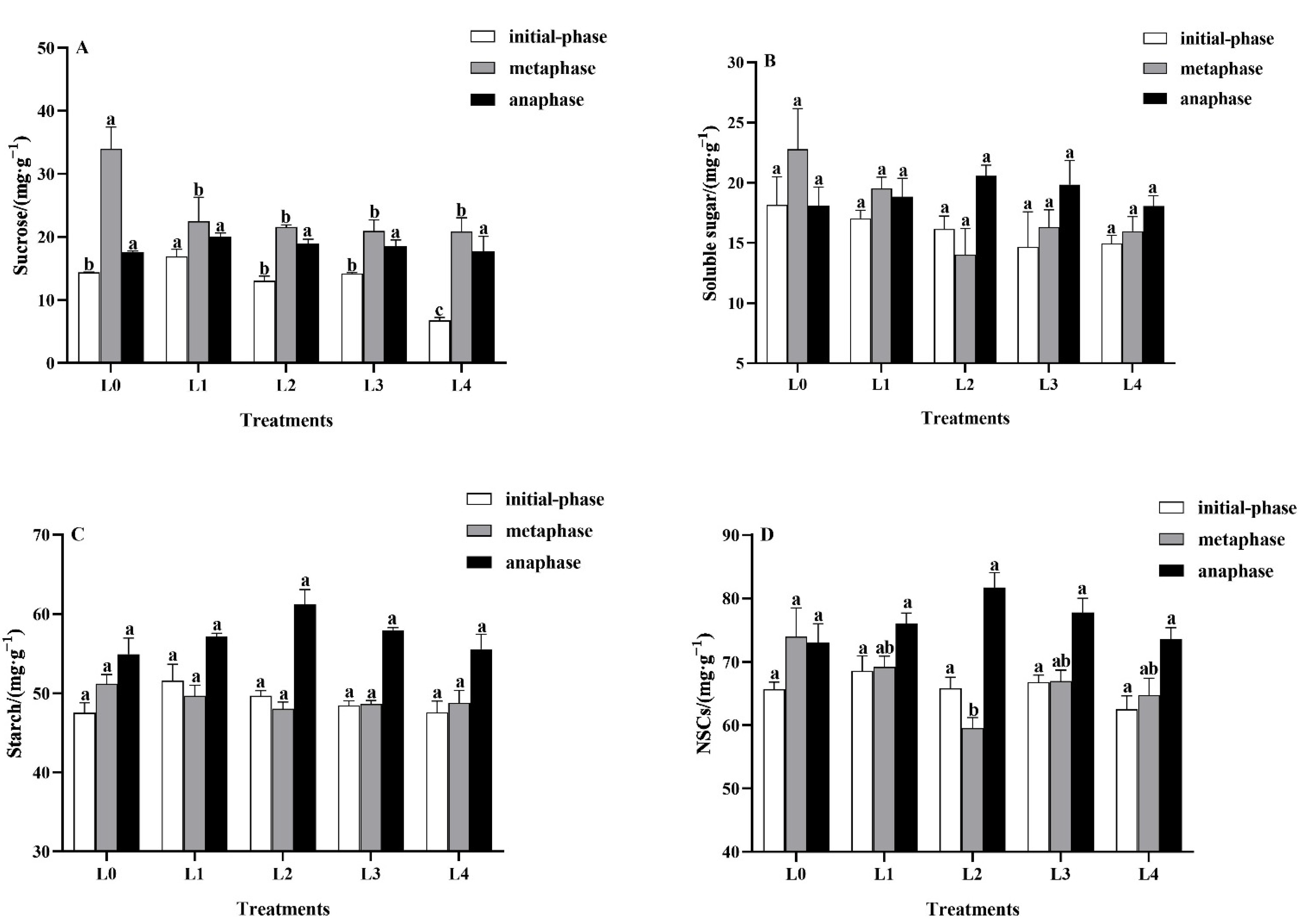
3.4. Effects of Light Intensity on Leaf Carbohydrates
3.5. Effects of Light Intensity on Leaf Endogenous Hormones
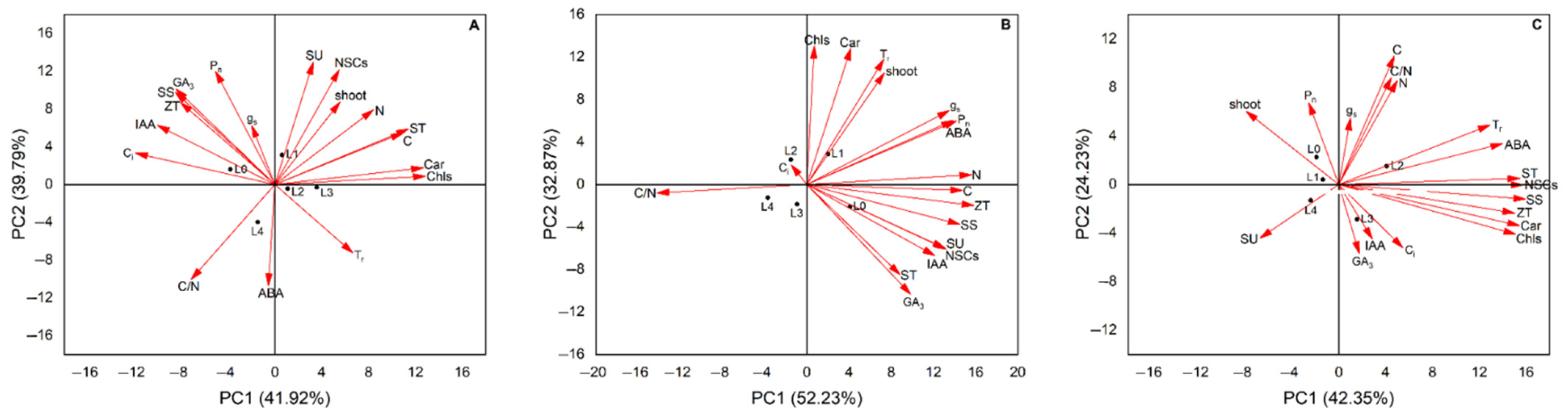
3.6. PCA of Light Intensity on the Number of Germinated Shoots and Leaf Biochemical Parameters
4. Discussion
4.1. Photosynthetic Mechanism on Shoot Germination of Ma Bamboo under Light Intensity
4.2. The Effects of Leaf Photosynthesis Products on Shoot Germination of Ma Bamboo under Light Intensity
4.3. The Effects of Leaf Endogenous Hormones on Shoot Germination of Ma Bamboo under Light Intensity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
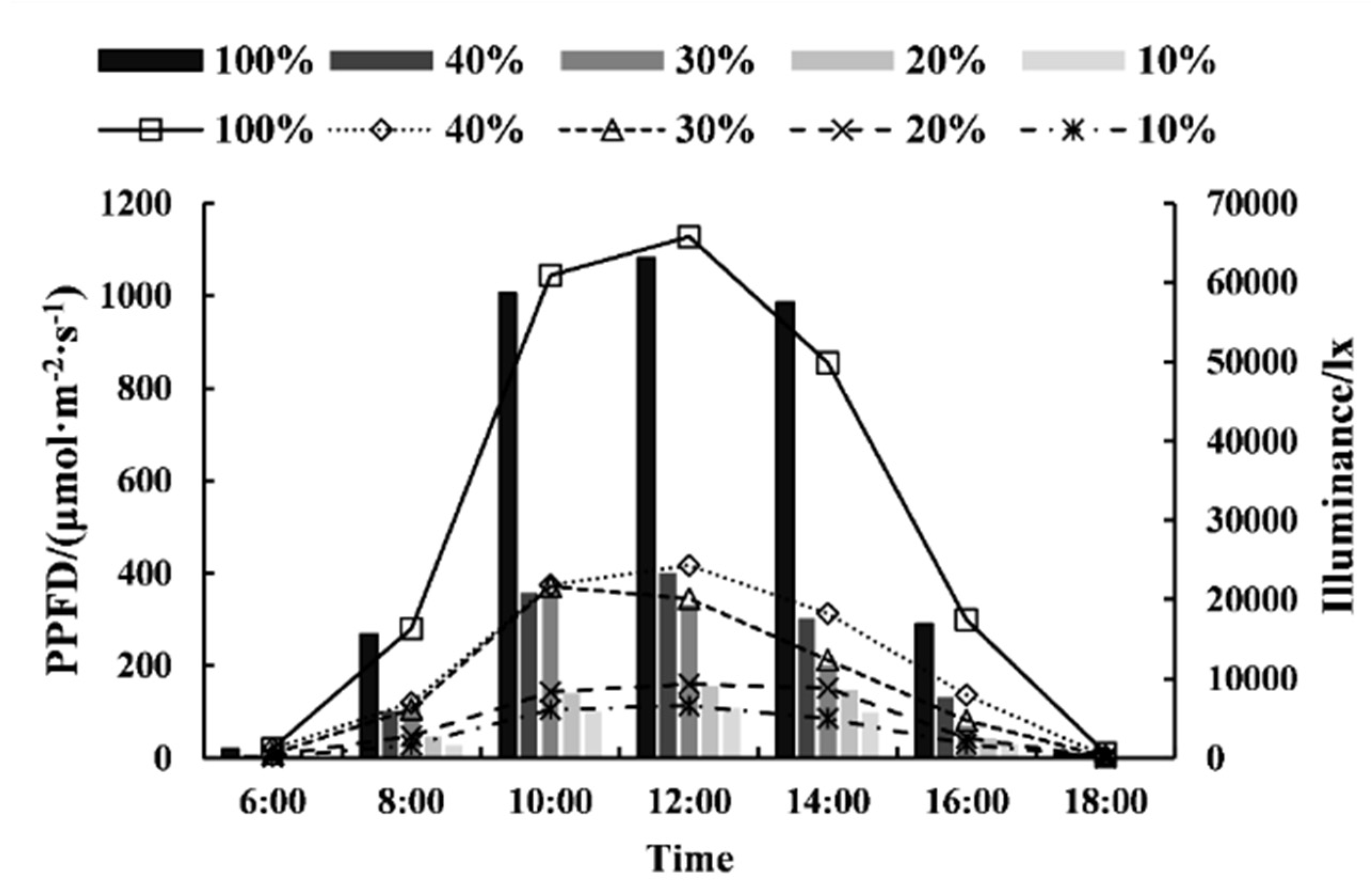

| Treatments | Light Intensities (%) | Illuminance (lx) | Average PPFD (μmol·m−2·s−1) | The PPFD at the Time Corresponds to the Average PPFD (μmol·m−2·s−1) | |
|---|---|---|---|---|---|
| 8:00 | 15:50 | ||||
| L0 | 100 | 30491.43 ± 569.73 a | 519.62 ± 64.20 a | 532.55 ± 20.37 a | 545.78 ± 33.59 a |
| L1 | 40 | 11394.89 ± 580.98 b | 203.89 ± 10.56 b | 202.76 ± 10.97 b | 206.65 ± 34.02 b |
| L2 | 30 | 9070.34 ± 570.49 b | 164.57 ± 9.53 bc | 177.18 ± 8.35 c | 157.03 ± 10.25 c |
| L3 | 20 | 6248.78 ± 503.85 b | 110.34 ± 26.06 c | 79.87 ± 20.27 cd | 83.66 ± 7.39 cd |
| L4 | 10 | 3129.54 ± 498.35 b | 53.64 ± 13.01 c | 53.25 ± 10.04 d | 54.58 ± 11.25 d |
References
- Meier, S.; Grand, L.; Schoeneberger, M.; Reinert, R.; Bruck, R. Growth, ectomycorrhizae and nonstructural carbohydrates of loblolly pine seedlings exposed to ozone and soil water deficit. Environ. Pollut. 1990, 64, 11–27. [Google Scholar] [CrossRef]
- Xie, H.; Yu, M.; Cheng, X. Leaf Non-Structural Carbohydrate Allocation and C:N:P Stoichiometry in Response to Light Acclimation in Seedlings of Two Subtropical Shade-Tolerant Tree Species. Plant Physiol. Biochem. 2018, 124, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Song, L.; Yu, W.; Hu, Y.; Liu, Y.; Wu, J.; Ying, Y. Growth, Physiological, and Biochemical Responses of Camptotheca Acuminata Seedlings to Different Light Environments. Front. Plant Sci. 2015, 6, 321. [Google Scholar] [CrossRef]
- Truong, T.H.H.; Marschner, P. Plant Residues Differing in C/N Ratio in Mulch and Soil-the Effect of the Mulch on Nutrient Availability and Microbial Biomass Is More Pronounced with Higher Leaching Amount. Soil Ecol. Lett. 2020, 2, 317–326. [Google Scholar] [CrossRef]
- Hu, C.; Pan, X. On the Density of Shoot Producing Ph. Pubescens Forests. J. Bamboo Res. 1983, 2, 53–61. [Google Scholar]
- Liao, Z.Q. Effect of Shade and Sunlight on the Development of Fargesia Denudata. J. Bamboo Res. 1993, 12, 58–63. [Google Scholar]
- Shuanlin, C.; Qingping, Y.; Ziwu, G. Influence of Principal Environmental Factors on Shooting, Growth and Abnormal Culm Rate of Bambusa Ventricosa. J. Sichuan Agric. Univ. 2008, 26, 117–120. [Google Scholar]
- Gao, G.; Zhong, H.; Pan, Y.; Wu, L.; Wu, Z.; Wen, X. Effects of Ecological Factors on Biomass Allocation of Indocalamus Decorus Pot. J. Nanjing For. Univ. Nat. Sci. Ed. 2017, 41, 35–41. [Google Scholar]
- Jin, K.; Wang, Y.; Zhuo, R.; Xu, J.; Lu, Z.; Fan, H.; Huang, B.; Qiao, G. TCP Transcription Factors Involved in Shoot Development of Ma Bamboo (Dendrocalamus Latiflorus Munro). Front. Plant Sci. 2022, 13, 884443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Li, J.; Wang, R. Optimum Planting Density Improves Resource Use Efficiency and Yield Stability of Rainfed Maize in Semiarid Climate. Front. Plant Sci. 2021, 12, 752606. [Google Scholar] [CrossRef]
- Matsuo, T.; Martínez-Ramos, M.; Bongers, F.; Sande, M.T.; Poorter, L. Forest Structure Drives Changes in Light Heterogeneity during Tropical Secondary Forest Succession. J. Ecol. 2021, 109, 2871–2884. [Google Scholar] [CrossRef] [PubMed]
- Sercu, B.K.; Baeten, L.; van Coillie, F.; Martel, A.; Lens, L.; Verheyen, K.; Bonte, D. How Tree Species Identity and Diversity Affect Light Transmittance to the Understory in Mature Temperate Forests. Ecol. Evol. 2017, 7, 10861–10870. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Chen, C.Y.; Wang, W.H.; Yang, Q.P. Study on Shooting Hastening Techniques of Dendrocalamopsis Oldhami. J. Southwest For. Univ. Nat. Sci. 2004, 24, 17–20. [Google Scholar]
- Fan, L.; Tarin, M.W.K.; Hu, W.; Han, Y.; Rong, J.; Chen, L.; He, T.; Zheng, Y. Changes in Light Intensity Affect Leaf Gas Exchange, Chlorophyll Fluorescence, and Nonstructural Carbohydrates of Ma Bamboo (Dendrocalamus Latiflorus Munro). Appl. Ecol. Environ. Res. 2022, 20, 1269–1284. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid Growth of Moso Bamboo (Phyllostachys Edulis): Cellular Roadmaps, Transcriptome Dynamics, and Environmental Factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Yrjälä, K.; Vinod, K.K.; Sharma, A.; Cho, J.; Satheesh, V.; Zhou, M. Genetics and Genomics of Moso Bamboo (Phyllostachys Edulis): Current Status, Future Challenges, and Biotechnological Opportunities toward a Sustainable Bamboo Industry. Food Energy Secur. 2020, 9, e229. [Google Scholar] [CrossRef]
- Katayama, N.; Kishida, O.; Sakai, R.; Hayakashi, S.; Miyoshi, C.; Ito, K.; Naniwa, A.; Yamaguchi, A.; Wada, K.; Kowata, S.; et al. Response of a Wild Edible Plant to Human Disturbance: Harvesting Can Enhance the Subsequent Yield of Bamboo Shoots. PLoS ONE 2015, 10, e0146228. [Google Scholar] [CrossRef]
- Gao, J. Experimental Guide of Plant Physiology; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Li, L.; Shao, T.; Yang, H.; Chen, M.; Gao, X.; Long, X.; Shao, H.; Liu, Z.; Rengel, Z. The Endogenous Plant Hormones and Ratios Regulate Sugar and Dry Matter Accumulation in Jerusalem Artichoke in Salt-Soil. Sci. Total Environ. 2017, 578, 40–46. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving Spinach, Radish, and Lettuce Growth under Red Light-Emitting Diodes (LEDs) with Blue Light Supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W.; Cohu, C.M.; Polutchko, S.K.; Lombardi, E.M.; Demmig-Adams, B. Differences in Light-Harvesting, Acclimation to Growth-Light Environment, and Leaf Structural Development between Swedish and Italian Ecotypes of Arabidopsis Thaliana. Planta 2015, 242, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, W.; Bai, Z.; Guo, Z.; Ren, W.; Huang, J.; Xu, Y.; Yao, J.; Ding, Y.; Zang, R. Competition and Facilitation Co-Regulate the Spatial Patterns of Boreal Tree Species in Kanas of Xinjiang, Northwest China. For. Ecol. Manag. 2020, 467, 118167. [Google Scholar] [CrossRef]
- Jiao, L.; Lu, B.; Zhou, R.; Bai, Z.; Liang, H.; Zhen, H. Effects of Shading on Net Photosynthetic Rate and Chlorophyll Fluorescence Parameters of Leaf in David Maple (Acer Davidii Franch.). Acta Hortic. Sin. 2007, 31, 173–178. [Google Scholar]
- Tarin, M.W.K.; Fan, L.; Shen, L.; Lai, J.; Li, J.; Deng, Z.; Chen, L.; He, T.; Rong, J.; Zheng, Y. Rice Straw Biochar Impact on Physiological and Biochemical Attributes of Fokienia Hodginsii in Acidic Soil. Scand. J. For. Res. 2020, 35, 59–68. [Google Scholar] [CrossRef]
- Honggang, S.; Yitai, C. Root Growth Patterns of Four Coastal Shelter Forest Tree Species in Response to Salt Stress. Chin. J. Ecol. 2010, 29, 2365–2372. [Google Scholar] [CrossRef]
- Katahata, S.I.; Naramoto, M.; Kakubari, Y.; Mukai, Y. Photosynthetic Capacity and Nitrogen Partitioning in Foliage of the Evergreen Shrub Daphniphyllum Humile along a Natural Light Gradient. Tree Physiol. 2007, 27, 199–208. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.; Liu, H. Interaction Effects of Light Intensity and Nitrogen Concentration on Growth, Photosynthetic Characteristics and Quality of Lettuce Lactuca. Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Fatbardha, B.; Gabriele, L. Chlorophyll Fluorescence Imaging of Photosynthetic Activity in Sun and Shade Leaves of Trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant. 2004, 120, 999–1014. [Google Scholar] [CrossRef]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem II. Inactivation, Protein Damage and Turnover. Biochim. Et Biophys. Acta BBA-Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- He, M.; Zhang, K.; Tan, H.; Hu, R.; Su, J.; Wang, J.; Huang, L.; Zhang, Y.; Li, X. Nutrient Levels within Leaves, Stems, and Roots of the Xeric Species Reaumuria Soongorica in Relation to Geographical, Climatic, and Soil Conditions. Ecol. Evol. 2015, 5, 1494–1503. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; ben El Hadj, S.; Braham, M.; Lemeur, R.; van Labeke, M.C. Effects of Nitrogen Deficiency on Leaf Photosynthesis, Carbohydrate Status and Biomass Production in Two Olive Cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Terfa, M.T.; Solhaug, K.A.; Gislerød, H.R.; Olsen, J.E.; Torre, S. A High Proportion of Blue Light Increases the Photosynthesis Capacity and Leaf Formation Rate of Rosa × Hybrida but Does Not Affect Time to Flower Opening. Physiol. Plant. 2013, 148, 146–159. [Google Scholar] [CrossRef]
- Greer, D.H. Photosynthetic Light Responses of Apple (Malus Domestica) Leaves in Relation to Leaf Temperature, CO2 and Leaf Nitrogen on Trees Grown in Orchard Conditions. Funct. Plant Biol. 2018, 45, 1149. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Christiaens, A.; de Keyser, E.; Pauwels, E.; de Riek, J.; Gobin, B.; van Labeke, M.-C. Suboptimal Light Conditions Influence Source-Sink Metabolism during Flowering. Front. Plant Sci. 2016, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Kitajima, K. Carbohydrate Storage and Light Requirements of Tropical Moist and Dry Forest Tree Species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- Cifuentes, L.; Moreno, F. Trait Coordination at Leaf Level Explains the Resistance to Excess Light Stress in Shade-Tolerant Tropical Tree Species. Tree Physiol. 2022, 42, 1325–1336. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, B.; Li, Q.; Kong, F.; Du, L.; Zhou, F.; Shi, H.; Ke, Y.; Liu, Q.; Feng, D.; et al. Non-Structural Carbohydrates in Maize with Different Nitrogen Tolerance Are Affected by Nitrogen Addition. PLoS ONE 2019, 14, e0225753. [Google Scholar] [CrossRef]
- Hu, X.; Sun, L.; Luo, S.; Liu, Y. Effect of Integrated Nutrient Management on Accumulation and Distribution of Soluble Sugar and Yield of Soybean. J. Northeast. Agric. Univ. 2011, 42, 8–12. [Google Scholar] [CrossRef]
- Chantuma, P.; Lacointe, A.; Kasemsap, P.; Thanisawanyangkura, S.; Gohet, E.; Clement, A.; Guilliot, A.; Ameglio, T.; Thaler, P. Carbohydrate Storage in Wood and Bark of Rubber Trees Submitted to Different Level of C Demand Induced by Latex Tapping. Tree Physiol. 2009, 29, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Allard, V.; Gaju, O.; le Gouis, J.; Foulkes, M.J.; Martre, P. Acclimation of Leaf Nitrogen to Vertical Light Gradient at Anthesis in Wheat Is a Whole-Plant Process That Scales with the Size of the Canopy. Plant Physiol. 2012, 160, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Yoshinaga, S.; Takanashi, J.I.; Terao, T. Effects of Dry Matter Production, Translocation of Nonstructural Carbohydrates and Nitrogen Application on Grain Filling in Rice Cultivar Takanari, a Cultivar Bearing a Large Number of Spikelets. Plant Prod. Sci. 2001, 4, 173–183. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis Mineralogical, Organic and Inorganic Methods: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 3540312110. [Google Scholar]
- Cai, T.; Meng, X.; Liu, X.; Liu, T.; Wang, H.; Jia, Z.; Yang, D.; Ren, X. Exogenous Hormonal Application Regulates the Occurrence of Wheat Tillers by Changing Endogenous Hormones. Front. Plant Sci. 2018, 9, 1886. [Google Scholar] [CrossRef]
- Rook, F.; Corke, F.; Card, R.; Munz, G.; Smith, C.; Bevan, M.W. Impaired Sucrose-Induction Mutants Reveal the Modulation of Sugar-Induced Starch Biosynthetic Gene Expression by Abscisic Acid Signalling. Plant J. 2001, 26, 421–433. [Google Scholar] [CrossRef]
- Brunetti, C.; Sebastiani, F.; Tattini, M. Review: ABA, Flavonols, and the Evolvability of Land Plants. Plant Sci. 2019, 280, 448–454. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on Plant Abiotic Stress Responses in the Post-Genome Era: Past, Present and Future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Vasantha, S.; Shekinah, D.E.; Gupta, C.; Rakkiyappan, P. Tiller Production, Regulation and Senescence in Sugarcane (Saccharum Species Hybrid) Genotypes. Sugar Tech. 2012, 14, 156–160. [Google Scholar] [CrossRef]
- Shan, F.; Zhang, R.; Zhang, J.; Wang, C.; Lyu, X.; Xin, T.; Yan, C.; Dong, S.; Ma, C.; Gong, Z. Study on the Regulatory Effects of GA3 on Soybean Internode Elongation. Plants 2021, 10, 1737. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Guo, B.; Li, L.; Ma, G.; Wu, K.; Yang, F.; Zhu, G.; Fang, L.; Zeng, S. Exogenous GA3 Promotes Flowering in Paphiopedilum Callosum (Orchidaceae) through Bolting and Lateral Flower Development Regulation. Hortic. Res. 2022, 9, uhac091. [Google Scholar] [CrossRef] [PubMed]
- van Meeteren, U.; Kaiser, E.; Malcolm Matamoros, P.; Verdonk, J.C.; Aliniaeifard, S. Is Nitric Oxide a Critical Key Factor in ABA-Induced Stomatal Closure? J. Exp. Bot. 2020, 71, 399–410. [Google Scholar] [CrossRef]
- Teng, R.; Wang, Y.; Li, H.; Lin, S.; Liu, H.; Zhuang, J. Effects of Shading on Lignin Biosynthesis in the Leaf of Tea Plant (Camellia Sinensis (L.) O. Kuntze). Mol. Genet. Genom. 2021, 296, 165–177. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Li, B.; Han, Y.; Chen, L.; He, T.; Zheng, Y.; Rong, J. Lower Light Intensities Increase Shoot Germination with Improved Leaf Biosynthesis in Ma Bamboo (Dendrocalamus latiflorus Munro). Forests 2022, 13, 1723. https://doi.org/10.3390/f13101723
Fan L, Li B, Han Y, Chen L, He T, Zheng Y, Rong J. Lower Light Intensities Increase Shoot Germination with Improved Leaf Biosynthesis in Ma Bamboo (Dendrocalamus latiflorus Munro). Forests. 2022; 13(10):1723. https://doi.org/10.3390/f13101723
Chicago/Turabian StyleFan, Lili, Bingjun Li, Yongzhen Han, Liguang Chen, Tianyou He, Yushan Zheng, and Jundong Rong. 2022. "Lower Light Intensities Increase Shoot Germination with Improved Leaf Biosynthesis in Ma Bamboo (Dendrocalamus latiflorus Munro)" Forests 13, no. 10: 1723. https://doi.org/10.3390/f13101723
APA StyleFan, L., Li, B., Han, Y., Chen, L., He, T., Zheng, Y., & Rong, J. (2022). Lower Light Intensities Increase Shoot Germination with Improved Leaf Biosynthesis in Ma Bamboo (Dendrocalamus latiflorus Munro). Forests, 13(10), 1723. https://doi.org/10.3390/f13101723






