Responses of Rhizosphere Soil Chemical Properties and Bacterial Community Structure to Major Afforestation Tree Species in Xiong’an New Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling Method
2.3. Soil Chemical Analysis
2.4. Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Bacterial Community Composition
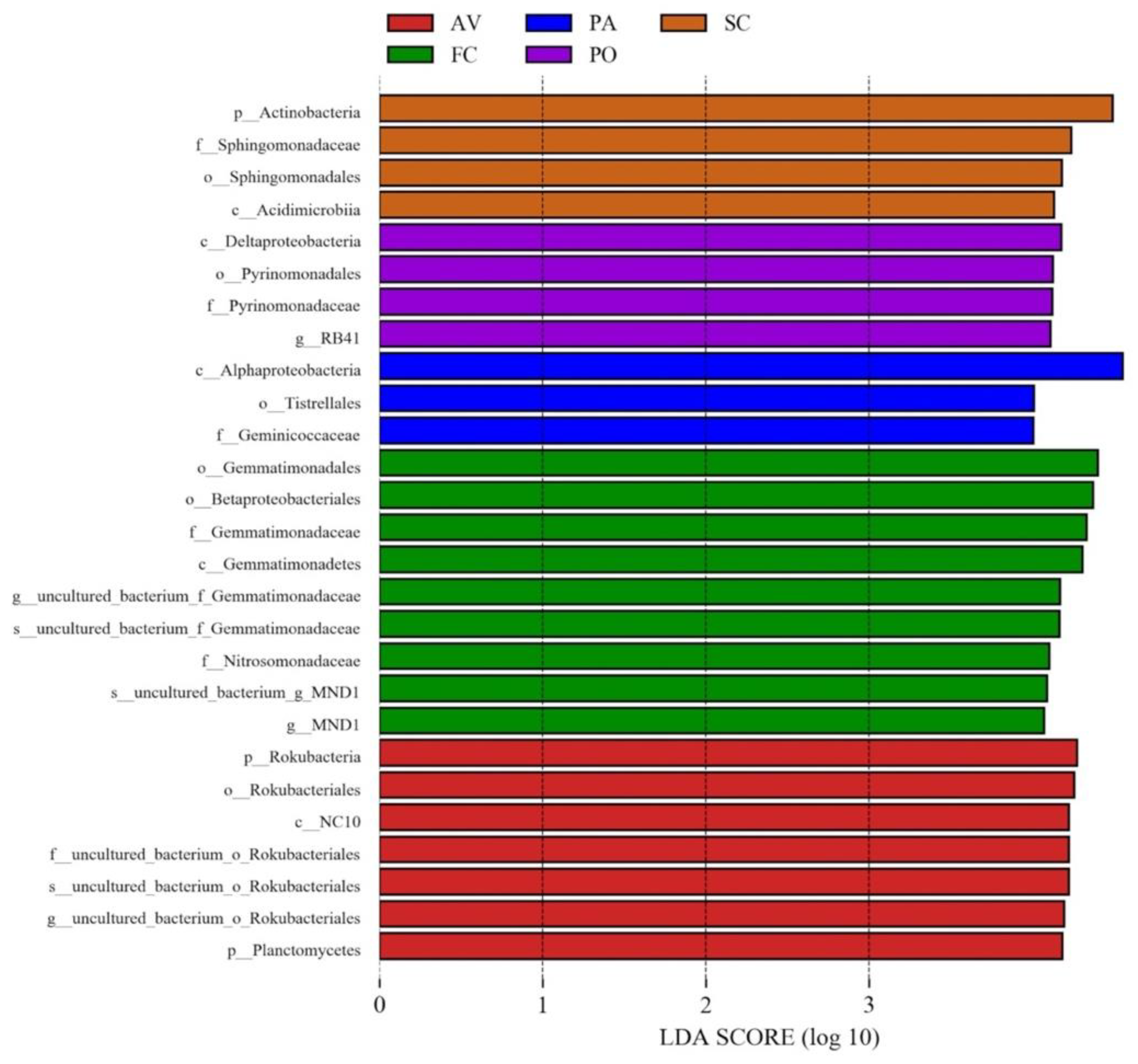
3.3. Biomarkers
3.4. α-Diversity
3.5. β-Diversity
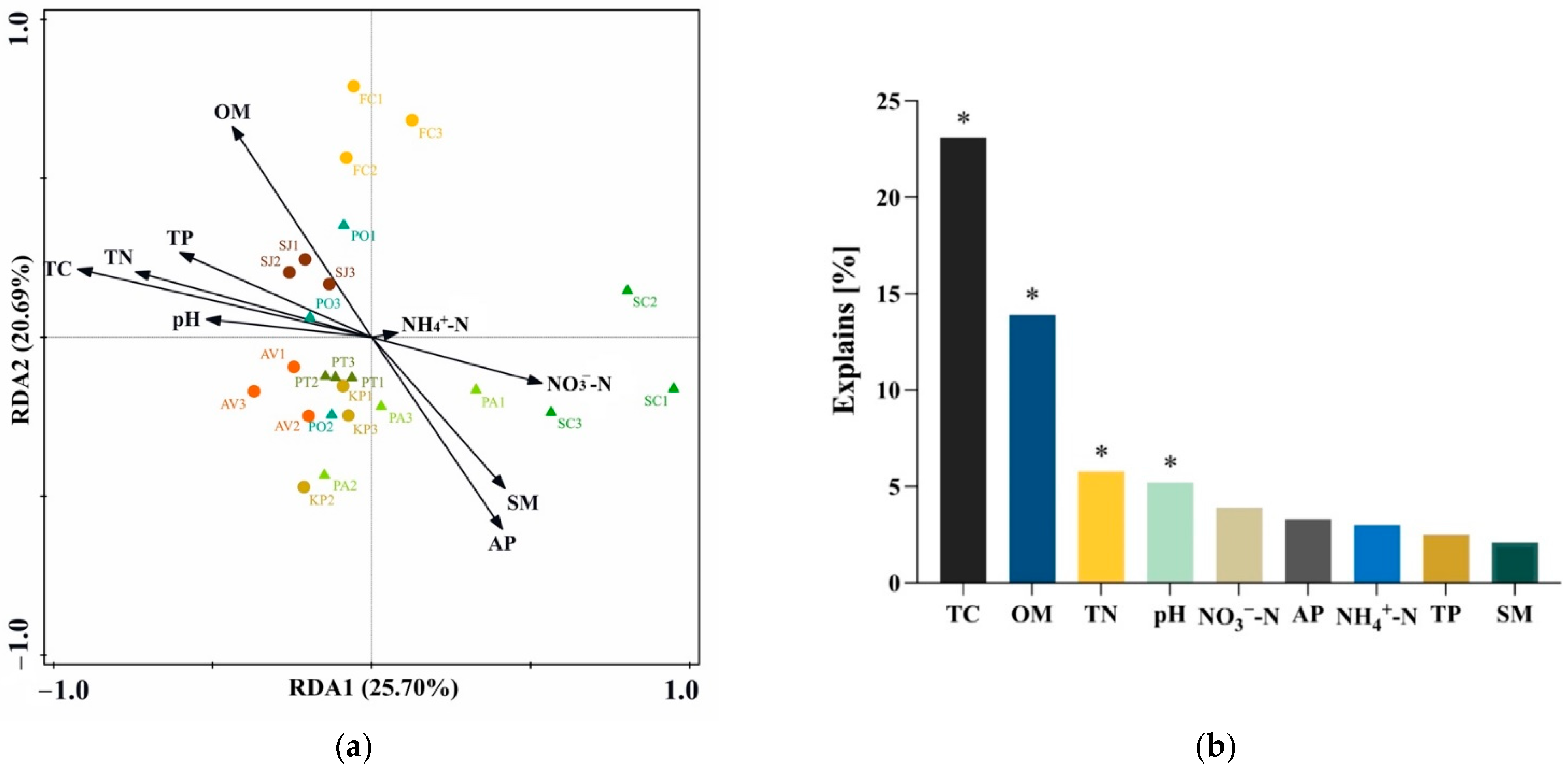
3.6. Driving Factors of Bacterial Community Structure
4. Discussion
4.1. Influences of Tree Species on Rhizosphere Soil Chemical Properties
4.2. Dominant Taxa in Studied Stands
4.3. Effects of Tree Species on Soil Bacterial Diversity and Community Structure
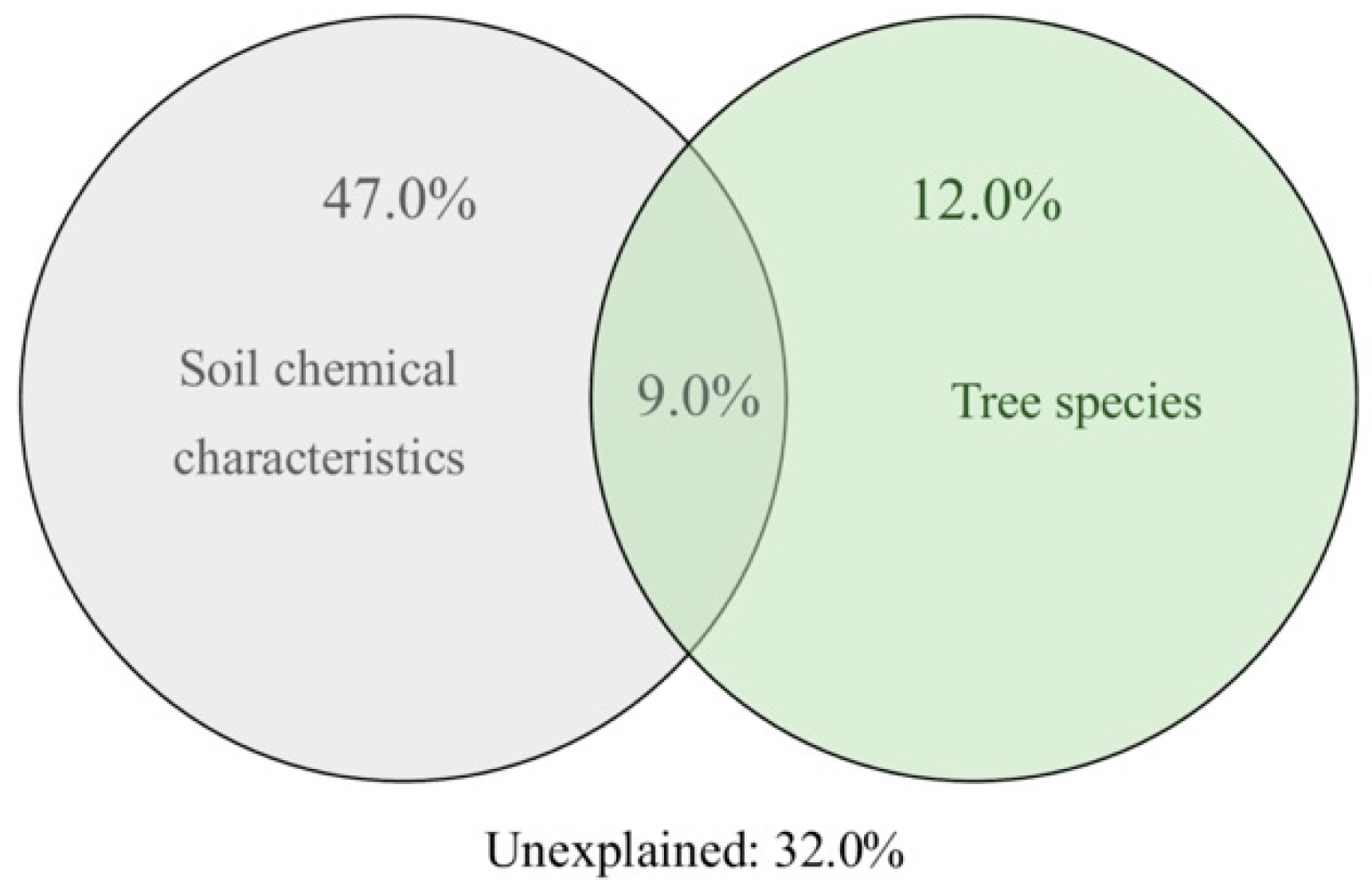
4.4. Interplay among Trees, Soil Chemical Properties, and Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for global biodiversity in the 21st century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, B.A.; Ye, Y.Q.; Zhang, J.E.; Connor, J.D. Land-use change impacts on ecosystem services value: Incorporating the scarcity effects of supply and demand dynamics. Ecosyst. Serv. 2018, 32, 144–157. [Google Scholar] [CrossRef]
- Alkama, R.; Cescatti, A. Biophysical climate impacts of recent changes in global forest cover. Science 2016, 351, 600–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhan, J.Y.; Zhao, F.; Yan, H.M.; Zhang, F.; Wei, X.Q. Impacts of urbanization-induced land-use changes on ecosystem services: A case study of the Pearl River Delta Metropolitan Region, China. Ecol. Indic. 2019, 98, 228–238. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Ferraz, S.F.B. Role of eucalypt and other planted forests in biodiversity conservation and the provision of biodiversity-related ecosystem services. Forest Ecol. Manag. 2013, 301, 43–50. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FRA 2015 Terms and Definitions. Forest Resources Assessment Working Paper 180; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Peng, S.S.; Piao, S.L.; Zeng, Z.Z.; Ciais, P.; Zhou, L.M.; Li, L.Z.X.; Myneni, R.B.; Yin, Y.; Zeng, H. Afforestation in China cools local land surface temperature. Proc. Natl. Acad. Sci. USA 2014, 111, 2915–2919. [Google Scholar] [CrossRef] [Green Version]
- Heilmayr, R.; Echeverría, C.; Lambin, E.F. Impacts of Chilean forest subsidies on forest cover, carbon and biodiversity. Nat. Sustain. 2020, 3, 701–709. [Google Scholar] [CrossRef]
- Jian, S.Q.; Zhao, C.Y.; Fang, S.M.; Yu, K. Effects of different vegetation restoration on soil water storage and water balance in the Chinese Loess Plateau. Agr. Forest Meteorol. 2015, 206, 85–96. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Ge, J.L.; Wang, Y.; Xu, W.T.; Xie, Z.Q. Latitudinal patterns and climatic drivers of leaf litter multiple nutrients in Chinese broad-leaved tree species: Does leaf habit matter? Ecosystems 2017, 20, 1124–1136. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.Q.; Yu, Z.Y.; Zeng, D.H. Rhizosphere organic phosphorus fractions of Simon poplar and Mongolian pine plantations in a semiarid sandy land of northeastern China. J. Arid. Land 2015, 7, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.D.; He, M.; Jiang, C.L.; Li, C.L.; Liu, F. Microbial community structure in rhizosphere soil rather than that in bulk soil characterizes aggregate-associated organic carbon under long-term forest conversion in subtropical region. Rhizosphere 2021, 20, 100438. [Google Scholar] [CrossRef]
- Martin-Guay, M.-O.; Belluau, M.; Côté, B.; Handa, I.T.; Jewell, M.D.; Khlifa, R.; Munson, A.D.; Rivest, M.; Whalen, J.K.; Rivest, D. Tree identity and diversity directly affect soil moisture and temperature but not soil carbon ten years after planting. Ecol. Evol. 2022, 12, e8509. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Purahong, W.; Durka, W.; Fischer, M.; Dommert, S.; Schöps, R.; Buscot, F.; Wubet, T. Tree species, tree genotypes and tree genotypic diversity levels affect microbe-mediated soil ecosystem functions in a subtropical forest. Sci. Rep. 2016, 6, 36672. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Cheng, X.L.; Hui, D.F.; Zhang, Q.; Li, M.; Zhang, Q.F. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. R. 2017, 81, e00063–e00016. [Google Scholar] [CrossRef]
- Cai, W.X.; He, N.P.; Li, M.X.; Xu, L.; Wang, L.Z.; Zhu, J.H.; Zeng, N.; Yan, P.; Si, G.X.; Zhang, X.Q.; et al. Carbon sequestration of Chinese forests from 2010 to 2060: Spatiotemporal dynamics and its regulatory strategies. Sci. Bull. 2022, 67, 836–843. [Google Scholar] [CrossRef]
- Liang, B.Y.; Wang, J.; Zhang, Z.Y.; Zhang, J.; Zhang, J.P.; Cressey, E.L.; Wang, Z. Planted forest is catching up with natural forest in China in terms of carbon density and carbon storage. Fund. Res. 2022, 2, 688–696. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.Y.; Shao, Q.Q.; Xu, X.L. Carbon sequestration by forestation across China: Past, present, and future. Renew. Sust. Energ. Rev. 2012, 16, 1291–1299. [Google Scholar] [CrossRef]
- Xie, S.L.; Su, Y.B.; Xu, W.H.; Cai, W.B.; Wang, X.K.; Lu, F.; Ouyang, Z.Y. The effect of habitat changes along the urbanization gradient for breeding birds: An example from the Xiong’an New Area. PeerJ 2019, 7, e7961. [Google Scholar] [CrossRef]
- Li, H.P.; Wickham, J.D.; Bushley, K.; Wang, Z.G.; Zhang, B.; Sun, J.H. New approaches in urban forestry to minimize invasive species impacts: The case of Xiongan New Area in China. Insects 2020, 11, 300. [Google Scholar] [CrossRef]
- Wang, K.F.; Qiu, Z.L.; Zhang, M.; Li, X.Y.; Fang, X.; Zhao, M.Y.; Shi, F.C. Effect of afforestation mode on rhizosphere soil physicochemical properties and bacterial community structure of two major tree species in Xiong’an New Area. For. Ecol. Manag. 2022, 520, 120361. [Google Scholar] [CrossRef]
- Lu, C.; Li, W. A comprehensive city-level GHGs inventory accounting quantitative estimation with an empirical case of Baoding. Sci. Total Environ. 2019, 651, 601–613. [Google Scholar] [CrossRef]
- Wang, C.; Masoudi, A.; Wang, M.; Yang, J.; Shen, R.W.; Man, M.; Yu, Z.J.; Liu, J. Community structure and diversity of the microbiomes of two microhabitats at the root-soil interface: Implications of meta-analysis of the root-zone soil and root endosphere microbial communities in Xiong’an New Area. Can. J. Microbiol. 2020, 66, 605–622. [Google Scholar] [CrossRef]
- Farooq, A.; Xie, M.W.; Stoilova, S.; Ahmad, F.; Guo, M.; Williams, E.J.; Gahlot, V.K.; Yan, D.; Issa, A.M. Transportation planning through GIS and multicriteria analysis: Case study of Beijing and XiongAn. J. Adv. Transport. 2018, 2018, 2696037. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Xie, H.L.; Lin, L.J.; Hu, Q.Y.; Qian, Y.; Zhang, S.R.; Wang, G.L.; Li, J.G.; Tan, C.X.; Guo, H.P.; et al. The environmental geological conditions of land resources in the Beijing-Tianjin-Hebei region. Geol. China 2017, 44, 857–873. [Google Scholar] [CrossRef]
- Xu, H.Q.; Wang, M.Y.; Shi, T.T.; Guan, H.D.; Fang, C.Y.; Lin, Z.L. Prediction of ecological effects of potential population and impervious surface increases using a remote sensing based ecological index (RSEI). Ecol. Indic. 2018, 93, 730–740. [Google Scholar] [CrossRef]
- Luo, J.S.; Ma, X.W.; Chu, Q.F.; Xie, M.; Cao, Y.J. Characterizing the up-to-date land-use and land-cover change in Xiong’an New Area from 2017 to 2020 using the multi-temporal sentinel-2 images on Google Earth Engine. ISPRS Int. J. Geo-Inf. 2021, 10, 464. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, M.; Bai, W.J.; Wei, B.F.; Shi, D.Y.; Li, H.P. Investigation on the host species and damages of Hyphantria cuneain the Xiongan New Area. For. Ecol. Sci. 2022, 37, 107–114. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Analysis in Agricultural Chemistry; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Phillips, R.P.; Fahey, T.J. The influence of soil fertility on rhizosphere effects in northern hardwood forest soils. Soil Sci. Soc. Am. J. 2008, 72, 453–461. [Google Scholar] [CrossRef] [Green Version]
- HJ615-2011; Soil Determination of Orgaic Carbon-Potassium Dichromate Oxidation Spectrophotometric Method. Ministry of Environmental Protection, PRC: Beijing, China, 2011.
- HJ634-2012; Soil Determination of Ammonium, Nitrite and Nitrate by Extraction with Potassium Chloride Solution-Spectrophotometric Methods. Ministry of Environmental Protection, PRC: Beijing, China, 2012.
- NY/T 1121.7-2014; Soil Testing-Method for Determination of Available Phosphorus in Soil. Ministry of Agriculture, PRC: Beijing, China, 2014.
- GB/T 32737-2016; Determination of Nitrate Nitrogen in Soil Ultraviolet Spectrophotometry Method. Standardization Administration of China: Beijing, China, 2016.
- NY/T 1377-2007; Determination of pH in Soil. Ministry of Agriculture, PRC: Beijing, China, 2007.
- GB 7172-1987; Method for Determination of Soil Water Content. Standardization Administration of China: Beijing, China, 1987.
- Zhuang, W.; Yu, X.L.; Hu, R.W.; Luo, Z.W.; Liu, X.Y.; Zheng, X.F.; Xiao, F.S.; Peng, Y.S.; He, Q.; Tian, Y.; et al. Diversity, function and assembly of mangrove root-associated microbial communities at a continuous fine-scale. NPJ Biofilms Microbi. 2020, 6, 52. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome. Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Wambsganss, J.; Freschet, G.T.; Beyer, F.; Bauhus, J.; Scherer-Lorenzen, M. Tree diversity, initial litter quality, and site conditions drive early-stage fine-root decomposition in European forests. Ecosystems 2021, 24, 1–17. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Ma, S.Y.; Rambal, S.; Misson, L.; Ourciva, J.-M.; Limousin, J.-M.; Pereira, J.; Papale, D. On the differential advantages of evergreenness and deciduousness in mediterranean oak woodlands: A flux perspective. Ecol. Appl. 2010, 20, 1583–1597. [Google Scholar] [CrossRef]
- Karst, J.; Gaster, J.; Wiley, E.; Landhäusser, S.M. Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiol. 2017, 37, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Zhao, X.; Chao, Z.; Wang, Y.C.; Zhang, X.P.; Wang, D.X. The linkages of plant, litter and soil C:N:P stoichiometry and nutrient stock in different secondary mixed forest types in the Qinling Mountains, China. PeerJ 2020, 8, e9274. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.S.; Gu, H.J.; Zhang, C.C.; Zhang, Y.; Singh, A.N.; Fang, X.M.; Fan, J.; Wang, H.M.; Chen, F.S. Mixed broadleaved tree species increases soil phosphorus availability but decreases the coniferous tree nutrient concentration in subtropical China. Forests 2020, 11, 461. [Google Scholar] [CrossRef]
- Augusto, L.; Schrijver, A.D.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2015, 90, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.Z.; Cui, C.; Hou, E.Q.; Li, F.B.; Liu, W.J.; Jiang, L.F.; Luo, Y.Q.; Xu, X.N. Acidification of soil due to forestation at the global scale. Forest Ecol. Manag. 2022, 505, 119951. [Google Scholar] [CrossRef]
- Chen, Y.P.; Xia, J.B.; Zhao, X.M.; Zhuge, Y.P. Effect of different plantation types on soil ecological stoichiometry in Yellow Delta. Chin. J. Soil Sci. 2017, 48, 392–398. [Google Scholar] [CrossRef]
- Xia, J.B.; Ren, J.Y.; Zhang, S.Y.; Wang, Y.H.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Luo, Z.Y. Characteristics of Soil Phosphorus Transformation Microbial Community under Different Vegetation Restoration Models in Manganese Mine Wasteland; Central South University of Forestry and Technology: Changsha, China, 2021. [Google Scholar]
- Mi, C.H. Effect of Litter Decomposition and Nutrient Circulation on Soil Polarization in the Planted Pure Forests of Loess Plateau; Northwest Agriculture and Forestry University: Xianyang, China, 2014. [Google Scholar]
- Yin, Y.; Li, Q.L.; Du, H.T. Near-natural transformation of Pinus tabuliformis better improve soil nutrients and soil microbial community. PeerJ 2021, 9, e12098. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, X.P.; Cheng, Y.; Wang, M.T.; Zhong, Q.L.; Li, M.; Cheng, D.L. Leaf and fine root economics spectrum across 49 woody plant species in Wuyi Mountains. Chin. J. Plant Ecol. 2021, 45, 242–252. [Google Scholar] [CrossRef]
- Qi, J.H.; Fan, Z.X.; Fu, P.L.; Zhang, Y.J.; Sterck, F. Differential determinants of growth rates in subtropical evergreen and deciduous juvenile trees: Carbon gain, hydraulics and nutrient-use efficiencies. Tree Physiol. 2020, 41, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Tian, H.Q.; Chen, G.S.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodribb, T.J.; Pittermann, J.; Coomes, D.A. Elegance versus speed: Examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 2012, 173, 673–694. [Google Scholar] [CrossRef] [Green Version]
- Schwendenmann, L.; Pendall, E.; Sanchez-Bragado, R.; Kunert, N.; Hölscher, D. Tree water uptake in a tropical plantation varying in tree diversity: Interspecific differences, seasonal shifts and complementarity. Ecohydrology 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.Q.; Huang, D.K.; Cheng, J.Z. Ammonium nitrogen in surface soil of arid and semiarid Central East Asia. Geochimica 2008, 37, 572–580. [Google Scholar] [CrossRef]
- Liu, X.J.; Trogisch, S.; He, J.S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.Y.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. Royal Soc. B Biol. Sci. 2018, 285, 20181240. [Google Scholar] [CrossRef] [Green Version]
- Guénon, R.; Day, T.A.; Velazco-Ayuso, S.; Gros, R. Mixing of Aleppo pine and Holm oak litter increases biochemical diversity and alleviates N limitations of microbial activity. Soil Biol. Biochem. 2017, 105, 216–226. [Google Scholar] [CrossRef]
- Luc, N.T.; Liu, Z.W.; Bing, Y.H.; Zhang, X.X.; Nguyen, T.H. The control of soil polarization in Populus simonii and Quercus liaotungensis forests by forage litter on the Loess Plateau, P.R. China. J. For. Res. 2015, 26, 687–695. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Júnior, G.V.; Noronha, M.F.; Cabral, L.; Delforno, T.P.; de Sousa, S.T.P.; Fernandes-Júnior, P.I.; Melo, I.S.; Oliveira, V.M. Land use and seasonal effects on the soil microbiome of a Brazilian dry forest. Front. Microbiol. 2019, 10, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.J.; Deng, J.J.; Yin, Y.; Qin, S.J.; Zhu, W.X.; Zhou, Y.B.; Wang, B.; Ruan, H.H.; Jin, L. Bacterial community changes associated with land use type in the forest montane region of northeast China. Forests 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. R. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Sun, M.L.; Zhang, H.; Xu, N.; Sun, G.Y. Use of mulberry-soybean intercropping in salt–alkali soil impacts the diversity of the soil bacterial community. Microb. Biotechnol. 2016, 9, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Buckley, D.H.; Huangyutitham, V.; Nelson, T.A.; Rumberger, A.; Thies, J.E. Diversity of Planctomycetes in soil in relation to soil history and environmental heterogeneity. Appl. Environ. Microb. 2006, 72, 4522–4531. [Google Scholar] [CrossRef] [Green Version]
- Ettwig, K.F.; Butler, M.K.; Paslier, D.L.; Pelletier, E.; Mangenot, S.; Kuypers, M.M.M.; Schreiber, F.; Dutilh, B.E.; Zedelius, J.; de Beer, D.; et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010, 464, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Norman, J.S.; Barrett, J.E. Substrate and nutrient limitation of ammonia-oxidizing bacteria and archaea in temperate forest soil. Soil Biol. Biochem. 2014, 69, 141–146. [Google Scholar] [CrossRef]
- Li, W.K.; Liu, X.D.; Niu, S.K. Differential responses of the acidobacterial community in the topsoil and subsoil to fire disturbance in Pinus tabulaeformis stands. PeerJ 2019, 7, e8047. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Liu, L.J.; Leng, P.S.; Hu, Z.H. Feasible and effective reuse of municipal sludge for vegetation restoration: Physiochemical characteristics and microbial diversity. Sci. Rep. 2019, 9, 879. [Google Scholar] [CrossRef]
- Yu, M.J.; Su, W.Q.; Huang, L.B.; Parikh, S.J.; Tang, C.X.; Dahlgrenc, R.A.; Xu, J.M. Bacterial community structure and putative nitrogen-cycling functional traits along a charosphere gradient under waterlogged conditions. Soil Biol. Biochem. 2021, 162, 108420. [Google Scholar] [CrossRef]
- Wang, W.J.; Liu, A.R.; Fu, W.T.; Peng, D.L.; Wang, G.; Ji, J.; Jin, C.; Guan, C.F. Tobacco-associated with Methylophilus sp. FP-6 enhances phytoremediation of benzophenone-3 through regulating soil microbial community, increasing photosynthetic capacity and maintaining redox homeostasis of plant. J. Hazard. Mater. 2022, 431, 128588. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Kang, S.M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces Gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Kumar, A.; Chen, L.J.; Jiang, Y.J.; Ling, N.; Wang, R.Z.; Pan, Q.; Singh, B.P.; Redmile-Gordon, M.; Luan, L.; et al. Rhizosphere microbiome modulated effects of biochar on ryegrass 15N uptake and rhizodeposited 13C allocation in soil. Plant Soil 2021, 463, 359–377. [Google Scholar] [CrossRef]
- Huang, H.Y.; Jiang, Y.M.; Zhao, J.H.; Li, S.S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef]
- Hu, H.; Li, D.L.; Wang, Y.Y.; Liu, Y.G.; Wang, Y. Environmental response of dominant bacterial community in sediment of arsenic-rich lakeside wetland to wind-wave disturbance. Chin. J. Appl. Environ. Biol. 2021, 27, 1492–1499. [Google Scholar] [CrossRef]
- Urbanová, M.; Snajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, 35, 2190–2204. [Google Scholar] [CrossRef]
- Li, W.Q.; Wu, Z.J.; Zong, Y.Y.; Wang, G.G.; Chen, F.S.; Liu, Y.Q.; Li, J.J.; Fang, X.M. Tree species mixing enhances rhizosphere soil organic carbon mineralization of conifers in subtropical plantations. Forest Ecol. Manag. 2022, 516, 120238. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. A plant economics spectrum of litter decomposability. Funct. Ecol. 2012, 26, 56–65. [Google Scholar] [CrossRef]
- Cherif, M.; Loreau, M. Stoichiometric constraints on resource use, competitive interactions, and elemental cycling in microbial decomposers. Am. Nat. 2007, 169, 709–724. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Shi, C.; Zhao, M.Y.; Wang, K.F.; Zhang, M.; Wang, T.T.; Shi, F.C. Improving effects of afforestation with different forest types on soil nutrients and bacterial community in Barren Hills of North China. Sustainability 2022, 14, 1202. [Google Scholar] [CrossRef]
- Kurm, V.; Geisen, S.; Hol, W.H.G. A low proportion of rare bacterial taxa responds to abiotic changes compared with dominant taxa. Environ. Microbiol. 2019, 21, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Paszkiewicz, K.; Field, D.; Knight, R.; Gilbert, J.A. The Western English Channel contains a persistent microbial seed bank. ISME J. 2012, 6, 1089–1093. [Google Scholar] [CrossRef] [Green Version]
- Goh, Y.K.; Zoqratt, M.Z.H.M.; Goh, Y.K.; Ayub, Q.; Ting, A.S.Y. Determining soil microbial communities and their influence on Ganoderma disease incidences in Oil Palm (Elaeis guineensis) via high-throughput sequencing. Biology 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, M.; Pascual, J.; González, I.; Genilloud, O. Draft genome sequence and biosynthetic potential of the newly described strain Longimicrobium terrae CB-286315T. Microbiol. Resour. Ann. 2020, 9, e00512–e00520. [Google Scholar] [CrossRef]
- Bañeras, L.; Llorens, L.; Díaz-Guerra, L.; Gispert, M.; Amo, E.H.; Massart, S.; Verdaguer, D. Resilience of microbial communities in Mediterranean soil after induced drought and manipulated UV radiation. Eur. J. Soil Sci. 2022, 73, e13218. [Google Scholar] [CrossRef]
- Xu, X.T.; Wang, L.; Huang, P.; Wan, Y.X.; Li, J.Z.; Wan, X.C. Effects of wind and irradiation on growth and morphogenesis of Platycladus orientalis and Quercus variabilis. Sci. Silvae Sin. 2014, 50, 164–168. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.B.; Yin, J.; Huang, S.M. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Serna-Chavez, H.M.; Fierer, N.; van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Global Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Shen, C.C.; Ni, Y.Y.; Liang, W.J.; Wang, J.J.; Chu, H.Y. Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front. Microbiol. 2015, 6, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braos, L.B.; Ruiz, J.G.C.L.; Lopes, I.G.; Ferreira, M.E.; da Cruz, M.C.P. Mineralization of nitrogen in soils with application of acid whey at different pH. J. Soil Sci. Plant Nut. 2020, 20, 1102–1109. [Google Scholar] [CrossRef]
- Camenzind, T.; Hattenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Knelman, J.E.; Legg, T.M.; O’Neill, S.P.; Washenberger, C.L.; González, A.; Cleveland, C.C.; Nemergut, D.R. Bacterial community structure and function change in association with colonizer plants during early primary succession in a glacier forefield. Soil Biol. Biochem. 2012, 46, 172–180. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Bastida, F.; Crowther, T.W.; Prieto, I.; Routh, D.; García, C.; Jehmlich, N. Climate shapes the protein abundance of dominant soil bacteria. Sci. Total Environ. 2018, 640–641, 18–21. [Google Scholar] [CrossRef]
- Wilson, M.J. The importance of parent material in soil classification: A review in a historical context. Catena 2019, 182, 104131. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Cajthaml, T.; Põlme, S.; Hiiesalu, I.; Anslan, S.; Harend, H.; Buegger, F.; Pritsch, K.; Koricheva, J.; et al. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 2016, 10, 346–362. [Google Scholar] [CrossRef] [Green Version]
- Kemner, J.E.; Adams, M.B.; McDonald, L.M.; Peterjohn, W.T.; Kelly, C.N. Fertilization and tree species influence on stable aggregates in forest soil. Forests 2021, 12, 39. [Google Scholar] [CrossRef]
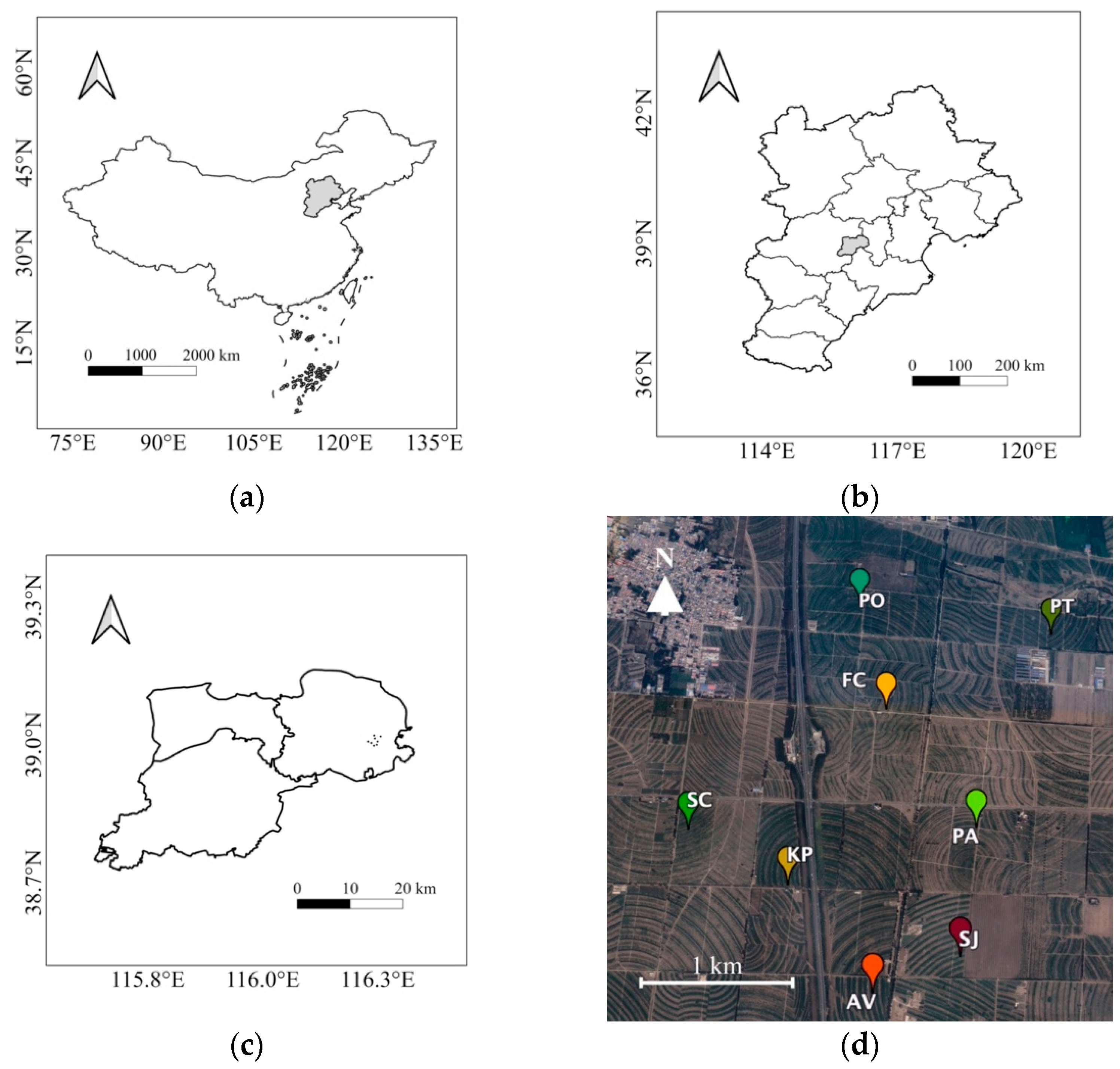




| Tree Species Composition | Area (ha) | Density (Stems·ha−1) | Location |
|---|---|---|---|
| Armeniaca vulgaris | 0.83 | 1963 | 38.997856 N, 116.241925 E |
| Sophora japonica | 0.91 | 1930 | 38.999703 N, 116.248072 E |
| Koelreuteria paniculata | 1.30 | 1873 | 39.003558 N, 116.236375 E |
| Fraxinus chinensis | 0.85 | 1908 | 39.013431 N, 116.243931 E |
| Platycladus orientalis | 0.82 | 1824 | 39.019797 N, 116.242372 E |
| Pinus tabuliformis | 1.35 | 1927 | 39.017867 N, 116.256533 E |
| Picea asperata | 0.96 | 1897 | 39.006622 N, 116.249847 E |
| Sabina chinensis | 0.65 | 1912 | 39.006608 N, 116.229425 E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Qiu, Z.; Zhang, M.; Li, X.; Fang, X.; Zhao, M.; Shi, F. Responses of Rhizosphere Soil Chemical Properties and Bacterial Community Structure to Major Afforestation Tree Species in Xiong’an New Area. Forests 2022, 13, 1822. https://doi.org/10.3390/f13111822
Wang K, Qiu Z, Zhang M, Li X, Fang X, Zhao M, Shi F. Responses of Rhizosphere Soil Chemical Properties and Bacterial Community Structure to Major Afforestation Tree Species in Xiong’an New Area. Forests. 2022; 13(11):1822. https://doi.org/10.3390/f13111822
Chicago/Turabian StyleWang, Kefan, Zhenlu Qiu, Mei Zhang, Xueying Li, Xin Fang, Mingyuan Zhao, and Fuchen Shi. 2022. "Responses of Rhizosphere Soil Chemical Properties and Bacterial Community Structure to Major Afforestation Tree Species in Xiong’an New Area" Forests 13, no. 11: 1822. https://doi.org/10.3390/f13111822




