The Growth and Physiological Characteristics of the Endangered CAM Plant, Nadopungnan (Sedirea japonica), under Drought and Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
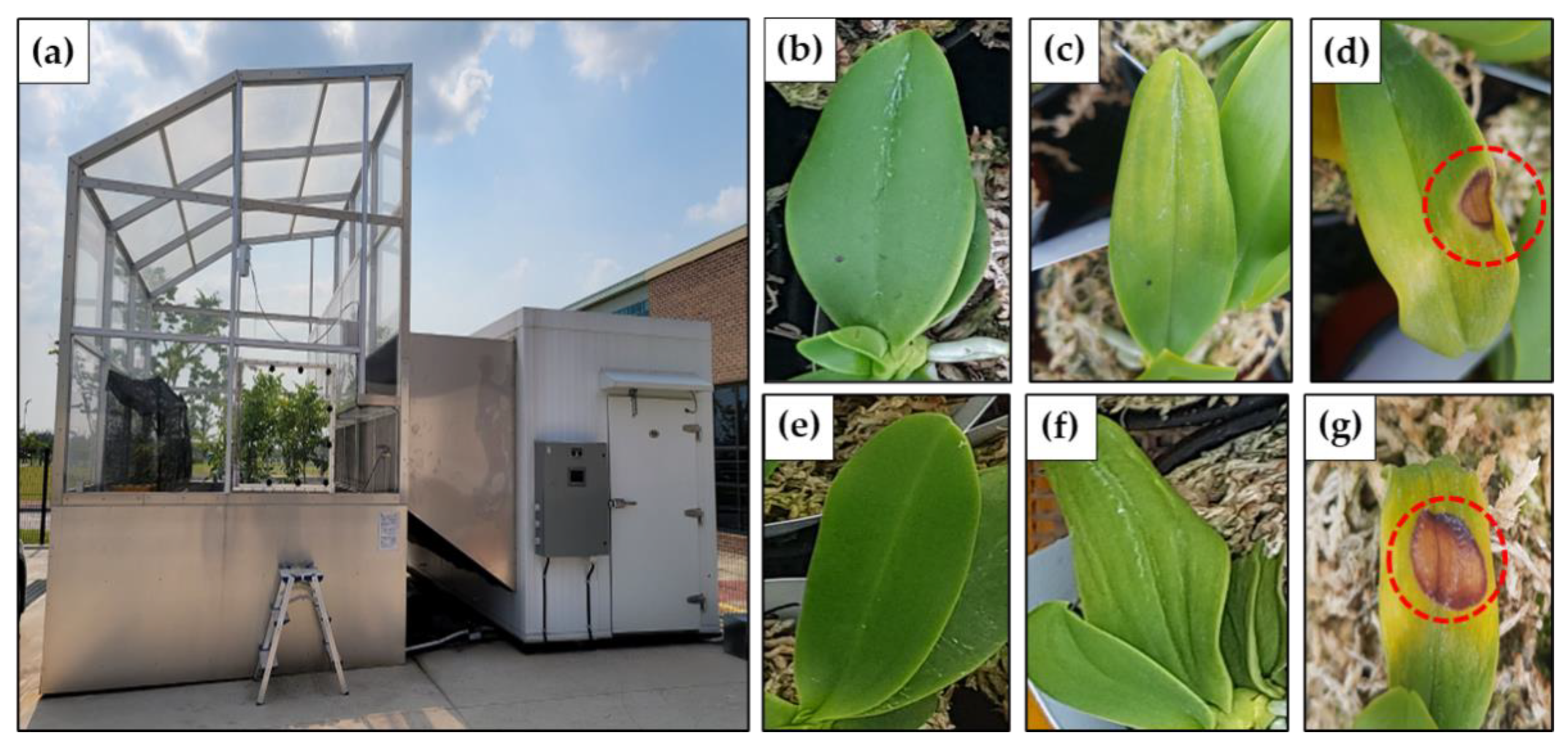
2.1. Plant Materials and Growth Conditions
2.2. Nocturnal Total CO2 Uptake and Transpiration
2.3. Growth Characteristics
2.4. Chlorophyll and Carotenoid Content
2.5. Chlorophyll a Fluorescence
2.6. Statistical Analysis
3. Results
3.1. Nocturnal Total CO2 Uptake and Transpiration
3.2. Growth Characteristics
3.3. Chlorophyll and Carotenoid Content
3.4. Chlorophyll a Fluorescence
4. Discussion
4.1. Nocturnal Total CO2 Uptake and Transpiration
4.2. Growth Characteristics
4.3. Chlorophyll and Carotenoid Content
4.4. Chlorophyll a Fluorescence
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Group I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; pp. 1–151. [Google Scholar]
- Korea Meteorological Administration. Analysis of Climate Change Forecasts on the Korean Peninsula; Korea Meteorological Administration: Seoul, Korea, 2018; p. 16.
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2006; pp. 180–183. [Google Scholar]
- Rathore, A.; Jasrai, Y.T. Growth and chlorophyll levels of selected plants with varying photosynthetic pathways (C3, C4, and CAM). Int. J. Sci. Eng. Res. 2013, 4, 1–4. [Google Scholar]
- Osmond, C.B. Crassulacean acid metabolism: A curiosity in context. Annu. Rev. Plant Physiol. 1978, 29, 379–414. [Google Scholar] [CrossRef]
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; Van den Ende, W.; Ceusters, J. Performance index and PSII connectivity under drought and contrasting light regimes in the CAM orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley and Sons: New York, NY, USA, 2008; pp. 205–208. [Google Scholar]
- Hogewoning, S.W.; van den Boogaart, S.A.; van Tongerlo, E.; Trouwborst, G. CAM-physiology and carbon gain of the orchid Phalaenopsis in response to light intensity, light integral, and CO2. Plant Cell Environ. 2021, 44, 762–774. [Google Scholar] [CrossRef]
- Lee, K.C.; An, J.; Hwang, J.E.; Kim, P.B.; Park, H.B.; Kim, S.; Park, H.J.; Lee, C.W.; Lee, B.D.; Kim, N.Y. Effects of light condition on growth and physiological characteristics of the endangered species Sedirea japonica under RCP 6.0 climate change scenarios. Plants 2021, 10, 1891. [Google Scholar]
- Davis, S.C.; Simpson, J.; Gil Vega, K.d.C.; Niechayev, N.A.; van Tongerlo, E.; Castano, N.H.; Dever, L.V.; Búrquez, A.A. Undervalued potential of crassulacean acid metabolism for current and future agricultural production. J. Exp. Bot. 2019, 70, 6521–6537. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Cushman, J.C.; Borland, A.M.; Edwards, E.J.; Wullschleger, S.D.; Tuskan, G.A.; Owen, N.A.; Griffiths, H.; Smith, J.A.; De Paoli, H.C.; et al. A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol. 2015, 207, 491–504. [Google Scholar] [CrossRef]
- Suetsugu, K.; Tanaka, K. Pollination of Sedirea japonica (Orchidaceae) by Bombus diversus (Hymenoptera: Apidae). Eur. J. Entomol. 2013, 110, 545. [Google Scholar] [CrossRef] [Green Version]
- Shim, Y.J.; Park, Y.S.; Jang, R.H.; Yoon, Y.J.; Kim, S.R.; Han, S.H. The development of Habitat Suitability Index Model of Class I endangered wildlife, Sedirea japonica. J. Korean Isl. 2020, 32, 153–172. [Google Scholar] [CrossRef]
- Lee, S.I.; Lee, E.P.; Kim, E.J.; Park, J.H.; Cho, K.T.; Lee, S.Y.; You, Y.H. Growth response and variation of ecological niche breadth of Hibiscus hamabo, the endangered plant, according to light, moisture, and nutrient under elevated CO2 concentration and temperature. Korean J. Environ. Ecol. 2017, 31, 279–286. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jo, S.J.; Kim, K.J. Phylogenetic position of Neofinetia and Sedirea (Orchidaceae) and their species identification using the chloroplast matK and the nuclear ITS sequences. Korean J. Plant Taxon. 2014, 44, 39–50. [Google Scholar] [CrossRef]
- Guo, W.J.; Lee, N. Effect of leaf and plant age, and day/night temperature on net CO2 uptake in Phalaenopsis amabilis var. formosa. J. Am. Soc. Hortic. Sci. 2006, 131, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation, and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK; New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Males, J.; Griffiths, H. Stomatal biology of CAM plants. Plant Physiol. 2017, 174, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Dodd, A.N.; Borland, A.M.; Haslam, R.P.; Griffiths, H.; Maxwell, K. Crassulacean acid metabolism: Plastic, fantastic. J. Exp. Bot. 2002, 53, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Hartwell, J. The coordination of central plant metabolism by the circadian clock. Biochem. Soc. Trans. 2005, 33, 945–948. [Google Scholar] [CrossRef] [Green Version]
- Von Caemmerer, S.; Griffiths, H. Stomatal responses to CO2 during a diel Crassulacean acid metabolism cycle in Kalanchoe daigremontiana and Kalanchoe pinnata. Plant Cell Environ. 2009, 32, 567–576. [Google Scholar] [CrossRef]
- Ceusters, J.; Borland, A.M.; De Proft, M.P. Drought adaptation in plants with crassulacean acid metabolism involves the flexible use of different storage carbohydrate pools. Plant Signal. Behav. 2009, 4, 212–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drennan, P.M.; Nobel, P.S. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant Cell Environ. 2000, 23, 767–781. [Google Scholar] [CrossRef] [Green Version]
- Nobel, P.S.; Israel, A.A.; Wang, N. Growth, CO2 uptake, and responses of the carboxylating enzymes to inorganic carbon in two highly productive CAM species at current and doubled CO2 concentrations. Plant Cell Environ. 1996, 19, 585–592. [Google Scholar] [CrossRef]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of agricultural crops to free-air CO2 enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar]
- Winter, K.; Smith, J.A.C. Crassulacean acid metabolism: Current status and perspectives. In Crassulacean Acid Metabolism: Biochemistry. Ecophysiology and Evolution; Winter, K., Smith, J.A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 230–246. [Google Scholar]
- Taylor, G.; Ranasinghe, S.; Bosac, C.; Gardner, S.D.L.; Ferris, R. Elevated CO2 and plant growth: Cellular mechanisms and responses of whole plants. J. Exp. Bot. 1994, 45, 1761–1774. [Google Scholar] [CrossRef]
- Pritchard, S.; Rogers, H.; Prior, S.A.; Peterson, C. Elevated CO2 and plant structure: A review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Cho, A.R.; Park, K.S.; Kim, Y.J. Effect of CO2 enrichment on growth and flowering of Phalaenopsis. Horticult. J. 2017, 86, 1–6. [Google Scholar]
- Cui, M.; Miller, P.M.; Nobel, P.S. CO2 exchange and growth of the Crassulacean acid metabolism plant Opuntia ficus-indica under elevated CO2 in open-top chambers. Plant Physiol. 1993, 103, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Holtum, J.A.M.; Smith, J.A.C.; Neuhaus, H.E. Intracellular transport and pathways of carbon flow in plants with crassulacean acid metabolism. Funct. Plant. Biol. 2005, 32, 429–449. [Google Scholar] [CrossRef]
- Popp, M.; Janett, H.P.; Lüttge, U.; Medina, E. Metabolite gradients and carbohydrate translocation in rosette leaves of CAM and C3 bromeliads. New Phytol. 2003, 157, 649–656. [Google Scholar] [CrossRef]
- Lim, N.H.; Lee, H.B.; An, S.K.; Kim, K.S. Acclimation of young Doritaenopsis Queen Beer ‘Mantefon’ plants under increased CO2 concentration. Flower Res. J. 2018, 26, 11–18. [Google Scholar] [CrossRef]
- Saraswathi, S.G.; Paliwal, K. Drought induced changes in growth, leaf gas exchange, and biomass production in Albizia lebbeck and Cassia siamea seedlings. J. Environ. Biol. 2011, 32, 173–178. [Google Scholar] [PubMed]
- Brunetti, C.; Tattini, M.; Guidi, L.; Velikova, V.; Ferrini, F.; Fini, A. An integrated overview of physiological and biochemical responses of Celtis australis to drought stress. Urban For. Urban Green 2019, 46, 126480. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol. 2003, 131, 1816–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukhov, N.G.; Sabat, S.C.; Mohanty, P. Analysis of chlorophyll a fluoresence changes in weak light in heat treated Amaranthus chloroplasts. Photosynth. Res. 1990, 23, 81–87. [Google Scholar] [CrossRef]
- Boureima, S.; Oukarroum, A.; Diouf, M.; Cisse, N.; Van Damme, P. Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ. Exp. Bot. 2012, 81, 37–43. [Google Scholar] [CrossRef]
- Evans, J.R. The dependence of quantum yield on wavelength and growth irradiance. Funct. Plant Biol. 1987, 14, 69–79. [Google Scholar] [CrossRef]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, P.; Yang, W.; Sui, X.; Li, X.; Li, W.; Zhang, R.; Gu, S.; Xu, N. Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J. For. Res. 2018, 29, 1049–1059. [Google Scholar] [CrossRef]
- Lotfi, R.; Kalaji, H.M.; Valizadeh, G.R.; Khalilvand Behrozyar, E.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica 2018, 56, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Rapacz, M.; Sasal, M.; Kalaji, H.M.; Kościelniak, J. Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and Triticale under variable winter environments? PLoS ONE 2015, 10, e0134820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapacz, M.; Sasal, M.; Gut, M. Chlorophyll fluorescence-based studies of frost damage and the tolerance for cold-induced photoinhibition in freezing tolerance analysis of Triticale (×Triticosecale Wittmack). J. Agron. Crop Sci. 2011, 197, 378–389. [Google Scholar] [CrossRef]
- Begovic, L.; Galic, V.; Abicic, I.; Loncaric, Z.; Lalic, A.; Mlinaric, S. Implications of intra-seasonal climate variations on chlorophyll a fluorescence and biomass in winter barley breeding program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Lee, K.C.; Kweon, H.; Sung, J.W.; Kim, Y.S.; Song, Y.G.; Cha, S.; Koo, N. Physiological response analysis for the diagnosis of drought and waterlogging damage in Prunus yedoensis. For. Sci. Technol. 2022, 18, 14–25. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.; Li, S.; Zhu, Q.; Zhang, H.; Wang, H.; Yu, C.; St. Martin, S.K.; Xie, F. Effect of shade on leaf photosynthetic capacity, light-intercepting, electron transfer and energy distribution of soybeans. Plant Growth Regul. 2017, 83, 409–416. [Google Scholar] [CrossRef]
- Lopes, M.N.; Gomes, G.M.F.; Maranhão, T.D.; Silveira, W.M.; Pompeu, R.C.F.F.; Silva, R.G.D.; Bezerra, M.A.; Cândido, M.J.D. Photochemical activity modulated by water, nitrogen, and phosphorus status in cactus pear. Rev. Bras. Zootecn. 2021, 50, 1–14. [Google Scholar] [CrossRef]
- Borland, A.M.; Hartwell, J.; Jenkins, G.I.; Wilkins, M.B.; Nimmo, H.G. Metabolite control overrides circadian regulation of phosphoenol pyruvate carboxylase kinase and CO2 fixation in Crassulacean acid metabolism. Plant Physiol. 1999, 121, 889–896. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Córdoba-Matson, M.V.; Villegas-Espinoza, J.A.; Hernández-Montiel, L.G.; Troyo-Diéguez, E.; García-Hernández, J.L. Mineral content and biochemical variables of Aloe vera L. under salt stress. PLoS ONE 2014, 9, e94870. [Google Scholar]
- Becerril, G.A.; Valdivia, C.B.P. Physiological alterations induced by drought stress on prickly pear (Opuntia ficus-indica). Rev. Fitot. Mex. 2006, 29, 231–237. [Google Scholar]







| Parameters | Description |
|---|---|
| VK | Relative variable fluorescence at the K-step |
| VJ | Relative variable fluorescence at the J-step |
| VI | Relative variable fluorescence at the I-step |
| M0 | The initial slope of the fluorescence transient normalized on the maximal variable fluorescence |
| Sm | The normalized total complementary area above the OJIP transient or total electron carriers per RC |
| N | The total complementary area between the fluorescence induction curve and F = FM |
| φPo | The maximum quantum yield of primary photochemistry at t = 0 |
| ψEo | Efficiency with which a trapped exciton moves an electron into the ETC beyond QA at t = 0 |
| φEo | Quantum yield of electron transport at t = 0 |
| δRo | The efficiency with which an electron from the intersystem electron carriers is transferred to reduce end electron acceptors at the PSI acceptor side |
| φRo | Quantum yield of reduction of end electron acceptors at the PSI acceptor side |
| φDo | Quantum yield of energy dissipation at t = 0 |
| ρRo | The efficiency with which a trapped exciton can move an electron into the electron transport chain from QA– to the PSI end electron acceptors |
| 1-VI | The efficiency with which a PSII trapped electron is transferred to the PSI acceptor side |
| VK/VJ | The ratio of variable fluorescence in time 0.3 ms to variable fluorescence in time 2 ms as an indicator of the PSII donor-side limitation |
| RC/ABS | QA- reducing RCs per PSII antenna chlorophyll |
| ABS/RC | Absorption flux per RC |
| DI0/RC | Energy dissipation flux per RC |
| TR0/RC | Trapped energy flux per RC |
| ET0/RC | Electron transport flux from QA to QB per RC |
| RE0/RC | Electron transport flux until PSI acceptors per RC |
| RC/CS0 | Amount of active PSII RCs per CS at t = 0 |
| PIabs | Performance index on absorption basis |
| PIabs,Total | Total PI, measuring the performance up to the PSI end electron acceptors |
| DFabs | Driving force on absorption basis |
| SFIabs | Structural and functional index on absorption basis |
| Treatment | Dry Mass Production (g) | S/R | LWR | RWR | ||
|---|---|---|---|---|---|---|
| Leaf | Root | Total Dry Weight | ||||
| 2.6LVPD | 1.38 ± 0.30 b | 2.28 ± 0.70 b | 3.66 ± 0.93 b | 0.64 ± 0.16 ns | 0.38 ± 0.06 ns | 0.62 ± 0.06 ns |
| 2.6HVPD | 1.13 ± 0.32 ab | 1.68 ± 0.47 a | 2.81 ± 0.69 a | 0.70 ± 0.20 ns | 0.41 ± 0.07 ns | 0.59 ± 0.07 ns |
| 8.5LVPD | 1.34 ± 0.41 b | 2.42 ± 0.52 b | 3.76 ± 0.90 b | 0.55 ± 0.09 ns | 0.35 ± 0.04 ns | 0.65 ± 0.04 ns |
| 8.5HVPD | 0.92 ± 0.17 a | 1.58 ± 0.39 a | 2.5 ± 0.53 a | 0.59 ± 0.09 ns | 0.37 ± 0.03 ns | 0.63 ± 0.03 ns |
| Date | Treatment | Chl (mg·g−1) | Car | Chl a/b | T Chl/Car | SPAD | ||
|---|---|---|---|---|---|---|---|---|
| a | b | a + b | (mg·g−1) | |||||
| Aug 25 | 2.6LVPD | 2.38 ± 0.39 ns | 0.61 ± 0.11 ns | 2.99 ± 0.50 ns | 0.81 ± 0.08 ns | 3.88 ± 0.09 b | 3.65 ± 0.31 ns | 58.0 ± 6.1 c |
| 2.6HVPD | 2.04 ± 0.13 | 0.57 ± 0.04 | 2.61 ± 0.16 | 0.88 ± 0.08 | 3.60 ± 0.18 ab | 3.01 ± 0.43 | 50.2 ± 6.3 b | |
| 8.5LVPD | 2.26 ± 0.32 | 0.60 ± 0.10 | 2.86 ± 0.41 | 0.86 ± 0.13 | 3.82 ± 0.26 b | 3.38 ± 0.47 | 55.5 ± 7.0 bc | |
| 8.5HVPD | 2.05 ± 0.50 | 0.63 ± 0.17 | 2.68 ± 0.66 | 0.92 ± 0.11 | 3.28 ± 0.31 a | 2.86 ± 0.48 | 43.6 ± 9.8 a | |
| Sept 24 | 2.6LVPD | 3.04 ± 0.51 ns | 0.75 ± 0.23 ns | 3.79 ± 0.70 ns | 1.52 ± 0.11 ns | 4.24 ± 0.84 b | 2.48 ± 0.31 ns | 55.3 ± 8.0 ns |
| 2.6HVPD | 2.81 ± 0.37 | 1.16 ± 0.36 | 3.98 ± 0.57 | 1.55 ± 0.12 | 2.64 ± 0.81 ab | 2.56 ± 0.31 | 56.5 ± 6.0 | |
| 8.5LVPD | 2.66 ± 0.29 | 1.13 ± 0.42 | 3.79 ± 0.55 | 1.25 ± 0.17 | 2.69 ± 0.94 ab | 3.14 ± 0.87 | 55.3 ± 6.7 | |
| 8.5HVPD | 2.48 ± 0.20 | 1.17 ± 0.35 | 3.65 ± 0.18 | 1.43 ± 0.23 | 2.31 ± 0.69 a | 2.62 ± 0.43 | 51.0 ± 7.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.G.; Hwang, J.E.; An, J.; Kim, P.B.; Park, H.B.; Park, H.J.; Kim, S.; Lee, C.W.; Lee, B.D.; Kim, N.Y.; et al. The Growth and Physiological Characteristics of the Endangered CAM Plant, Nadopungnan (Sedirea japonica), under Drought and Climate Change Scenarios. Forests 2022, 13, 1823. https://doi.org/10.3390/f13111823
Song YG, Hwang JE, An J, Kim PB, Park HB, Park HJ, Kim S, Lee CW, Lee BD, Kim NY, et al. The Growth and Physiological Characteristics of the Endangered CAM Plant, Nadopungnan (Sedirea japonica), under Drought and Climate Change Scenarios. Forests. 2022; 13(11):1823. https://doi.org/10.3390/f13111823
Chicago/Turabian StyleSong, Yeong Geun, Jung Eun Hwang, Jiae An, Pyoung Beom Kim, Hyeong Bin Park, Hwan Joon Park, Seongjun Kim, Chang Woo Lee, Byoung Doo Lee, Nam Young Kim, and et al. 2022. "The Growth and Physiological Characteristics of the Endangered CAM Plant, Nadopungnan (Sedirea japonica), under Drought and Climate Change Scenarios" Forests 13, no. 11: 1823. https://doi.org/10.3390/f13111823





