The Separation Mechanism of Bamboo Bundles at Cellular Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
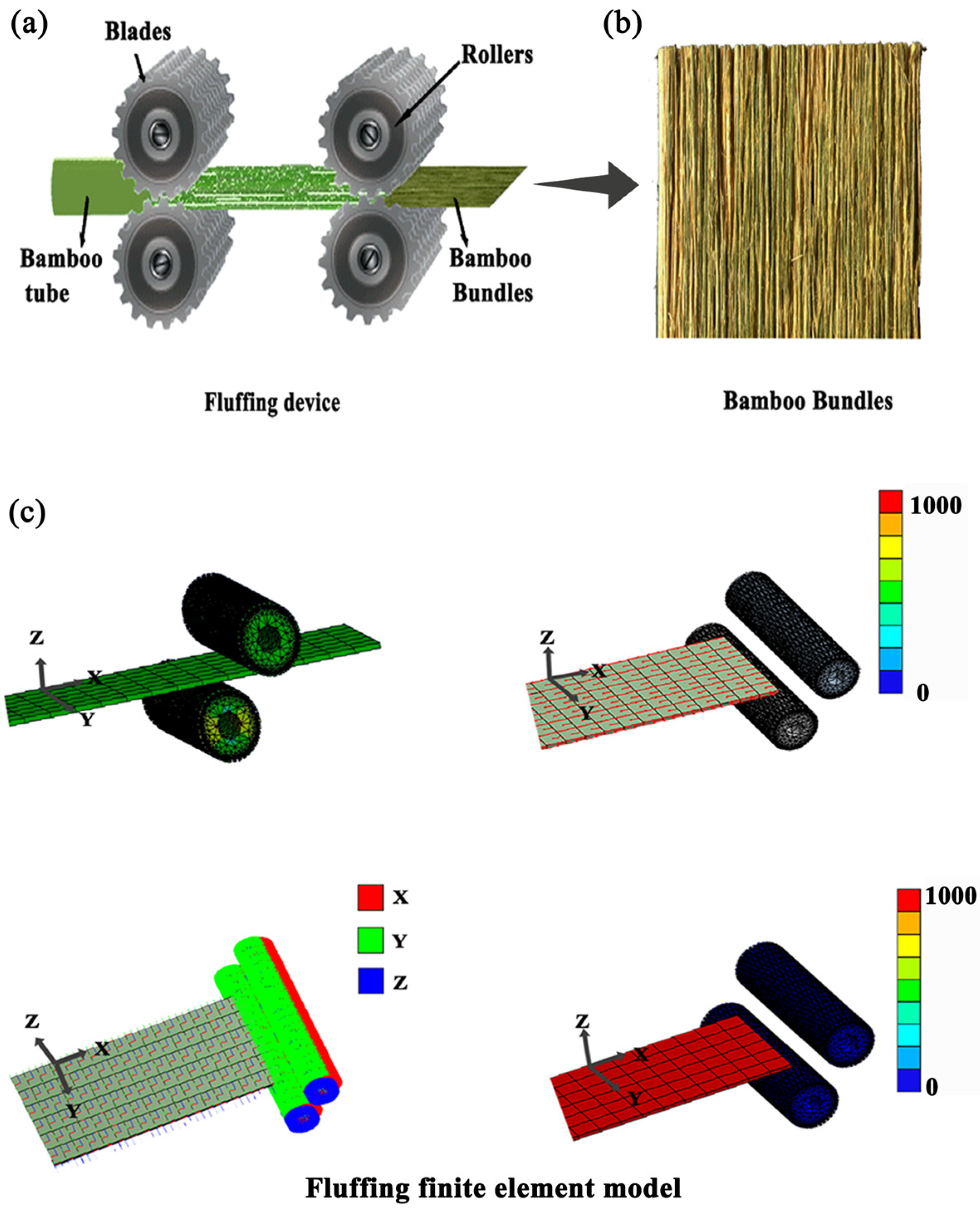
2.2. Three-Dimensional Reconstructed Geometries
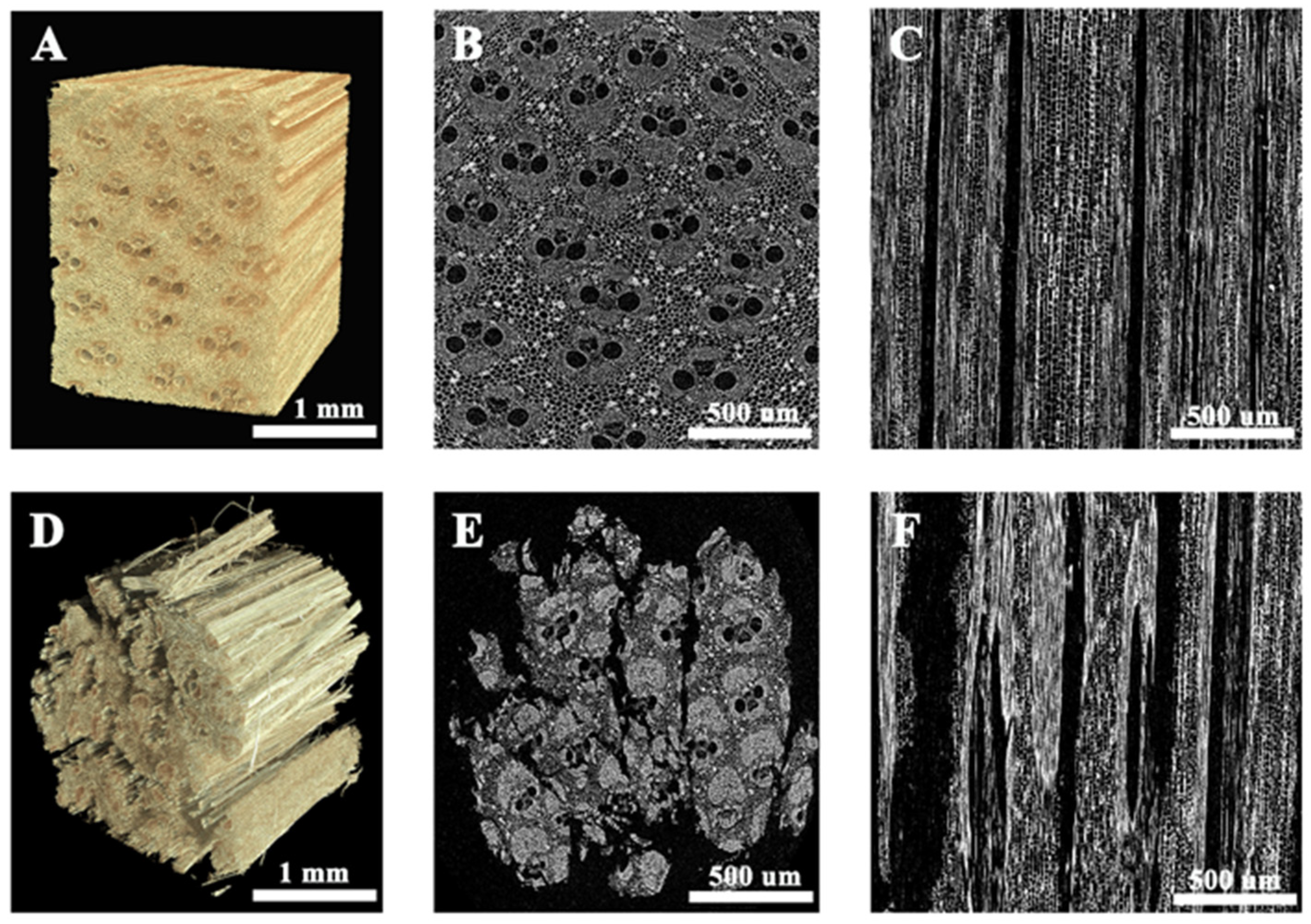
2.3. Morphology Characterization
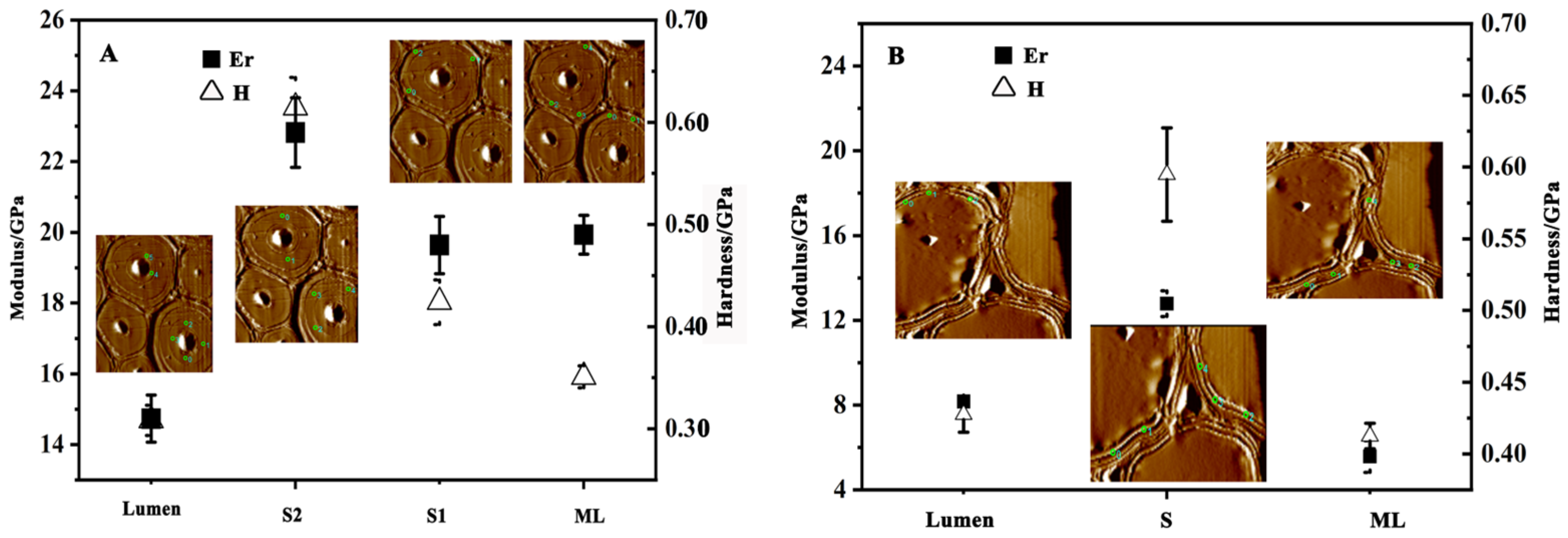
2.4. Nanoindentation and Modulus Mapping Test
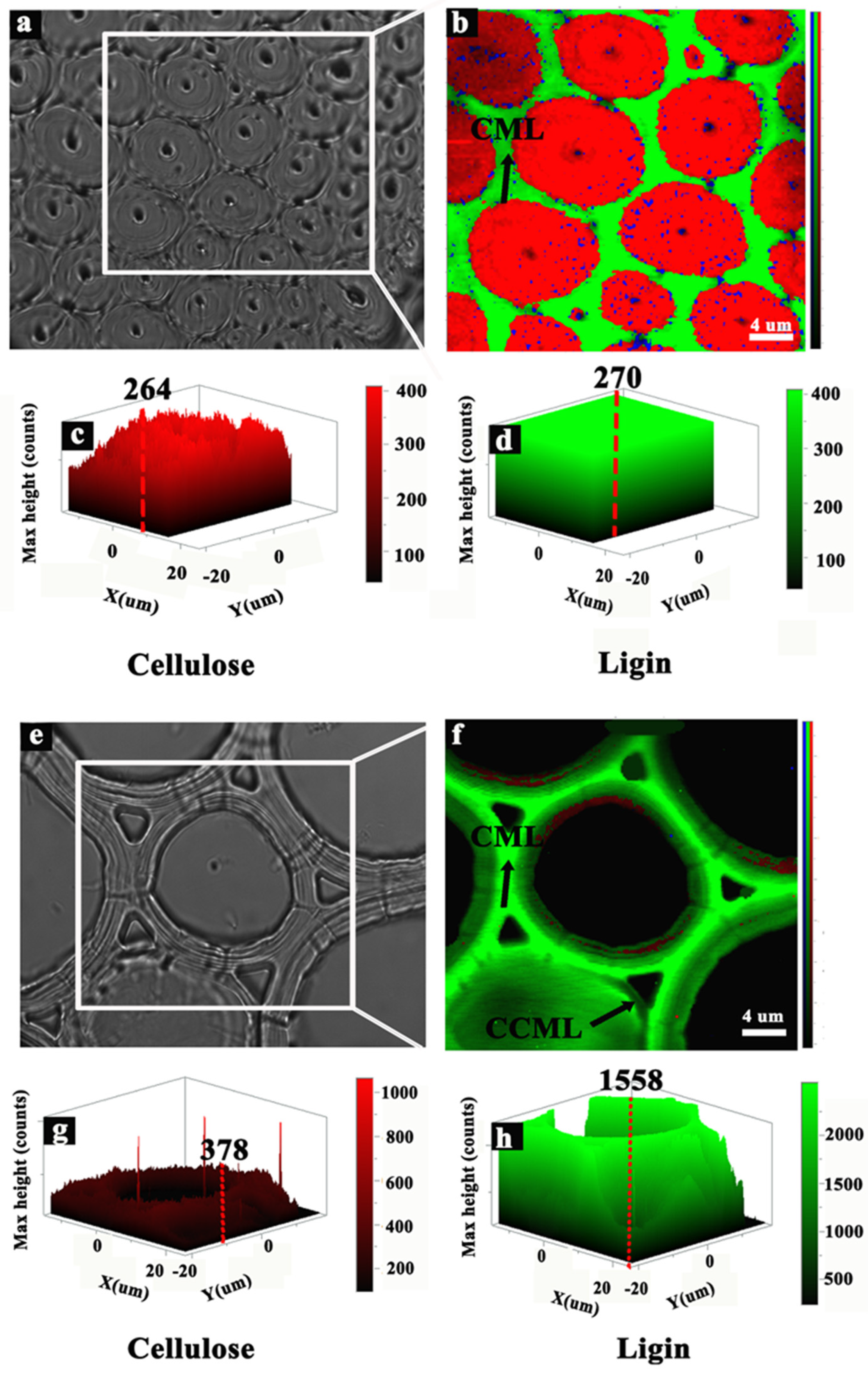
2.5. Raman Microscopy Analysis
3. Results
3.1. The 3D Morphology of Bamboo Bundles
3.2. Characteristics of Cells in the Bamboo Bundles
3.3. Micro-Mechanical Properties of Cell Walls
3.4. Chemical Components of the Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lugt, V.; Dobbelsteen, V.D. An environmental, economic and practical assessment of bamboo as a building material for supporting structures. Constr. Build. Mater. 2006, 20, 648–656. [Google Scholar] [CrossRef]
- Sharma, B.; Gatoo, A.; Bock, M.; Ramage, M. Engineered bamboo for structural applications. Constr. Build. Mater. 2015, 81, 66–73. [Google Scholar] [CrossRef]
- Ahmad, M.; Kamke, F.A. Analysis of Calcutta bamboo for structural composite materials: Physical and mechanical properties. Wood Sci. Technol. 2005, 39, 448–459. [Google Scholar] [CrossRef]
- Yu, W.; Yu, Y. Development and prospect of wood and bamboo scrimber industry in China. China Wood Ind. 2013, 27, 5–8. [Google Scholar]
- Zhang, J.; Wang, K.H.; Li, Q. Prospects of efficient and comprehensive utilization of bamboo and wood resources in China. China Timber 2006, 005, 24–26. [Google Scholar]
- Zhang, Y.; Huang, Y.; Zhang, Y.; Yu, W. Scrimber board (sb) manufacturing by a new method and characterization of sb’s mechanical properties and dimensional stability. Holzforschung 2018, 72, 283–289. [Google Scholar] [CrossRef]
- Yu, Y. Manufacturing Technology and Mechanism of High Performance Bamboo-Based Fiber Composites. Ph.D. Dissertation, Chinese Academy of Forestry, Beijing, China, 2014. [Google Scholar]
- Belayachi, N.; Hoxha, D.; Slaimia, M. Impact of accelerated climatic aging on the behavior of gypsum plaster-straw material for building thermal insulation. Constr. Build. Mater. 2015, 81, 66–73. [Google Scholar] [CrossRef]
- Bao, M.; Huang, X.; Zhang, Y.; Yu, W.; Yu, Y. Effect of density on the hygroscopicity and surface characteristics of hybrid poplar compreg. Wood Sci. Technol. 2016, 62, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Yu, W.; Xie, J.; Yu, Y.; Zhang, Y.; Sun, H. Morphologies of crushed bamboo veneer used for bamboo-based fiber composites. China Wood Ind. 2012, 26, 6–9. [Google Scholar]
- Wu, B.; Yu, Y.; Qi, J.; Yu, W. Effects of bamboo bundles treated with fine fluffing and carbonized treatment on the properties of bamboo scrimber. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2014, 38, 115–120. [Google Scholar]
- Li, J.; Wu, Z.; He, S.; Huang, C.; Chen, Y. Effect of bundle diameter on water absorption properties of bamboo scrimber. J. Zhejiang For. Sci. Technol. 2016, 36, 67–68. [Google Scholar]
- Wei, J.; Rao, F.; Zhang, Y.; Li, C.; Yu, W. Effect of veneer crushing process on physical and mechanical properties of scrimbers made of Pinus radiata and Populus tomentosa. China Wood Ind. 2019, 33, 55–58. [Google Scholar]
- Sutnaun, S.; Srisuwan, S.; Jindasai, P.; Cherdchim, B.; Matan, N. Macroscopic and microscopic gradient structures of bamboo culms. Walailak J. Sci. Technol. 2005, 2, 31–39. [Google Scholar]
- Lian, C.; Liu, R.; Zhang, S.; Yuan, J.; Luo, J.; Yang, F.; Fei, B. Ultrastructure of parenchyma cell wall in bamboo (Phyllostachys edulis) culms. Cellulose 2020, 27, 7321–7329. [Google Scholar] [CrossRef]
- Dixon, P.G.; Gibson, L.J.; Bock, M. The structure and mechanics of moso bamboo material. Sci. Rep. 2014, 11, 20140321. [Google Scholar] [CrossRef]
- Habibi, M.; Lu, Y. Cracks propagation in bamboo’s hierarchical cellular structure. Sci. Rep. 2014, 4, 5598. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Fang, C.; Huang, S. Tensile properties of moso bamboo (Phyllostachys pubescens) and its components with respect to its fiber-reinforced composite structure. Wood Sci. Technol. 2010, 44, 655–666. [Google Scholar] [CrossRef]
- Wang, F.; Shao, Z.; Bock, M. Study on the variation law of bamboo fibers’ tensile properties and the organization structure on the radial direction of bamboo stem. Ind. Crops Prod. 2020, 152, 112521. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Zhang, B.; Fei, B.; Burgert, I. Cell wall structure and formation of maturing fibres of moso bamboo (Phyllostachys pubescens) increase buckling resistance. J. R. Soc. Interface 2012, 9, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lin, L.; Fu, F. Fracture mechanisms of moso bamboo (Phyllostachys pubescens) under longitudinal tensile loading. Ind. Crops Prod. 2020, 153, 112574. [Google Scholar] [CrossRef]
- Parameswaran, N.; Liese, W. On the fine structure of bamboo fibres. Wood Sci. Technol. 1976, 10, 231–246. [Google Scholar] [CrossRef]
- Obataya, E.; Kitin, P.; Yamauchi, H. Bending characteristics of bamboo (Phyllostachys pubescens) with respect to its fiber–foam composite structure. Wood Sci. Technol. 2007, 41, 385–400. [Google Scholar] [CrossRef]
- Liu, X.; Tian, G.; Shang, L.; Yang, S.; Jiang, Z. Compression properties of vascular boundles and parenchyma of rattan (Plectocomia assamica Griff). Holzforschung 2014, 68, 927–932. [Google Scholar] [CrossRef]
- Chen, M.; Ye, L.; Wang, G.; Fang, C.; Dai, C.; Fei, B. Fracture modes of bamboo fiber bundles in three-point bending. Cellulose 2019, 26, 8101–8108. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Tian, X.; Li, S.; Yang, C.; Yu, Y.; Yu, W. The Separation Mechanism of Bamboo Bundles at Cellular Level. Forests 2022, 13, 1897. https://doi.org/10.3390/f13111897
Hao X, Tian X, Li S, Yang C, Yu Y, Yu W. The Separation Mechanism of Bamboo Bundles at Cellular Level. Forests. 2022; 13(11):1897. https://doi.org/10.3390/f13111897
Chicago/Turabian StyleHao, Xiu, Xinchi Tian, Shunong Li, Chunmei Yang, Yanglun Yu, and Wenji Yu. 2022. "The Separation Mechanism of Bamboo Bundles at Cellular Level" Forests 13, no. 11: 1897. https://doi.org/10.3390/f13111897






