Predicting the Future Age Distribution of Conifer and Broad-Leaved Trees Based on Survival Analysis: A Case Study on Natural Forests in Northern Japan
Abstract
:1. Introduction
2. Materials and Methods
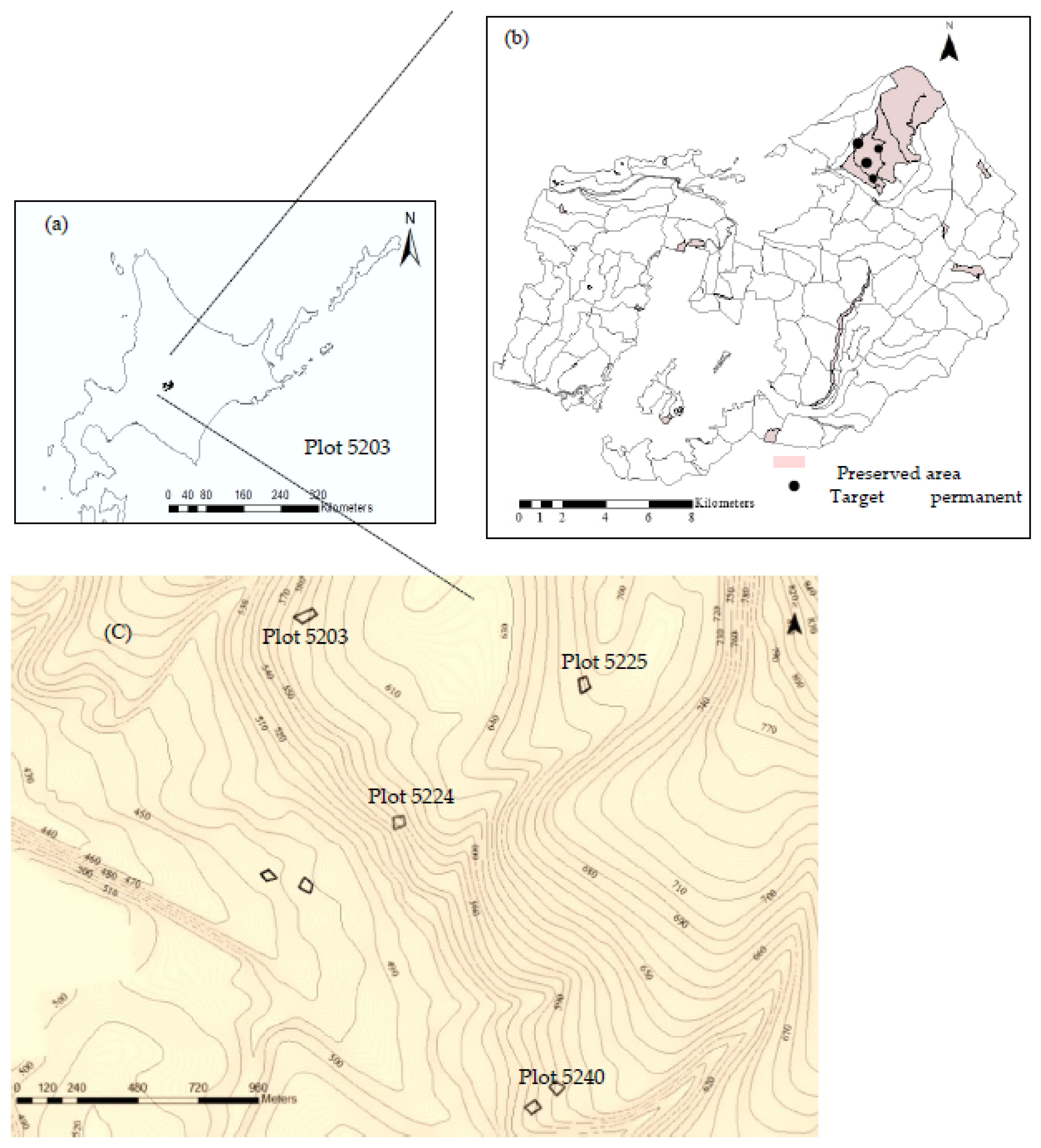
2.1. Study Area
2.2. Collection of Tree Age Data in Observation Periods
2.3. Survival Analysis
2.4. Future Predictions
3. Results
3.1. Tree Composition and Age-Class Distribution of Major Conifers, Broad-Leaved Trees, and All the Trees
3.2. Survival Analyses
4. Discussion
4.1. Stand Dynamics
4.2. Management Implications for SSMS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelstam, P.K. Maintaining and Restoring Biodiversity in European Boreal Forests by Developing Natural Disturbance Regimes. J. Veg. Sci. 1998, 9, 593–602. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Penttinen, A.; Leinonen, K.; Nygren, M. Statistical Opportunities for Comparing Stand Structural Heterogeneity in Managed and Primeval Forests: An Example from Boreal Spruce Forest in Southern Finland. Silva Fenn. 1996, 30, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Zenner, E.K.; Hibbs, D.E. A New Method for Modeling the Heterogeneity of Forest Structure. For. Ecol. Manag. 2000, 129, 75–87. [Google Scholar] [CrossRef]
- Agren, J.; Zackrisson, O. Age and Size Structure of Pinus Sylvestris Populations on Mires in Central and Northern Sweden. J. Ecol. 1990, 78, 1049. [Google Scholar] [CrossRef]
- Limaei, S.M.; Lohmander, P.; Olsson, L. Dynamic Growth Models for Continuous Cover Multi-Species Forestry in Iranian Caspian Forests. J. For. Sci. 2017, 63, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Wallenius, T.; Kuuluvainen, T.; Heikkilä, R.; Lindholm, T. Spatial Tree Age Structure and Fire History in Two Old-Growth Forests in Eastern Fennoscandia. Silva Fenn. 2002, 36, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Paul, C.; Brandl, S.; Friedrich, S.; Falk, W.; Härtl, F.; Knoke, T. Climate Change and Mixed Forests: How Do Altered Survival Probabilities Impact Economically Desirable Species Proportions of Norway Spruce and European Beech? Ann. For. Sci. 2019, 76, 14. [Google Scholar] [CrossRef] [Green Version]
- Moe, K.T.; Owari, T. Sustainability of High-Value Timber Species in Mixed Conifer-Broadleaf Forest Managed under Selection System in Northern Japan. Forests 2020, 11, 484. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and Process in the Plant Community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Villalba, R.; Veblen, T.T. Regional Patterns of Tree Population Age Structures in Northern Patagonia: Climatic and Disturbance Influences. J. Ecol. 1997, 85, 113–124. [Google Scholar] [CrossRef]
- Antos, J.A.; Parish, R.; Conley, K. Age Structure and Growth of the Tree-Seedling Bank in Subalpine Spruce-Fir Forests of South-Central British Columbia. Am. Midl. Nat. 2000, 143, 342–354. [Google Scholar] [CrossRef]
- Worbes, M.; Staschel, R.; Roloff, A.; Junk, W.J. Tree Ring Analysis Reveals Age Structure, Dynamics and Wood Production of a Natural Forest Stand in Cameroon. For. Ecol. Manag. 2003, 173, 105–123. [Google Scholar] [CrossRef]
- Loewenstein, E.F.; Johnson, P.S.; Garrett, H.E. Age and Diameter Structure of a Managed Uneven-Aged Oak Forest. Can. J. For. Res. 2000, 30, 1060–1070. [Google Scholar] [CrossRef]
- Yue, Q.; Hao, M.; Li, X.; Zhang, C.; von Gadow, K.; Zhao, X. Assessing Biotic and Abiotic Effects on Forest Productivity in Three Temperate Forests. Ecol. Evol. 2020, 10, 7887–7900. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N. The Stand-Based Silvicultural Management System: Its Theory and Practices, 1st ed.; Log Bee: Sapporo, Japan, 2001. [Google Scholar]
- The University of Tokyo Hokkaido Forest. 2021. Available online: http://www.uf.a.u-tokyo.ac.jp/files/gaiyo_hokkaido.pdf. (accessed on 26 July 2020).
- Owari, T.; Yasumura, N.; Ishibashi, S.; Kamoda, S.; Saito, H. The University of Tokyo Forests and Forest Science Education in Japan; SILVA Network Conference. Faculty of Forestry and Wood Sciences; Czech University of Life Sciences: Prague, Czech, 2019; pp. 38–50. [Google Scholar]
- Wijenayake, P.R.; Hiroshima, T. Survival Analyses of Individual Tree Populations in Natural Forest Stands to Evaluate the Maturity of Forest Stands: A Case Study of Preserved Forests in Northern Japan. J. For. Plan. 2022, 28, 1–13. [Google Scholar] [CrossRef]
- Wijenayake, P.R.; Hiroshima, T. Age-based Survival Analysis of Coniferous and Broad-leaved Trees: A Case Study of Preserved Forests in Northern Japan. Forests 2021, 12, 1014. [Google Scholar] [CrossRef]
- IUSS Working Group WRB-FAO. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; IUSS Working Group WRB-FAO: Rome, Italy, 2015. [Google Scholar]
- Nakagawa, M.; Kurahashi, A.; Kaji, M.; Hogetsu, T. The Effects of Selection Cutting on Regeneration of Picea Jezoensis and Abies Sachalinensis in the Sub-Boreal Forests of Hokkaido, Northern Japan. For. Ecol. Manag. 2001, 146, 15–23. [Google Scholar] [CrossRef]
- Hiroshima, T. Applying Age-Based Mortality Analysis to a Natural Forest Stand in Japan. J. For. Res. 2014, 19, 379–387. [Google Scholar] [CrossRef]
- Rinn, F. Eine Neue Bohrmethode Zur Holzuntersuchung. Holz-Zent. 1989, 34, 529–530. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Cox, D.R.; Oakes, D. Analysis of Survival Data; Chapman and Hall: London, UK, 1984. [Google Scholar]
- Crowder, M.J.; Kimber, A.C.; Smith, R.L. Statistical Analysis of Reliability Data; Chapman and Hall: London, UK, 1994; Volume 27. [Google Scholar]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis: Techniques for Censored and Truncated Data; Springer: New York, NY, USA, 1997. [Google Scholar]
- Kleinbaum, D.G.D.; Klein, M. Statistics for Biology and Health. In Survival Analysis: A Self-Learning Text, 3rd ed.; 2011; ISBN 1441966455. [Google Scholar]
- Fujikake, I. An Analyses of Harvesting Activities in a Forest Management Entity Using Harvesting Age Distribution (Tentative Translation by Author). Ph.D. Thesis, Kyoto University, Kyoto, Japan, 2000. [Google Scholar]
- Fujikake, I. Estimation of Gentan Probability Based on the Forest Resource Table. Proc. Inst. J. Stat. Math. 2003, 51, 95–109. [Google Scholar] [CrossRef]
- Hiroshima, T. Study on the Methodology of Calculating Mean and Variance of Felling Age in Forest Planning. Jpn. J. For. Plan. 2006, 40, 139–149. [Google Scholar] [CrossRef]
- Tiryana, T.; Tatsuhara, S.; Shiraishi, N. Empirical Models for Estimating the Stand Biomass of Teak Plantations in Java, Indonesia(Multipurpose Forest Management). J. For. Plan. 2011, 16, 35–44. [Google Scholar] [CrossRef]
- Suzuki, T. The Gentan Probability, a Model for the Improvement of the Normal Forest Concept and Forest Planning. In Proceedings of the IUFRO Symposium on Forest Management Planning and Managerial Economics, Tokyo, Japan, 15–19 October 1984; pp. 12–24. [Google Scholar]
- Wollenberg, E.; Edmunds, D.; Buck, L. Using Scenarios to Make Decisions about the Future: Anticipatory Learning for the Adaptive Co-Management of Community Forests. Landsc. Urban Plan. 2000, 47, 65–77. [Google Scholar] [CrossRef]
- Sadamoto, W. Hokkaido Natural Forest Sucession Pattern 1. Hokkaido For. 1970, 261, 349–356. [Google Scholar]
- Zhao, J.; He, C.; Qi, C.; Wang, X.; Deng, H.; Wang, C.; Liu, H.; Yang, L.; Tan, Z. Biomass Increment and Mortality Losses in Tropical Secondary Forests of Hainan, China. J. For. Res. 2019, 30, 647–655. [Google Scholar] [CrossRef]
- Bourque, C.P.A.; Bayat, M.; Zhang, C. An Assessment of Height–Diameter Growth Variation in an Unmanaged Fagus Orientalis-Dominated Forest. Eur. J. For. Res. 2019, 138, 607–621. [Google Scholar] [CrossRef]
- Selvin, S. Statistical Analysis of Epidemiologic Data; Oxford University Press: Oxford, UK, 2004; ISBN 9780199865697. [Google Scholar]
- Thurnher, C.; Klopf, M.; Hasenauer, H. MOSES—A Tree Growth Simulator for Modelling Stand Response in Central Europe. Ecol. Modell. 2017, 352, 58–76. [Google Scholar] [CrossRef]
- Narukawa, Y.; Iida, S.; Tanouchi, H.; Abe, S.; Yamamoto, S.I. State of Fallen Logs and the Occurrence of Conifer Seedlings and Saplings in Boreal and Subalpine Old-Growth Forests in Japan. Ecol. Res. 2003, 18, 267–277. [Google Scholar] [CrossRef]
- Kubota, Y.; Hara, T. Allometry and Competition between Saplings of Picea Jezoensis and Abies Sachalinensis in a Sub-Boreal Coniferous Forest, Northern Japan. Ann. Bot. 1996, 77, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Whitmore, T.C. Canopy Gaps and the Two Major Groups of Forest Trees. Ecol. Soc. Am. 2009, 70, 536–538. [Google Scholar] [CrossRef]
- Takahashi, M.; Takao, G.; Ishibashi, S.; Kawahara, T. Evaluation of Landscape-Level Genetic Diversity in Mixed Managed Forest in Hokkaido, Japan. J. For. Plan. 2011, 16, 215–221. [Google Scholar]
- Lähde, E.; Laiho, O.; Norokorpi, Y.; Saksa, T. The Structure of Advanced Virgin Forests in Finland. Scand. J. For. Res. 1990, 6, 527–537. [Google Scholar] [CrossRef]
- Hoernberg, G. Boreal Old-Growth Picea Abies Swamp-Forests in Sweden—Disturbance History, Structure and Regeneration Patterns-Thesis; Sveriges Lantbruksuniversitet: Uppsala, Sweden, 1996. [Google Scholar]
- Zackrisson, O. Influence of Forest Fires on the North Swedish Boreal Forest. Oikos 1977, 29, 22–32. [Google Scholar] [CrossRef]
- Yamamoto, H. Natural Forest Management Based on Selection Cutting and Natural Regeneration. In Proceedings of the IUFRO International Workshop on Sustainable Forest Managements, Furano, Japan, 17–21 October 1994; pp. 10–22. [Google Scholar]








| Plot Number | Plot Size (ha) | Elevation (m) | No. of Target Trees in Period 3 | Slope Aspect | Mean Slope Angle (°) |
|---|---|---|---|---|---|
| 5203 | 0.40 | 580 | 327 | Southwest | 15 |
| 5224 | 0.25 | 570 | 150 | Southwest | 18 |
| 5225 | 0.25 | 690 | 237 | Southwest | 20 |
| 5240 | 0.25 | 600 | 214 | Southwest | 25 |
| Period | Tree Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. sachalinensis | P. jezoensis | All Broad-Leaved Species | ||||||||||
| Log-Rank Test | Wilcoxon Test | Log-rRank Test | Wilcoxon Test | Log-Rank Test | Wilcoxon Test | |||||||
| p-Value | Chi-Square Value | p-Value | Chi-Square Value | p-Value | Chi-Square Value | p-Value | Chi-Square Value | p-Value | Chi-Square Value | p-Value | Chi-Square Value | |
| Periods 1 and 2 | 0.9161 | 0.0111 | 0.6542 | 0.2007 | 0.5023 | 0.4502 | 0.3674 | 0.8124 | 0.0194 * | 5.4637 | 0.0772 | 3.1236 |
| Periods 2 and 3 | 0.1034 | 2.6524 | 0.5685 | 0.3252 | 0.1538 | 2.0337 | 0.2549 | 1.2961 | 0.2691 | 1.2215 | 0.0610 | 3.5101 |
| Tree Category | No. of Trees (%) | |
|---|---|---|
| Living Trees | Dead Trees | |
| Conifer | ||
| P. jezoensis | 117 (12.62) | 22 (2.37) |
| A. sachalinensis | 219 (23.62) | 41 (4.42) |
| Broad-leaved | 429 (46.28) | 99 (10.68) |
| Total | 765 (82.52) | 162 (17.48) |
| Weibull Parameter | P. jezoensis | A. sachalinensis | Broad-Leaved | All Trees |
|---|---|---|---|---|
| m | 6.7133 | 7.3496 | 6.0360 | 6.8872 |
| k | 1.1600 | 1.3525 | 0.7279 | 0.9144 |
| Mean | 6.3732 | 6.7374 | 7.3722 | 7.1851 |
| Standard Deviation | 5.5101 | 5.0359 | 10.3135 | 7.5409 |
| Tree Category | Period 2 | |
|---|---|---|
| RMSE Living Trees | RMSPE (%) Living Trees | |
| P. jezoensis | 1.20 | 23.31 |
| A. sachalinensis | 1.30 | 8.96 |
| Broad-leaved | 4.91 | 36.67 |
| All trees | 6.28 | 17.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijenayake, P.R.; Hiroshima, T.; Takahashi, M.; Saito, H. Predicting the Future Age Distribution of Conifer and Broad-Leaved Trees Based on Survival Analysis: A Case Study on Natural Forests in Northern Japan. Forests 2022, 13, 1912. https://doi.org/10.3390/f13111912
Wijenayake PR, Hiroshima T, Takahashi M, Saito H. Predicting the Future Age Distribution of Conifer and Broad-Leaved Trees Based on Survival Analysis: A Case Study on Natural Forests in Northern Japan. Forests. 2022; 13(11):1912. https://doi.org/10.3390/f13111912
Chicago/Turabian StyleWijenayake, Pavithra Rangani, Takuya Hiroshima, Masayoshi Takahashi, and Hideki Saito. 2022. "Predicting the Future Age Distribution of Conifer and Broad-Leaved Trees Based on Survival Analysis: A Case Study on Natural Forests in Northern Japan" Forests 13, no. 11: 1912. https://doi.org/10.3390/f13111912





