Studies of the Photoprotection of Radiata Pine Wood Using Photocatalytic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Photoactivity Tests on Nanoparticles
2.3. Treatment of Wood Samples with Nanoparticles
2.4. UV Exposure
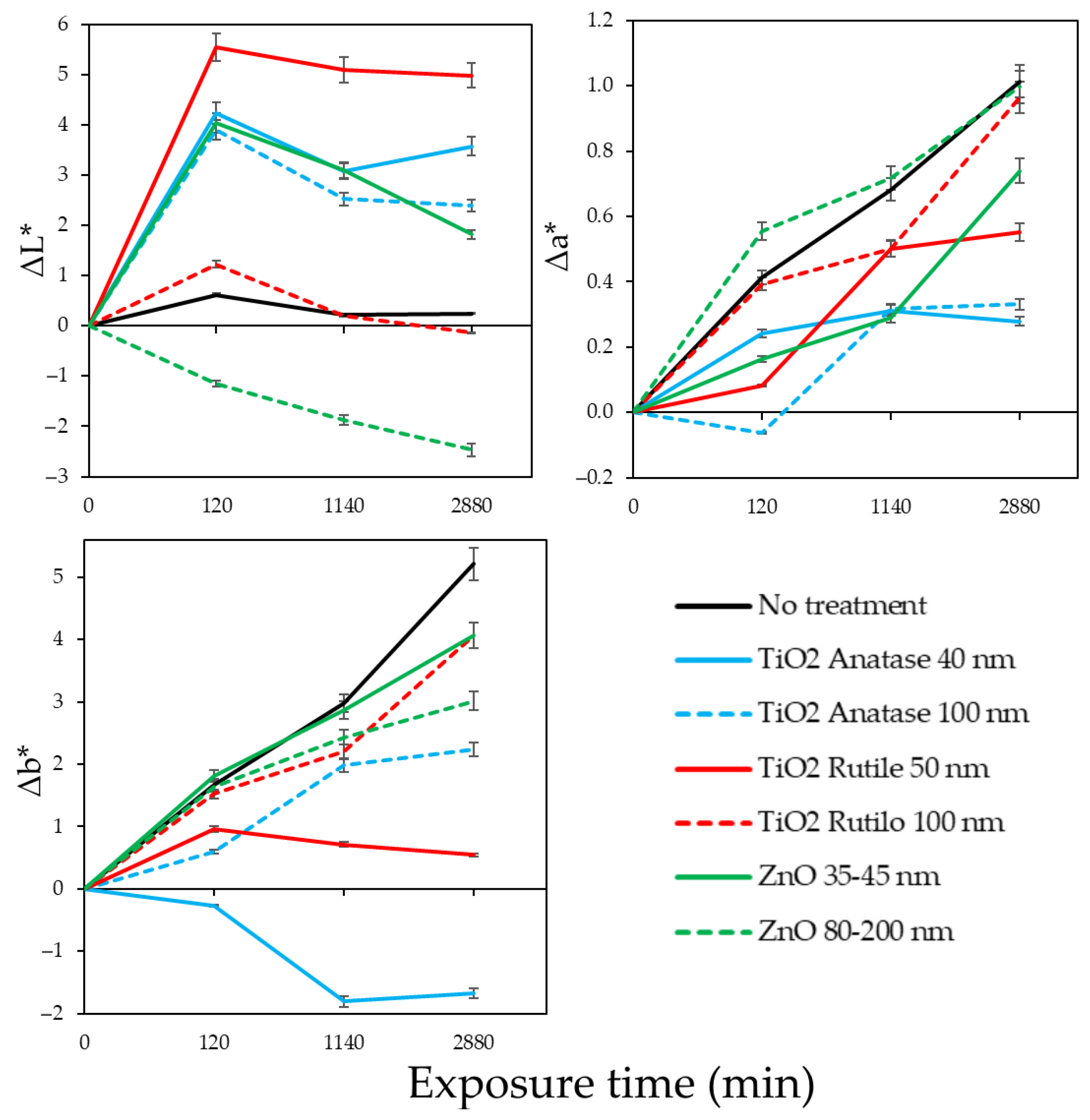
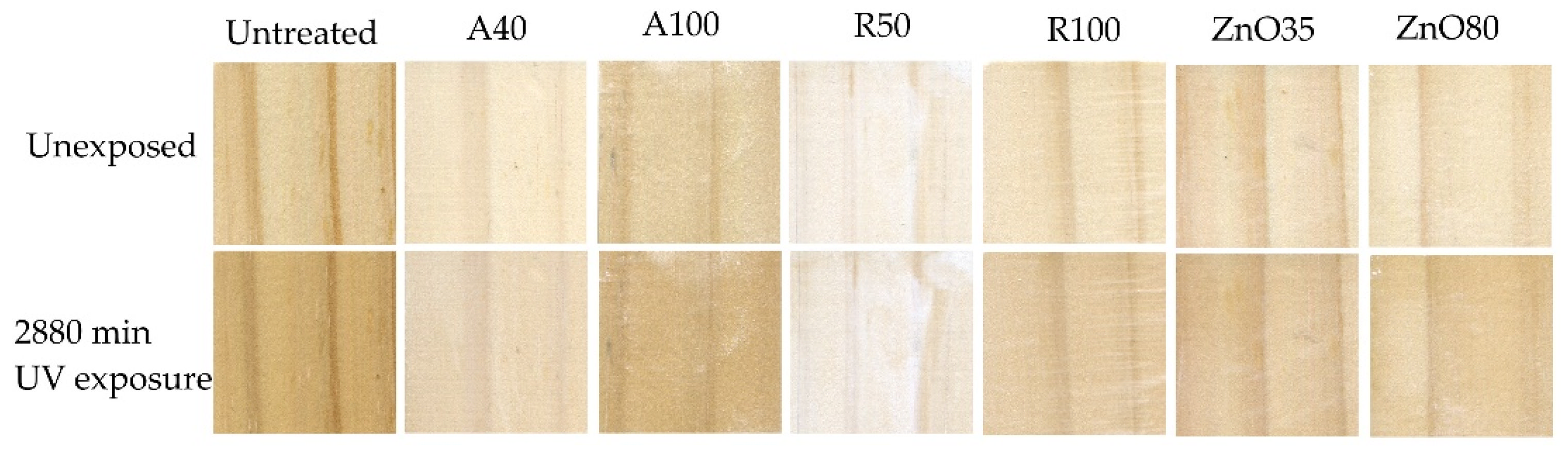
2.5. Wood Surface Color Change Measurements
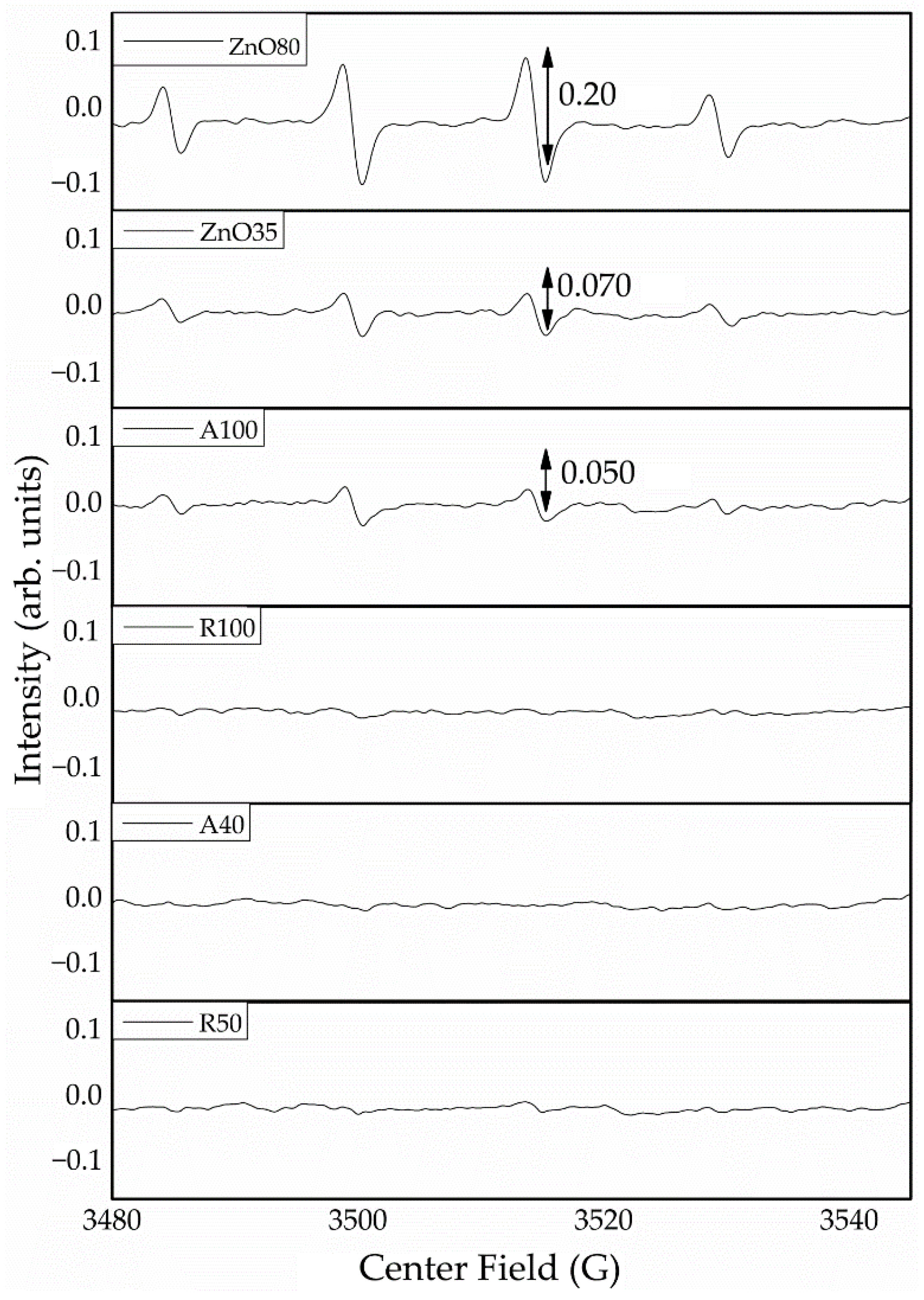
2.6. Chemical Changes on Wood Surfaces
2.7. UV Absorbance of Exposed Surfaces
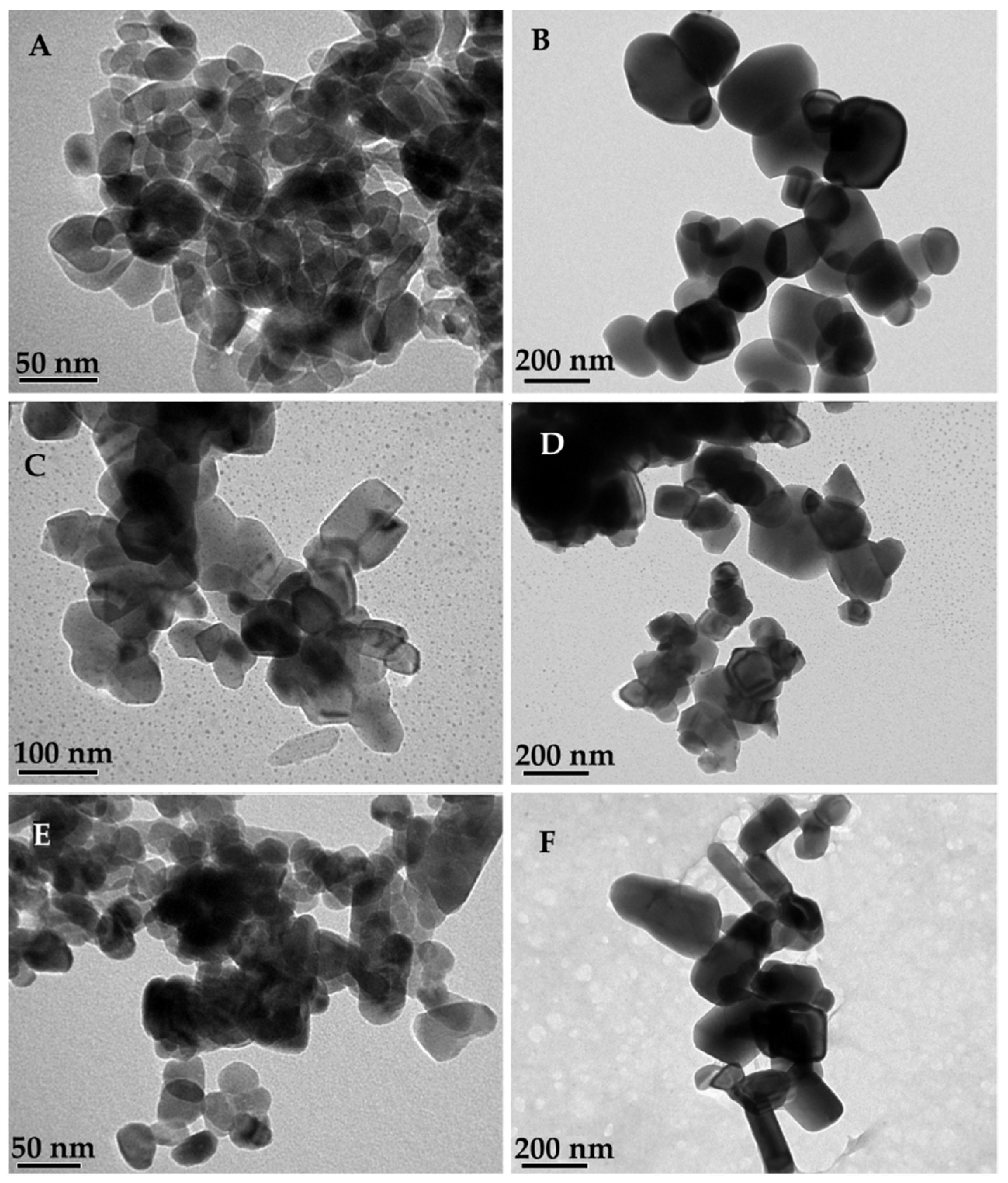
2.8. Transmission Electron Microscopy and Scanning Electron Microscopy
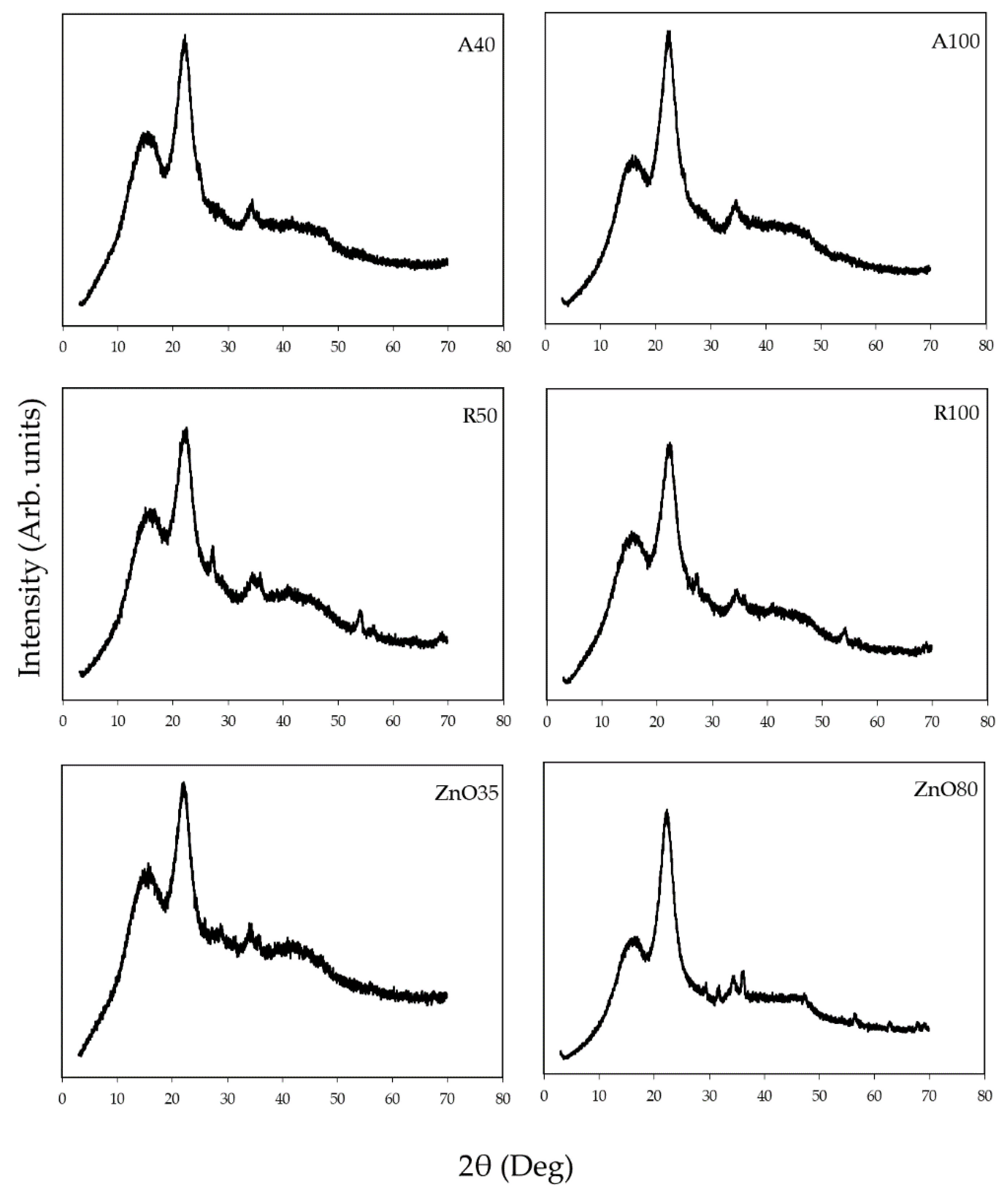
2.9. X-ray Diffraction Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feist, W.C.; Hon, D.N.S. Chemistry of Weathering and Protection. In The Chemistry of Solid Wood; American Chemical Society: Washington, DC, USA, 1984; pp. 401–451. [Google Scholar]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and Photostabilisation of Wood–the State of the Art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Evans, P.D. Weathering and Photoprotection of Wood. In Development of Commercial Wood Preservatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 69–117. [Google Scholar]
- Derbyshire, H.; Miller, E.R. The Photodegradation of Wood during Solar Irradiation. Holz Als Roh- Und Werkstoff 1981, 39, 341–350. [Google Scholar] [CrossRef]
- Diffey, B.L. Solar Ultraviolet Radiation Effects on Biological Systems. Phys. Med. Biol. 1991, 36, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.D.; Haase, J.G.; Seman, A.; Kiguchi, M. The Search for Durable Exterior Clear Coatings for Wood. Coatings 2015, 5, 830–864. [Google Scholar] [CrossRef] [Green Version]
- Jirouš-Rajković, V.; Miklečić, J. Enhancing Weathering Resistance of Wood—A Review. Polymers 2021, 13, 1980. [Google Scholar] [CrossRef]
- Petrič, M. Surface Modification of Wood: A Critical Review. Rev. Adh. Adhes. 2013, 1, 216–247. [Google Scholar] [CrossRef]
- Williams, R.S.; Feist, W.C. Wood Modified by Inorganic Salts: Mechanism and Properties. I. Weathering Rate, Water Repellency, and Dimensional Stability of Wood Modified with Chromium (III) Nitrate versus Chromic Acid. Wood Fiber Sci. 1985, 17, 184–198. [Google Scholar]
- Deka, M.; Humar, M.; Rep, G.; Kričej, B.; Šentjurc, M.; Petrič, M. Effects of UV Light Irradiation on Colour Stability of Thermally Modified, Copper Ethanolamine Treated and Non-Modified Wood: EPR and DRIFT Spectroscopic Studies. Wood Sci. Technol. 2008, 42, 5–20. [Google Scholar] [CrossRef]
- Zhu, Y.; Evans, P.D. Surface Protection of Wood with Metal Acetylacetonates. Coatings 2021, 11, 916. [Google Scholar] [CrossRef]
- Schaller, C.; Rogez, D. New Approaches in Wood Coating Stabilization. J. Coat. Technol. Res. 2007, 4, 401–409. [Google Scholar] [CrossRef]
- Evans, P.D.; Chowdhury, M. Photoprotection of Wood Using Polyester-Type UV-Absorbers Derived from the Reaction of 2-Hydroxy-4(2,3-Epoxypropoxy)-Benzophenone with Dicarboxylic Acid Anhydrides. J. Wood Chem. Technol. 2010, 30, 186–204. [Google Scholar] [CrossRef]
- Schauwecker, C.F.; McDonald, A.G.; Preston, A.F.; Morrell, J.J. Use of Iron Oxides to Influence the Weathering Characteristics of Wood Surfaces: A Systematic Survey of Particle Size, Crystal Shape and Concentration. Eur. J. Wood Prod. 2014, 72, 669–680. [Google Scholar] [CrossRef]
- Blackburn, S.R.; Meldrum, B.J.; Clayton, J. The Use of Fine Particle Titanium Dioxide for UV Protection in Wood Finishes. Faerg Och Lack Scand. 1991, 37, 192–196. [Google Scholar]
- Zanatta, P.; Lazarotto, M.; Gonzalez de Cademartori, P.; Cava, S.; Moreira, M.; Gatto, D. The Effect of Titanium Dioxide Nanoparticles Obtained by Microwave-Assisted Hydrothermal Method on the Color and Decay Resistance of Pinewood. Maderas Cienc. Tecnol. 2017, 19, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Fufa, S.M.; Jelle, B.P.; Hovde, P.J. Effects of TiO2 and Clay Nanoparticles Loading on Weathering Performance of Coated Wood. Prog. Org. Coat. 2013, 76, 1425–1429. [Google Scholar] [CrossRef]
- Egerton, T.A. UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences. Molecules 2014, 19, 18192–18214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Role of Nanoparticles in Photocatalysis. J. Nanopart. Res. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Brezová, V.; Gabčová, S.; Dvoranová, D.; Staško, A. Reactive Oxygen Species Produced upon Photoexcitation of Sunscreens Containing Titanium Dioxide (an EPR Study). J. Photochem. Photobiol. B Biol. 2005, 79, 121–134. [Google Scholar] [CrossRef]
- Gladis, F.; Schumann, R. A Suggested Standardised Method for Testing Photocatalytic Inactivation of Aeroterrestrial Algal Growth on TiO2-Coated Glass. Int. Biodeterior. Biodegrad. 2011, 65, 415–422. [Google Scholar] [CrossRef]
- Jain, A.; Vaya, D.; Jain, A.; Vaya, D. Photocatalytic Activity of TiO2 Nanomaterials. J. Chil. Chem. Soc. 2017, 62, 3683–3690. [Google Scholar] [CrossRef] [Green Version]
- Nevárez-Martínez, M.; Espinoza-Montero, P.; Quiroz-Chávez, F.; Ohtani, B. Fotocatálisis: Inicio, actualidad y perspectivas a través del TiO2. Av. En Quim. 2017, 12, 45–59. [Google Scholar]
- Hughes, W. Photodegradation of Paint Films Containing TiO2 Pigments; Verlag Chemie GmbH: Weinheim/Bergst, Germany, 1970; pp. 67–82. [Google Scholar]
- Allen, N.S.; McKellar, J.F.; Phillips, G.O.; Chapman, C.B. The TiO2 Photosensitized Degradation of Nylon 6, 6: Stabilizing Action of Manganese Ions. J. Polym. Sci. Polym. Lett. Ed. 1974, 12, 723–727. [Google Scholar] [CrossRef]
- Tsuzuki, T.; He, R.; Wang, J.; Sun, L.; Wang, X.; Hocking, R. Reduction of the Photocatalytic Activity of ZnO Nanoparticles for UV Protection Applications. Int. J. Nanotechnol. 2012, 9, 1017–1029. [Google Scholar] [CrossRef]
- Xiao, L.; Youji, L.; Feitai, C.; Peng, X.; Ming, L. Facile Synthesis of Mesoporous Titanium Dioxide Doped by Ag-Coated Graphene with Enhanced Visible-Light Photocatalytic Performance for Methylene Blue Degradation. RSC Adv. 2017, 7, 25314–25324. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Guo, B.; Yi, Z.; Wu, X.; Zhang, J.; Yang, H. Morphology Modulation of Hollow-Shell ZnSn(OH)6 for Enhanced Photodegradation of Methylene Blue. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129908. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Xu, P.; Tang, S.; Liu, C. Efficient Photocatalytic Degradation of Acid Orange 7 over N-Doped Ordered Mesoporous Titania on Carbon Fibers under Visible-Light Irradiation Based on Three Synergistic Effects. Appl. Catal. A Gen. 2016, 524, 163–172. [Google Scholar] [CrossRef]
- Hernandez, V.A.; Morales, C.; Sagredo, N.; Perez-Gonzalez, G.; Romero, R.; Contreras, D. Radical Species Production and Color Change Behavior of Wood Surfaces Treated with Suppressed Photoactivity and Photoactive TiO2 Nanoparticles. Coatings 2020, 10, 1033. [Google Scholar] [CrossRef]
- Numano, T.; Xu, J.; Futakuchi, M.; Fukamachi, K.; Alexander, D.B.; Furukawa, F.; Kanno, J.; Hirose, A.; Tsuda, H.; Suzui, M. Comparative Study of Toxic Effects of Anatase and Rutile Type Nanosized Titanium Dioxide Particles in Vivo and in Vitro. Asian Pac. J. Cancer Prev. 2014, 15, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, I.; Piette, L.H. ESR Spin-Trapping Studies on the Reaction of Fe2+ Ions with H2O2-Reactive Species in Oxygen Toxicity in Biology. J. Biol. Chem. 1990, 265, 13589–13594. [Google Scholar] [CrossRef]
- Hernandez, V.A.; Evans, P.D. Technical Note: Melanization of the Wood-Staining Fungus Aureobasidium pullulans in Response to UV Radiation. Wood Fiber Sci. 2015, 47, 1–5. [Google Scholar]
- Kropat, M.; Hubbe, M.A.; Laleicke, F. Natural, Accelerated, and Simulated Weathering of Wood: A Review. Bioresources 2020, 15, 9998–10062. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Harrington, K.J.; Higgins, H.G.; Michell, A.J. Infrared Spectra of Eucalyptus regnans F. Muell and Pinus Radiata D. Don. Holzforschung 1964, 18, 108–113. [Google Scholar] [CrossRef]
- Lionetto, F.; Del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring Wood Degradation during Weathering by Cellulose Crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef]
- Paulsson, M.; Parkas, J. Review: Light-Induced Yellowing of Lignocellulosic Pulps–Mechanisms and Preventive Methods. Bioresources 2012, 7, 5995–6040. [Google Scholar] [CrossRef] [Green Version]
- Gratzl, J. Lichtinduzierte Vergilbung von Zellstoffen: Ursachen Und Verhütung. Papier 1985, 39, 14–23. [Google Scholar]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of Water Resistance, Dimensional Stability, and Mechanical Properties of Poplar Wood by Rosin Impregnation. Eur. J. Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Zhu, X.; Kong, X.-Z. Preparation of Hollow TiO2 Nanoparticles through TiO2 Deposition on Polystyrene Latex Particles and Characterizations of Their Structure and Photocatalytic Activity. Nanoscale Res. Lett. 2012, 7, 646. [Google Scholar] [CrossRef] [Green Version]
- Khan, Y.A.; Singh, B.J.; Ullah, R.; Shoeb, M.; Naqvi, A.H.; Abidi, S.M.A. Anthelmintic Effect of Biocompatible Zinc Oxide Nanoparticles (ZnO NPs) on Gigantocotyle explanatum, a Neglected Parasite of Indian Water Buffalo. PLoS ONE 2015, 10, e0133086. [Google Scholar] [CrossRef] [Green Version]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.W.; Keller, A.A. Comparative Photoactivity of CeO2, γ-Fe2O3, TiO2 and ZnO in Various Aqueous Systems. Appl. Catal. B 2011, 102, 600–607. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Colvin, V.L. Photoactivity Tests of TiO2 and ZnO Sunscreen Ingredients. MRS Online Proc. Libr. 2012, 1413, 1–6. [Google Scholar] [CrossRef]
- Lanzalunga, O.; Bietti, M. Photo- and Radiation Chemical Induced Degradation of Lignin Model Compounds. J. Photochem. Photobiol. B Biol. 2000, 56, 85–108. [Google Scholar] [CrossRef]
- Fabbri, C.; Bietti, M.; Lanzalunga, O. Generation and Reactivity of Ketyl Radicals with Lignin Related Structures. On the Importance of the Ketyl Pathway in the Photoyellowing of Lignin Containing Pulps and Papers. J. Org. Chem. 2005, 70, 2720–2728. [Google Scholar] [CrossRef]
- Williams, R.S. Weathering of Wood. In Handbook of Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; pp. 139–185. [Google Scholar]
- Hon, D.N.S. Photochemical Degradation of Lignocellulosic Materials. In Developments in Polymer Degradation–3; Essex: Applied Science Ltd.: London, UK, 1981; pp. 229–281. [Google Scholar]
- Huang, Y.; Pagé, D.; Wayner, D.D.; Mulder, P. Radical-Induced Degradation of a Lignin Model Compound. Decomposition of 1-Phenyl-2-Phenoxyethanol. Can. J. Chem. 1995, 73, 2079–2085. [Google Scholar] [CrossRef]
- Hon, D.N.S.; Chang, S.T.; Feist, W.C. Participation of Singlet Oxygen in the Photodegradation of Wood Surfaces. Wood Sci. Technol. 1982, 16, 193–201. [Google Scholar] [CrossRef]
- Hon, D.N.S.; Feist, W.C. Hydroperoxidation in Photoirradiated Wood Surfaces. Wood Fiber Sci. 1992, 24, 448–455. [Google Scholar]
- Evans, P.D. Weathering of Wood and Wood Composites. In Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2013; pp. 151–200. [Google Scholar]
- Hon, D.N.S.; Ifju, G.; Feist, W.C. Characteristics of Free Radicals in Wood. Wood Fiber 1980, 12, 121–130. [Google Scholar]
- Baur, S.I.; Easteal, A.J. ESR Studies on the Free Radical Generation in Wood by Irradiation with Selected Sources from UV to IR Wavelength Regions. Holzforschung 2014, 68, 775–780. [Google Scholar] [CrossRef]
- Hon, D.N.S.; Ifju, G. Measuring Penetration of Light into Wood by Detection of Photo-Induced Free Radicals. Wood Sci. 1978, 11, 118–127. [Google Scholar]
- Backa, S.; Gierer, J.; Reitberger, T.; Nilsson, T. Hydroxyl Radical Activity in Brown-Rot Fungi Studied by a New Chemiluminescence Method. Holzforschung 1992, 46, 61–67. [Google Scholar] [CrossRef]
- Tanaka, H.; Itakura, S.; Enoki, A. Hydroxyl Radical Generation by an Extracellular Low-Molecular-Weight Substance and Phenol Oxidase Activity during Wood Degradation by the White-Rot Basidiomycete Trametes versicolor. J. Biotechnol. 1999, 75, 57–70. [Google Scholar] [CrossRef]
- Stafford, U.; Gray, K.A.; Kamat, P.V.; Varma, A. An in Situ Diffuse Reflectance FTIR Investigation of Photocatalytic Degradation of 4-Chlorophenol on a TiO2 Powder Surface. Chem. Phys. Lett. 1993, 205, 55–61. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Nair, S.; Nagarajappa, G.S.; Pandey, K.K. UV Stabilization of Wood by Nano Metal Oxides Dispersed in Propylene Glycol. J. Photochem. Photobiol. B Biol. 2018, 183, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yoshikawa, A.; Sandhu, A. Wide Bandgap Semiconductors: Fundamental Properties and Modern Photonic and Electronic Devices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-47235-3. [Google Scholar]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Analysis of Electronic Structures of 3d Transition Metal-Doped TiO2 Based on Band Calculations. J. Phys. Chem. Solids 2002, 63, 1909–1920. [Google Scholar] [CrossRef]
- Tanemura, S.; Miao, L.; Wunderlich, W.; Tanemura, M.; Mori, Y.; Toh, S.; Kaneko, K. Fabrication and Characterization of Anatase/Rutile–TiO2 Thin Films by Magnetron Sputtering: A Review. Sci. Technol. Adv. Mater. 2005, 6, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.W.; Kwok, H.S. Optical Properties of Epitaxially Grown Zinc Oxide Films on Sapphire by Pulsed Laser Deposition. J. Appl. Phys. 1999, 86, 408–411. [Google Scholar] [CrossRef]
- Lee, G.H.; Kawazoe, T.; Ohtsu, M. Difference in Optical Bandgap between Zinc-Blende and Wurtzite ZnO Structure Formed on Sapphire (0001) Substrate. Solid State Commun. 2002, 124, 163–165. [Google Scholar] [CrossRef]
- Popov, A.P.; Zvyagin, A.V.; Lademann, J.; Roberts, M.S.; Sanchez, W.; Priezzhev, A.V.; Myllylä, R. Designing Inorganic Light-Protective Skin Nanotechnology Products. J. Biomed. Nanotechnol. 2010, 6, 432–451. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Shokri, M.; Elham, H.; Zeininezhad, A. The Effect of Particle Size and Crystal Structure of Titanium Dioxide Nanoparticles on the Photocatalytic Properties. J. Environ. Sci. Health A 2008, 43, 460–467. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, V.A.; Romero, R.; Sagredo, N.; Contreras, D.; Evans, P.D. Studies of the Photoprotection of Radiata Pine Wood Using Photocatalytic Nanoparticles. Forests 2022, 13, 1922. https://doi.org/10.3390/f13111922
Hernandez VA, Romero R, Sagredo N, Contreras D, Evans PD. Studies of the Photoprotection of Radiata Pine Wood Using Photocatalytic Nanoparticles. Forests. 2022; 13(11):1922. https://doi.org/10.3390/f13111922
Chicago/Turabian StyleHernandez, Vicente A., Romina Romero, Nicole Sagredo, David Contreras, and Philip D. Evans. 2022. "Studies of the Photoprotection of Radiata Pine Wood Using Photocatalytic Nanoparticles" Forests 13, no. 11: 1922. https://doi.org/10.3390/f13111922
APA StyleHernandez, V. A., Romero, R., Sagredo, N., Contreras, D., & Evans, P. D. (2022). Studies of the Photoprotection of Radiata Pine Wood Using Photocatalytic Nanoparticles. Forests, 13(11), 1922. https://doi.org/10.3390/f13111922







