Simulation of Episodic Winter Warming on Dehardening of Boreal Forest Seedlings in Northern Forest Nurseries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Frost Tolerance of Black and White Spruce Seedlings at the End of the Fall
2.2.1. Electrical Conductivity and Index of Injury of Excised Apical Shoot Tips in Response to Different Artificial Freezing Temperatures
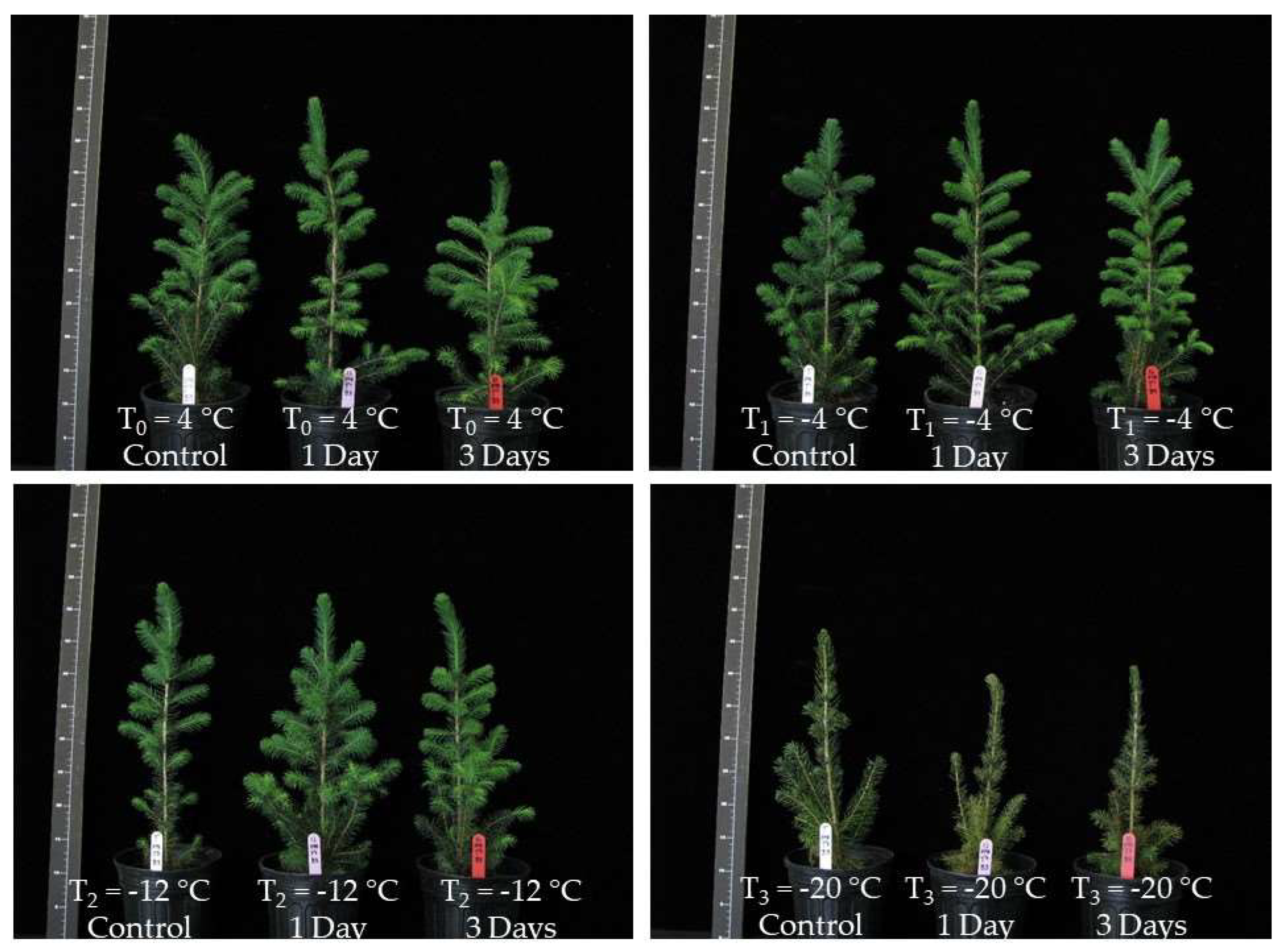
2.2.2. Growth of Seedlings, Bud Burst, and Initiation of New Roots in the Case of Whole-Seedling Freeze Treatments
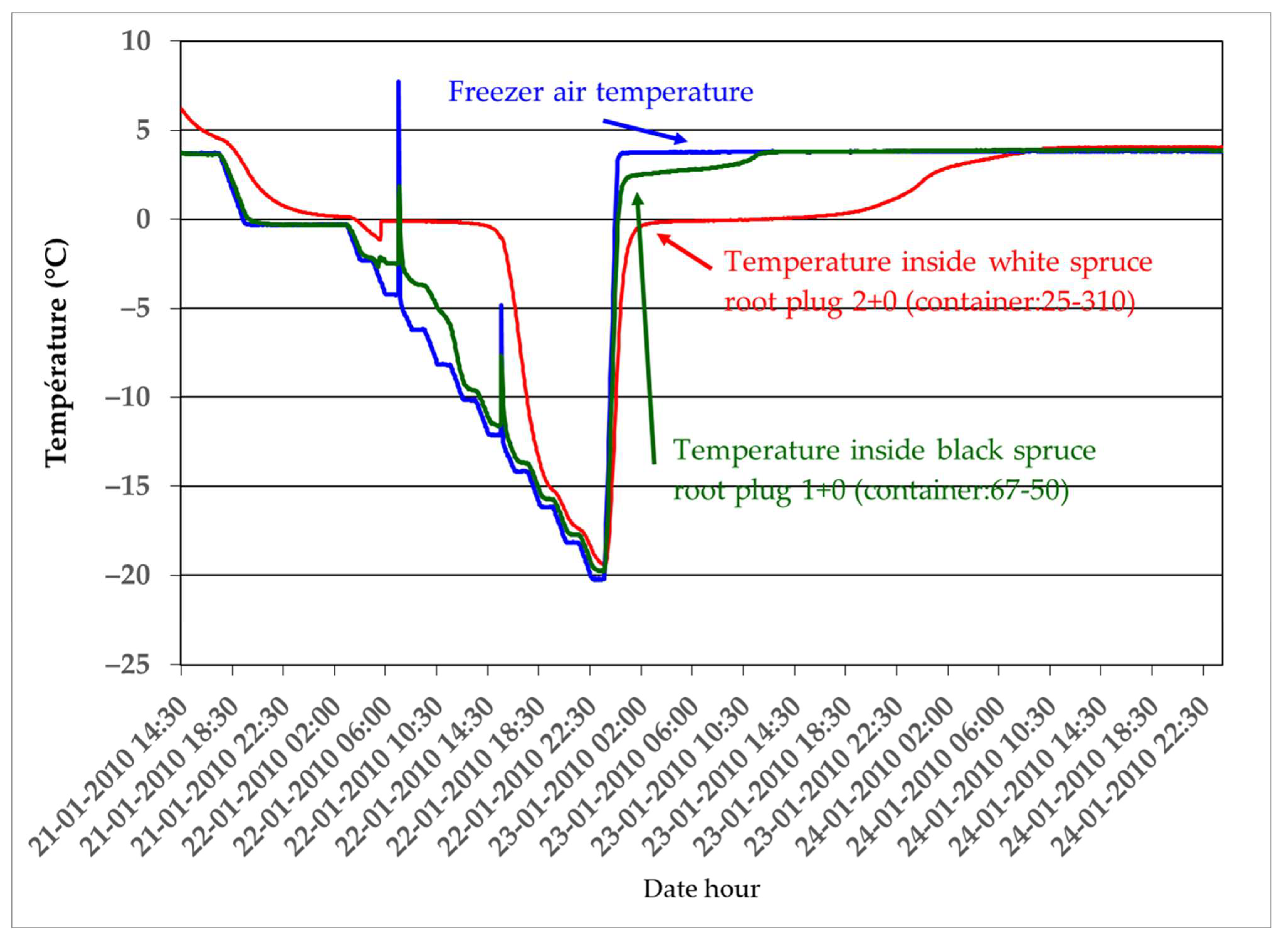
2.3. Simulation of Different Periods of Warm Weather and Their Effects on the Dehardening, Growth, and Mineral Nutrition of Black Spruce (1 + 0) and White Spruce (2 + 0) Seedlings at the Beginning and End of Winter
- -
- Treatment 1 (TR1): Control seedlings were kept in a cold room at 4 °C in the dark (thus simulating light conditions under a relatively deep snow cover).
- -
- Treatment 2 (TR2): Seedlings were placed for 1 day in a greenhouse at 10 °C under winter natural photoperiod conditions.
- -
- Treatment 3 (TR3): Seedlings were placed for 3 days in a greenhouse at 10 °C under winter natural photoperiod conditions.
- -
- -
- Electrical conductivity and an index of injury of excised terminal shoot tips of black spruce and white spruce seedlings in response to different artificial freezing temperatures (28 seedlings total; 3 shoot tips per Erlenmeyer flask per temperature per block distributed in four complete random blocks).
- -
- Artificial freezing of whole seedlings for the evaluation of growth variables (dry shoot dry mass, initial roots and new roots, total dry mass), bud burst and recovery (32 seedlings total per treatment per species at the rate of 2 seedlings per temperature per block distributed in four complete random blocks).
2.4. Mineral Nutrition
2.5. Statistical Analyses
3. Results
3.1. Assessment of Frost Tolerance of One-Year-Old Black Spruce (1 + 0) and Two-Year-Old White Spruce (2 + 0) Seedlings at the End of the Fall
3.2. Initiation of New Roots and Bud Break in the Case of Whole-Seedling Freeze Treatments at the End of the Fall
3.3. Frost Tolerance of Black Spruce (1 + 0) and White Spruce (2 + 0) Seedlings in Response to Different Periods of Warm Weather at the Beginning and End of Winter
3.3.1. Determination of Shoot Cold Tolerance of Black Spruce and White Spruce Using Electrolyte Conductivity Measurements in Response to Different Periods of Warm Weather at the Beginning and End of Winter
3.3.2. Determination of Whole-Seedling Freezing Temperature Effects on the Growth of New Roots, Shoot, and Bud Breaks in Response to Different Periods of Warm Weather at the Beginning and End of Winter
3.3.3. Mineral Nutrition in Autumn, Winter and towards the End of Winter in Response to Warming Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigras, F.J.; Colombo, S.J. (Eds.) Conifer Cold Hardiness; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; 596p. [Google Scholar]
- Colombo, S.J. Frost hardening spruce container stock for overwintering in Ontario. New For. 1997, 13, 449–467. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Veilleux, L.; Renaud, M. Élaboration des seuils de tolérance au gel des plants d’épinette blanche (1 + 0) en pépinière forestière selon les régions écologiques du Québec. Gouvernement du Québec, Ministère des Ressources naturelles. Mémoire De Rech. For. 2005, 147, 50. [Google Scholar]
- Lamhamedi, M.S.; Veilleux, L.; Renaud, M.; Desjardins, P. Prédiction et détermination des seuils de tolérance au gel en automne et techniques de protection contre le gel hivernal. In Production de Plants Forestiers au Québec: La Culture de L’innovation, Proceedings of the Colloque de Transfert de Connaissances et de Savoir-Faire, Carrefour Forêt Innovations, Québec, QC, Canada, 4–6 October 2011; Colas, F., Lamhamedi, M.S., Eds.; Gouvernement du Québec: Québec, QC, Canada, 2011; pp. 53–64. [Google Scholar]
- MFFP. Insectes, Maladies et Feux Dans les Forêts du Québec en 2021; Gouvernement du Québec: Québec, QC, Canada, 2022; pp. 1–70.
- Joly, E.; Les Serres coopératives de Guyenne, Guyenne, Québec, Canada. Personal communication, 2022.
- Bigras, F.J.; Ryyppö, A.; Lindström, A.; Stattin, E. Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 57–88. [Google Scholar]
- Colombo, S.J.; Menzies, M.I.; O’Reilly, C. Influence of nursery cultural practices on cold hardiness of coniferous forest tree seedlings. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 223–252. [Google Scholar]
- Margolis, H.A. Seedling Production and Reforestation in Québec. J. For. 1987, 85, 39–43. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; pp. 1–151. [Google Scholar]
- South, D.B.; Starkey, T.; Enebak, S. Warm Nighttime Temperatures (with Tents) Affect Terminal Buds of Loblolly Pine Seedlings; RR 09-04; Southern Forest Nursery Management Cooperative, Auburn University: Auburn, AL, USA, 2009; pp. 1–7. [Google Scholar]
- South, D.B.; Starkey, T.; Islam, M.A.; Jacobs, D.F. Warm Night Time Temperatures Affect the Ability of Loblolly Pine (Pinus taeda) Seedlings to Tolerate Freezing; RR 08-02; Southern Forest Nursery Management Cooperative, Auburn University: Auburn, AL, USA, 2008; pp. 1–10. [Google Scholar]
- Taulavuori, K.M.J.; Laine, K.; Taulavuori, E.B. Artificial deacclimation response of Vaccinium myrtillus in mid-winter. Ann. Bot. Fenn. 2002, 39, 143–147. [Google Scholar]
- Cunningham, S.M.; Nadeau, P.; Castonguay, Y.; Laberge, S.; Volenec, J.J. Raffinose and stachyose accumulation, galactinol synthase expression, and winter injury of contrasting alfalfa germplasms. Crop Sci. 2003, 43, 562–570. [Google Scholar] [CrossRef]
- Kalberer, S.R.; Wisniewski, M.; Arora, R. Deacclimation and reacclimation of cold-hardy plants: Current understanding and emerging concepts. Plant Sci. 2006, 171, 3–16. [Google Scholar] [CrossRef]
- Ögren, E. Relationship between temperature, respiratory loss of sugar and premature dehardening in dormant Scots pine seedlings. Tree Physiol. 1997, 17, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Strimbeck, G.R.; Schaberg, P.G.; DeHayes, D.H.; Shane, J.B.; Hawley, G.J. Midwinter dehardening of montane red spruce during a natural thaw. Can. J. For. Res. 1995, 25, 2040–2044. [Google Scholar] [CrossRef] [Green Version]
- Man, R.; Lu, P.; Dang, Q.-L. Cold tolerance of black spruce, white spruce, jack pine, and lodgepole pine seedlings at different stages of spring dehardening. New For. 2021, 52, 317–328. [Google Scholar] [CrossRef]
- Man, R.; Colombo, S.; Lu, P.; Dang, Q.-L. Effects of winter warming on cold hardiness and spring budbreak of four boreal conifers. Botany 2016, 94, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Man, R.; Lu, P.; Dang, Q.-L. Cold hardiness of white spruce, black spruce, jack pine, and lodgepole pine needles during dehardening. Can. J. For. Res. 2017, 47, 1116–1122. [Google Scholar] [CrossRef]
- Pike, C.C.; Warren, J.C.; Montgomery, R.A. Effects of artificial warming during quiescence on budbreak and growth of white spruce, Picea glauca. Can. J. For. Res. 2017, 47, 1538–1545. [Google Scholar] [CrossRef] [Green Version]
- Sevanto, S.; Suni, T.; Pumpanen, J.; Grönholm, T.; Kolari, P.; Nikinmaa, E.; Hari, P.; Vesala, T. Wintertime photosynthesis and water uptake in a boreal forest. Tree Physiol. 2006, 26, 749–757. [Google Scholar] [CrossRef] [PubMed]
- MFFP. Guide de Terrain. Inventaire de Qualification des Plants Résineux Cultivés en Récipients; Gouvernement du Québec: Québec, QC, Canada, 2019; p. 114.
- Burns, R.M.; Honkala, B.H. Silvics of North America: 1. Conifers; Agriculture Handbook 654; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; 675p.
- Lamhamedi, M.S.; Labbé, L.; Margolis, H.A.; Stowe, D.C.; Blais, L.; Renaud, M. Spatial variability of substrate water content and growth of white spruce seedlings. Soil Sci. Soc. Am. J. 2006, 70, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Lamhamedi, M.S.; Renaud, M.; Desjardins, P.; Veilleux, L. Root growth, plug cohesion, mineral nutrition, and carbohydrate content of (1 + 0) Picea mariana seedlings in response to a short-day treatment. Tree Plant. Notes 2013, 56, 35–46. [Google Scholar]
- Goulet, S. Utilisation du Canon à Neige en Conditions Opérationnelles. In Proceedings of the Colloque Forestier, Office des Producteurs de Plants Forestiers du Québec, Québec, QC, Canada, 23–24 February 2016; pp. 1–38. [Google Scholar]
- Lamhamedi, M.S. Mesures d’adaptation aux changements climatiques: Cas des extrêmes hivernaux dans les pépinières forestières du Québec. Ministère des Ressources naturelles, Direction de la recherche forestière. Avis Rech. For. 2012, 42, 1–2. [Google Scholar]
- Lamhamedi, M.S.; Renaud, M.; Auger, I.; Fortin, J.A. Granular calcite stimulates natural mycorrhization and growth of white spruce seedlings in peat-based substrates in forest nursery. Microorganisms 2020, 8, 1088. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Renaud, M.; Auger, I. Granular Calcite: A New Cultural Practice for the Improvement of the Physicochemistry of the Peat Substrate, Growth and Morphophysiological Quality of White Spruce Seedlings in Forest Nursery. Land 2021, 10, 661. [Google Scholar] [CrossRef]
- Lambany, G. La dynamique morphologique et physiologique de plants soumis à un entreposage de longue durée. Gouvernement du Québec, Ministère des Ressources naturelles. Mémoire Rech. For. 1994, 114, 33. [Google Scholar]
- Colombo, S.J.; Raitanen, E.M. Frost hardening in first-year eastern larch (Larix laricina) container seedlings. New For. 1993, 7, 55–61. [Google Scholar] [CrossRef]
- Colombo, S.J.; Glreum, C.; Webb, D.P. Frost hardiness testing: An operational manual for use with extended greenhouse culture. Ministry of Natural Resources of Ontario. For. Res. Rep. 1984, 110, 14. [Google Scholar]
- Palta, J.P.; Weiss, L.S. Ice formation and freezing injury: An overview on the survival mechanism and molecular aspects of injury and cold acclimation in herbaceous plants. In Advances in Plant Cold Hardiness; Li, P.H., Christersson, L., Eds.; CRC Press, Inc.: Boca Raton, FL, USA, 1993; pp. 143–176. [Google Scholar]
- Burr, K.E.; Hawkins, C.D.B.; L’Hirondelle, S.J.; Binder, W.; George, M.F.; Repo, T. Methods for measuring cold hardiness of conifers. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 369–401. [Google Scholar]
- Carles, S.; Lamhamedi, M.S.; Stowe, D.C.; Margolis, H.A.; Bernier, P.Y.; Veilleux, L.; Fecteau, B. Frost tolerance of two-year-old Picea glauca seedlings grown under different irrigation regimes in a forest nursery. Scand. J. For. Res. 2008, 23, 137–147. [Google Scholar] [CrossRef]
- Lindström, A.; Stattin, E. Root freezing tolerance and vitality of Norway spruce and Scots pine seedlings; influence of strorage duration, storage temperature, and prestorage root freezing. Can. J. For. Res. 1994, 24, 2477–2484. [Google Scholar] [CrossRef]
- Zhu, X.B.; Cox, R.M.; Bourque, C.-P.A.; Arp, P.A. Thaw effect on cold-hardiness parameters in yellow birch. Can. J. Bot. 2002, 80, 390–398. [Google Scholar] [CrossRef]
- Flint, H.L.; Boyce, B.R.; Beattie, D.J. Index of injury—A useful expression of freezing injury to plant tissues as determined by the electrolytic method. Can. J. Plant Sci. 1967, 47, 229–230. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Brotherton, P.; Hostetter, S.; Chapman, D.; Dyce SBelanger, J.; Johnson, B.; Duke, S. The operational planting stock quality testing program at Weyerhaeuser. New For. 1997, 13, 423–437. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Lambany, G.; Margolis, H.A.; Renaud, M.; Veilleux, L.; Bernier, P.Y. Growth, physiology and leachate losses in Picea glauca seedlings (1 + 0) grown in air-slit containers under different irrigation regimes. Can. J. For. Res. 2001, 31, 1968–1980. [Google Scholar] [CrossRef]
- Régnière, J.; St-Amant, R. Stochastic simulation of daily air temperature and precipitation from monthly normals in North America north of Mexico. Inter. J. Biometeorol. 2007, 51, 415–430. [Google Scholar] [CrossRef]
- Ouranos. Vers l’adaptation. Synthèse des Connaissances sur les Changements Climatiques au Québec. Partie 1: Évolution Climatique au Québec Edition 2015; Ouranos: Montréal, QC, Canada, 2015; p. 114. [Google Scholar]
- Timmer, V.R.; Armstrong, G.; Miller, B.D. Steady-state nutrient preconditioning and early outplanting performance of containerized black spruce seedlings. Can. J. For. Res. 1991, 21, 585–594. [Google Scholar] [CrossRef]
- Bernier-Cardou, M.; Bigras, F.B. The analysis of Cold Hardiness Experiments. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 403–435. [Google Scholar]
- Milliken, G.A.; Johnsen, D.E. Analysis of Messy Data. Vol 1. Designed Experiments; Van Nostrand Reinhold Company: New York, NY, USA, 1994. [Google Scholar]
- Westfall, P.H.; Tobias, R.D.; Russell, D.W. Multiple Comparisons and Multiple Tests Using SAS®, 2nd ed.; SAS Institute, Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Carles, S.; Lamhamedi, M.S.; Stowe, D.C.; Margolis, H.A. An operational method for estimating cold tolerance thresholds of white spruce seedlings in forest nurseries. For. Chron. 2012, 88, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Lamhamedi, M.S.; Margolis, H.A.; Renaud, M.; Veilleux, L.; Auger, I. Effets de différentes régies d’irrigation sur la croissance, la nutrition minérale et le lessivage des éléments nutritifs des semis d’épinette noire (1 + 0) produits en récipients à parois ajourées en pépinière forestière. Can. J. For. Res. 2003, 33, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Lamhamedi, M.S.; Gagnon, J. Comment Gérer la Fertilisation et L’irrigation en vue D’améliorer la Tolérance au gel des Plants Produits en Récipients? Ministère des Ressources Naturelles. Gouvernement du Québec: Québec, QC, Canada, 2002; 15p.
- Stowe, D.C.; Lamhamedi, M.S.; Margolis, H. Water relations, cuticular transpiration, and bud characteristics of air-slit containerized Picea glauca seedlings in response to controlled irrigation regime. Can. J. For. Res. 2001, 31, 1922–1929. [Google Scholar] [CrossRef]
- Bergeron, O.; Lamhamedi, M.S.; Margolis, H.A.; Bernier, P.Y.; Stowe, D.C. Irrigation control and physiological responses of nursery-grown black spruce seedlings (1 + 0) cultivated in air-slit containers. Hort. Sci. 2004, 39, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Calmé, S.; Margolis, H.A.; Bigras, F.J. Influence of cultural practices on the relationship between frost tolerance and water content of containerized black spruce, white spruce, and jack pine seedlings. Can. J. For. Res. 1993, 23, 503–511. [Google Scholar] [CrossRef]
- Colombo, S.J. Bud dormancy status, frost hardiness, shoot moisture content, and readiness of black spruce container seedlings for frozen storage. J. Am. Soc. Hort. Sci. 1990, 115, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Aitken, S.; Hannerz, M. Genecology and gene resource management strategies for conifer cold hardiness. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 23–54. [Google Scholar]
- Hänninen, H.; Beuker, E.; Johnsen, Ø.; Leinonen, I.; Murray, M.; Sheppard, L.; Skroppa, T. Impacts of climate change on cold hardiness. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 305–334. [Google Scholar]
- Bigras, F.J.; Margolis, H.A. Shoot and root sensitivity of containerized black spruce, white spruce and jack pine seedlings to late fall freezing. New For. 1997, 13, 29–49. [Google Scholar] [CrossRef]
- Tumanov, I.I.; Krasavtsev, O.A. Hardening of northern woody plants by treatment at temperature below 0 °C. Sov. Plant Physiol. 1959, 6, 654–667. [Google Scholar]
- Cannell, M.G.R.; Smith, R.I. Thermal time, chill days and prediction of budburst in Picea sitchensis. J. Appl. Ecol. 1983, 20, 951–963. [Google Scholar] [CrossRef]
- Koski, V. The timing of hardening and dehardening of forest trees. Acta Hort. 1985, 168, 117–124. [Google Scholar] [CrossRef]
- Glerum, C. Annual trends in frost hardiness and electrical impedance for seven coniferous species. Can. J. Plant Sci. 1973, 53, 881–889. [Google Scholar] [CrossRef]
- Bigras, F.; Dumais, D. Root-Freezing damage in the containerized nursery: Impact on plantation sites—A review. New For. 2005, 30, 167–184. [Google Scholar] [CrossRef]
- Dumais, D.; Coursolle, C.; Bigras, F.; Margolis, H. Simulated root freezing in the nursery: Effects on the growth and physiology of containerized boreal conifer seedlings after outplanting. Can. J. For. Res. 2002, 32, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Van den Driessche, R. Importance of current photosynthate to new root growth in planted conifer seedlings. Can. J. For. Res. 1987, 17, 776–782. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Carles, S.; Lamhamedi, M.S.; Stowe, D.C.; Bernier, P.Y.; Veilleux LMargolis, H.A. Relationships between frost hardiness, root growth potential and photosynthesis of nursery-grown white spruce seedlings. Ann. For. Sci. 2011, 68, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Merry, R.; Jerrard, J.; Frebault, J.; Verhoeven, A. A comparison of pine and spruce in recovery from winter stress; changes in recovery kinetics, and the abundance and phosphorylation status of photosynthetic proteins during winter. Tree Physiol. 2017, 37, 1239–1250. [Google Scholar] [CrossRef]
- Wallin, E.; Gräns, D.; Stattin, E.; Verhoef, N.; Mikusiński, G.; Lindström, A. Evaluating methods for storability assessment and determination of vitality status of container grown Norway spruce transplants after frozen storage. Scan. J. For. Res. 2019, 34, 417–426. [Google Scholar] [CrossRef]
- Nadel, R.; Payne, N.D.; Stokes, T.A.; Enebak, S.A. Lifting dates, chilling hours, and storage duration on slash pine seedlings root growth potential, growth, and survival. Tree Plan. Notes. 2020, 63, 80–90. [Google Scholar]
- Turbocristal. Artificial Snowmaking to Minimize Seedlings Loss in Tree Nursery; Turbocristal Projet Description: Québec, QC, Canada, 1993; pp. 1–2. [Google Scholar]
- MRNF. Recherche de Nouvelles Méthodes de Protection Hivernale Pour la Production de Plants en Récipient; Gouvernement du Québec: Québec, QC, Canada, 2009; pp. 1–33.






| Black Spruce (1 + 0) | White Spruce (2 + 0) | |
|---|---|---|
| Container type | Model IPL 67–50 | Model IPL 25–310 |
| Number of cavities/container | 67 | 25 |
| Volume/cavity (cm3) | 50 | 310 |
| Seedling size | ||
| Height (cm) | 15.35 ± 0.27 | 40.25 ± 0.69 |
| Root-collar diameter (mm) | 1.65 ± 0.03 | 5.65 ± 0.12 |
| Dry mass (mg/seedling) | ||
| Shoot | 457.83 ± 13.47 | 8681.81 ± 359.65 |
| Roots | 253.92 ± 8.80 | 1986.79 ± 89.80 |
| Total | 711.75 ± 20.72 | 10,668.60 ± 440.57 |
| Ratio of dry mass to fresh mass (DM/FM, %) | 43.04 ± 0.49 | 46.63 ± 0.49 |
| Shoot nutrient concentrations | ||
| N (%) | 1.99 ± 0.05 | 2.06 ± 0.07 |
| P (%) | 0.29 ± 0.01 | 0.20 ± 0.01 |
| K (%) | 0.84 ± 0.02 | 0.59 ± 0.02 |
| Ca (%) | 0.19 ± 0.01 | 0.23 ± 0.01 |
| Mg (%) | 0.14 ± 0.00 | 0.14 ± 0.00 |
| −20 °C | −15 °C | −10 °C | −5 °C | 4 °C | ||
|---|---|---|---|---|---|---|
| RC (%) | WS (2 + 0) | 2.48 ± 0.20 a 1 | 2.56 ± 0.20 a | 2.42 ± 0.20 a | 2.75 ± 0.20 a | 2.48 ± 0.20 a |
| BS (1 + 0) | 3.58 ± 0.20 a | 3.77 ± 0.20 a | 4.09 ± 0.20 a | 3.19 ± 0.20 a | 3.40 ± 0.20 a | |
| It | WS (2 + 0) | 0.00 ± 0.12 a | 0.08 ± 0.12 a | −0.06 ± 0.12 a | 0.27 ± 0.12 a | |
| BS (1 + 0) | 0.18 ± 0.35 a | 0.37 ± 0.35 a | 0.71 ± 0.35 a | −0.23 ± 0.35 a | ||
| Black Spruce (1 + 0) | White Spruce (2 + 0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 = 4 °C | T1 = −5 °C | T2 = −10 °C | T3 = −15 °C | T4 = −20 °C | T0 = 4 °C | T1 = −5 °C | T2 = −10 °C | T3 = −15 °C | T4 = −20 °C | |
| Dry mass of new white roots (mg) | 37.5 ± 5.1 a 1 | 38.3 ± 5.1 a | 27.3 ± 5.1 ab | 18.4 ± 5.1 ab | 15.9 ± 5.1 b | 62.4 ± 15.14 a | 85.8 ± 15.14 a | 79.6 ± 15.14 a | 85.1 ± 15.14 a | 35.1 ± 15.14 b |
| Initial root dry mass (mg) | 251.0 ± 18.3 a | 216.3 ± 18.3 a | 236.8 ± 18.3 a | 196.9 ± 18.3 a | 186.8 ± 18.3 a | 1989.5 ± 248.1 a | 2330.0 ± 248.1 a | 1958.3 ± 248.1 a | 2596.4 ± 248.1 a | 1987.9 ± 248.1 a |
| Total root dry mass (mg) | 288.5 ± 22.8 a | 254.5 ± 22.8 ab | 264.0 ± 22.8 ab | 215.3 ± 22.8 ab | 202.6 ± 22.8 b | 2051.9 ± 257.8 a | 2415.8 ± 257.8 a | 2037.9 ± 257.8 a | 2681.5 ± 257.8 a | 2023.0 ± 257.8 a |
| Shoot dry mass (g) | 0.67 ± 0.05 a | 0.60 ± 0.05 a | 0.62 ± 0.05 a | 0.53 ± 0.05 a | 0.58 ± 0.05 a | 10.16 ± 0.98 a | 11.57 ± 0.98 a | 9.61 ± 0.98 a | 11.95 ± 0.98 a | 10.16 ± 0.98 a |
| Total dry mass (g) | 0.96 ± 0.06 a | 0.86 ± 0.06 a | 0.88 ± 0.06 a | 0.74 ± 0.06 a | 0.79 ± 0.06 a | 12.22 ± 1.19 a | 13.98 ± 1.19 a | 11.65 ± 1.19 a | 14.63 ± 1.19 a | 12.18 ± 1.19 a |
| Relative Electrical Conductivity (%) | Index of Injury | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WS 2 + 0 | BS 1 + 0 | WS 2 + 0 | BS 1 + 0 | |||||||
| Source of Variation | dln 1 | dld | Pr > F | dld | Pr > F | dln | dld | Pr > F | dld | Pr > F |
| Period | 1 | 6 | 0.0427 | 6 | 0.0778 | 1 | 18 | 0.9492 | 18 | 0.0391 |
| Treatment | 2 | 12 | 0.1866 | 12 | 0.0139 | 2 | 18 | 0.0557 | 18 | 0.0391 |
| Period × Treatment | 2 | 12 | 0.5206 | 12 | 0.0409 | 2 | 18 | 0.2919 | 18 | 0.7038 |
| Temperature | 3 | 54 | 0.1098 | 54 | 0.4875 | 2 | 36 | 0.2587 | 36 | 0.6295 |
| Period × Temperature | 3 | 54 | 0.5516 | 54 | 0.1045 | 2 | 36 | 0.3411 | 36 | 0.6515 |
| Treatment × Temperature | 6 | 54 | 0.2293 | 54 | 0.0636 | 4 | 36 | 0.9011 | 36 | 0.3974 |
| Period × Treatment × Temperature | 6 | 54 | 0.5235 | 54 | 0.3738 | 4 | 36 | 0.6630 | 36 | 0.2057 |
| RC (%) | WS 2 + 0 | Mid-January | Mid-March | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.94 | ±0.15 | b 2 | 3.49 | ±0.15 | a | ||||||||||||||
| BS 1 + 0 | Mid-January | Mid-March | |||||||||||||||||
| One day | 3 days | Control | One day | 3 days | Control | ||||||||||||||
| 3.24 | ±0.23 | ab | 3.22 | ±0.23 | ab | 3.13 | ±0.23 | b | 3.77 | ±0.23 | ab | 4.25 | ±0.23 | a | 3.38 | ±0.23 | b | ||
| It | BS 1 + 0 | One day | 3 days | Control | |||||||||||||||
| 0.30 | ±0.18 | a | 0.34 | ±0.18 | a | −0.28 | ±0.18 | a | |||||||||||
| Mid-January | Mid-March | ||||||||||||||||||
| −0.11 | ±0.14 | b | 0.35 | ±0.14 | a | ||||||||||||||
| Shoot Dry Mass (mg) | Dry Mass of New Roots (mg) | Total Root Dry Mass (mg) | Total Dry Mass of Seedling (mg) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS 2 + 0 | BS 1 + 0 | WS 2 + 0 | BS 1 + 0 | WS 2 + 0 | BS 1 + 0 | WS 2 + 0 | BS 1 + 0 | ||||||||||
| Source of Variation | dln 1 | dld | Pr > F | dld | Pr > F | dld | Pr > F | dld | Pr > F | dld | Pr > F | dld | Pr > F | dld | Pr > F | dld | Pr > F |
| Period | 1 | 18 | 0.9184 | 21.4 | 0.4062 | 72 | 0.0401 | 24 | 0.0002 | 18 | 0.2280 | 6 | 0.0762 | 18 | 0.7931 | 20.1 | 0.8152 |
| Treatment | 2 | 18 | 0.8173 | 14.3 | 0.1117 | 72 | 0.5013 | 48 | 0.4314 | 18 | 0.4471 | 66 | 0.1280 | 18 | 0.6680 | 13.6 | 0.0993 |
| Period × Treatment | 2 | 18 | 0.6220 | 14.3 | 0.2499 | 72 | 0.9538 | 48 | 0.4529 | 18 | 0.2910 | 66 | 0.0604 | 18 | 0.8633 | 13.6 | 0.1743 |
| Temperature | 3 | 54 | <0.0001 | 19.2 | 0.0004 | 72 | <0.0001 | 24 | 0.0002 | 54 | <0.0001 | 66 | 0.0005 | 54 | <0.0001 | 18.6 | 0.0005 |
| Period × Temperature | 3 | 54 | <0.0001 | 19.2 | 0.0012 | 72 | 0.0429 | 24 | 0.1977 | 54 | 0.0666 | 66 | 0.0006 | 54 | <0.0001 | 18.6 | 0.0012 |
| Treatment × Temperature | 6 | 54 | 0.2458 | 36.9 | 0.5266 | 72 | 0.3370 | 48 | 0.1401 | 54 | 0.5086 | 66 | 0.8650 | 54 | 0.3127 | 38.2 | 0.6768 |
| Period × Treatment × Temperature | 6 | 54 | 0.5690 | 36.9 | 0.7818 | 72 | 0.4332 | 48 | 0.8765 | 54 | 0.5230 | 66 | 0.9975 | 54 | 0.6289 | 38.2 | 0.9153 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamhamedi, M.S.; Lambert, M.-C.; Renaud, M. Simulation of Episodic Winter Warming on Dehardening of Boreal Forest Seedlings in Northern Forest Nurseries. Forests 2022, 13, 1975. https://doi.org/10.3390/f13121975
Lamhamedi MS, Lambert M-C, Renaud M. Simulation of Episodic Winter Warming on Dehardening of Boreal Forest Seedlings in Northern Forest Nurseries. Forests. 2022; 13(12):1975. https://doi.org/10.3390/f13121975
Chicago/Turabian StyleLamhamedi, Mohammed S., Marie-Claude Lambert, and Mario Renaud. 2022. "Simulation of Episodic Winter Warming on Dehardening of Boreal Forest Seedlings in Northern Forest Nurseries" Forests 13, no. 12: 1975. https://doi.org/10.3390/f13121975




