Effects of Water Deficit Stress on Growth Parameters of Robinia pseudoacacia L. Selected Clones under In Vitro Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Statistical Analysis
3. Results
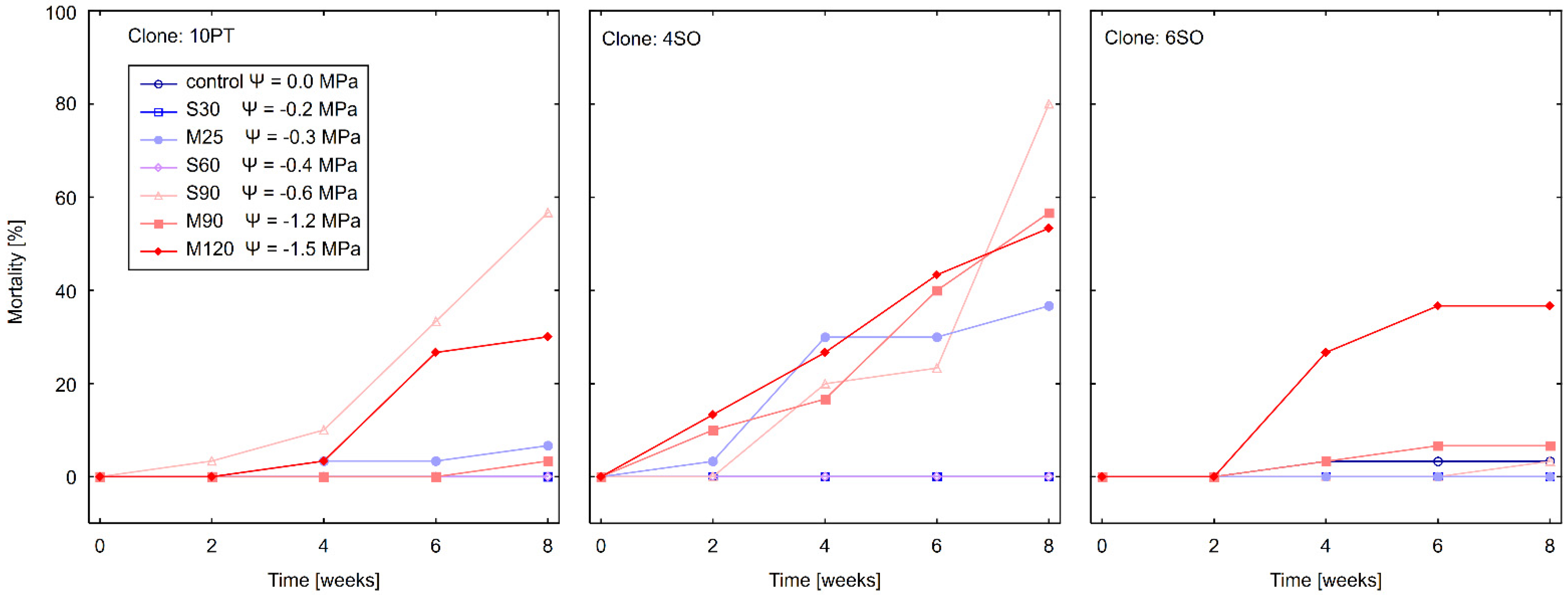
3.1. Effect of Water Stress on Mortality
3.2. Effect of Drought Stress on Total Shoot Length (TSL)
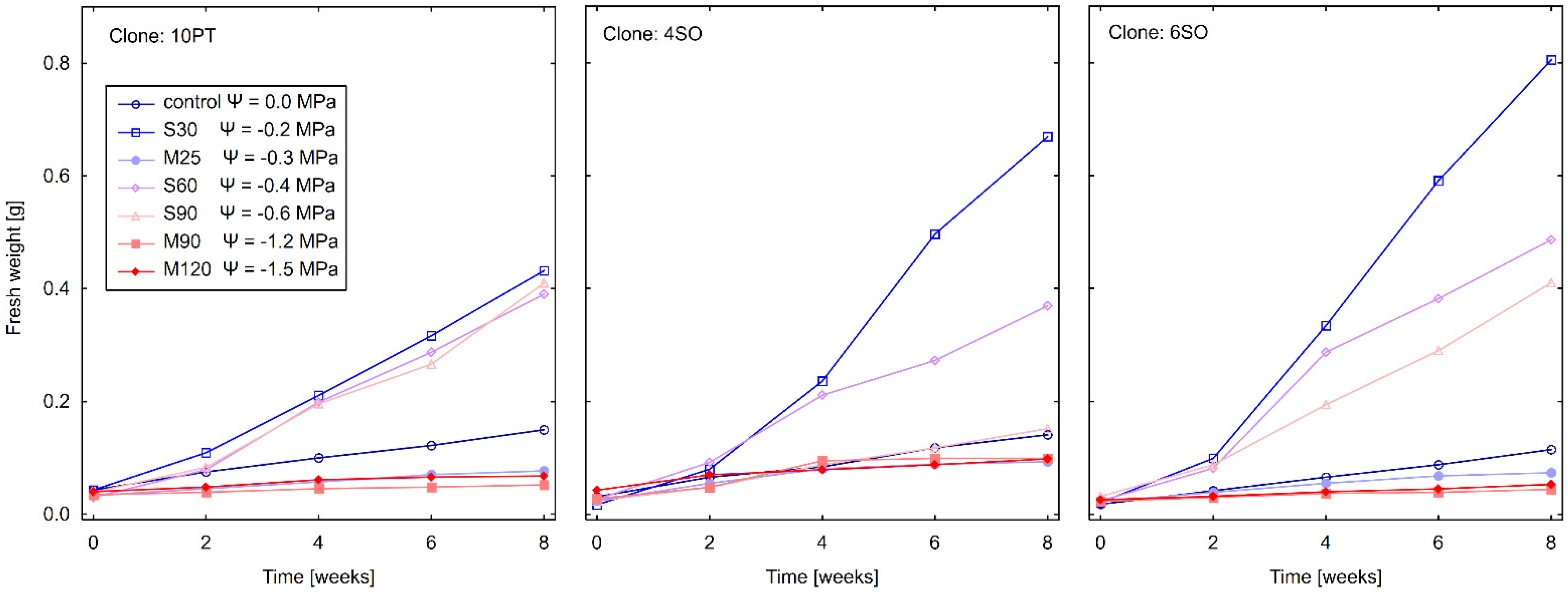
3.3. Effect of Drought Stress on Total Fresh Weight (FW)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Zhao, M.; Wang, N.; Liu, S.; Wang, J.; Zhang, W.; Yang, N.; Fan, P.; Wang, R.; Wang, H.; et al. Water use strategies and drought intensity define the relative contributions of hydraulic failure and carbohydrate depletion during seedling mortality. Plant Physiol. Biochem. 2020, 153, 106–118. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.P.; Allen, C.D.; Millar, C.I.; Swetnam, T.W.; Michaelsen, J.; Still, C.J.; Leavitt, S.W. Forest responses to increasing aridity and warmth in the southwestern United States. Proc. Natl. Acad. Sci. USA 2010, 107, 21289–21294. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, R.M.; Mankin, J.S.; Lesk, C.; Coffel, E.; Raymond, C. A Review of Recent Advances in Research on Extreme Heat Events. Curr. Clim. Chang. Rep. 2016, 2, 242–259. [Google Scholar] [CrossRef] [Green Version]
- Crouchet, S.E.; Jensen, J.; Schwartz, B.F.; Schwinning, S. Tree Mortality After a Hot Drought: Distinguishing Density-Dependent and -Independent Drivers and Why It Matters. Front. For. Glob. Chang. 2019, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Running, S.W. Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 Through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Robakowski, P.; Wyka, T.P.; Kowalkowski, W.; Barzdajn, W.; Pers-Kamczyc, E.; Jankowski, A.; Politycka, B. Practical implications of different phenotypic and molecular responses of evergreen conifer and broadleaf deciduous forest tree species to regulated water deficit in a container nursery. Forests 2020, 11, 1011. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Kramer, P.J. Fifty Years of Progress in Water Relations Research. Plant Physiol. 1974, 54, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.B.; Theeuwen, T.P.J.M.; Oortwijn, M.; Visser, R.G.F.; van der Linden, C.G. Carbon partitioning mechanisms in POTATO under drought stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.J.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant–Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Klisz, M.; Puchałka, R.; Netsvetov, M.; Prokopuk, Y.; Vítková, M.; Sádlo, J.; Matisons, R.; Mionskowski, M.; Chakraborty, D.; Olszewski, P.; et al. Variability in climate-growth reaction of Robinia pseudoacacia in Eastern Europe indicates potential for acclimatisation to future climate. For. Ecol. Manag. 2021, 492, 119194. [Google Scholar] [CrossRef]
- Nicolescu, V.N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastien, J.C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef] [Green Version]
- Vítková, M.; Sádlo, J.; Roleček, J.; Petřík, P.; Sitzia, T.; Müllerová, J.; Pyšek, P. Robinia pseudoacacia-dominated vegetation types of Southern Europe: Species composition, history, distribution and management. Sci. Total Environ. 2020, 707, 134857. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, L.; Wan, X. Relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality caused by drought. AoB Plants 2018, 10, plx069. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering drought resistance in forest trees. Front. Plant Sci. 2019, 9, 1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, D.; Veste, M.; Freese, D. Black locust (Robinia pseudoacacia L.) ecophysiological and morphological adaptations to drought and their consequence on biomass production and water-use efficiency. N. Z. J. For. Sci. 2014, 44, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Grünewald, H.; Böhm, C.; Quinkenstein, A.; Grundmann, P.; Eberts, J.; von Wühlisch, G. Robinia pseudoacacia L.: A lesser known tree species for biomass production. Bioenergy Res. 2009, 2, 123–133. [Google Scholar] [CrossRef]
- Inman-Bamber, N.G.; Smith, D.M. Water relations in sugarcane and response to water deficits. Field Crops Res. 2005, 92, 185–202. [Google Scholar] [CrossRef]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. Food Energy Soc. Third Ed. 2007, 54, 183–200. [Google Scholar] [CrossRef]
- Demeter, Z.; Kanalas, P.; Máthé, C.; Cseke, K.; Szollosi, E.; M-Hamvas, M.; Jámbrik, K.; Kiss, Z.; Mészáros, I. Osmotic stress responses of individual white oak (Quercus section, Quercus subgenus) genotypes cultured in vitro. J. Plant Physiol. 2014, 171, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovalikova, Z.; Jiroutova, P.; Toman, J.; Dobrovolna, D.; Drbohlavova, L. Physiological Responses of Apple and Cherry In Vitro Culture under Different Levels of Drought Stress. Agronomy 2020, 10, 1689. [Google Scholar] [CrossRef]
- Lawlor, D.W. Genetic engineering to improve plant performance under drought: Physiological evaluation of achievements, limitations, and possibilities. J. Exp. Bot. 2013, 64, 83–108. [Google Scholar] [CrossRef] [Green Version]
- Claeys, H.; Van Landeghem, S.; Dubois, M.; Maleux, K.; Inzé, D. What Is Stress? Dose-response effects in commonly used in vitro stress assays. Plant Physiol. 2014, 165, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Deikman, J.; Petracek, M.; Heard, J.E. Drought tolerance through biotechnology: Improving translation from the laboratory to farmers’ fields. Curr. Opin. Biotechnol. 2012, 23, 243–250. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.O. Plant Physiology, 3rd ed.; Sinauer Associates Incorporated: Franklin County, MA, USA, 2003; Volume 91, p. 750. [Google Scholar] [CrossRef] [Green Version]
- Haswell, E.S.; Verslues, P.E. The ongoing search for the molecular basis of plant osmosensing. J. Gen. Physiol. 2015, 145, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojda, T.; Klisz, M.; Jastrzębowski, S.; Mionskowski, M.; Szyp-Borowska, I.; Szczygieł, K. The Geographical Distribution Of The Black Locust (Robinia Pseudoacacia L.) In Poland And Its Role On Non-Forest Land. Pap. Glob. Chang. IGBP 2015, 22, 101–113. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Szyp-Borowska, I.; Banha, C.; Wojda, T.; Szczygiel, K. Micropropagation of black locust (Robinia pseudoacacia L.) and genetic stability of long term cultivated plants. Folia For. Pol. Ser. A 2016, 58, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Siddique, K.H.M.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and Characterization of Contrasting Genotypes/Cultivars for Developing Heat Tolerance in Agricultural Crops: Current Status and Prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef] [PubMed]
- Kulpa, D.; Gawlik, A.; Matuszk-Slamani, R.; Włodarczyk, M.; Bejger, R.; Sienkiewicz, M.; Gołębiowska, D.; Semeniuk, A. Effect of Mannitol and Sorbitol on Soybean in Vitro Development. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2018, 341, 41–48. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, D. Entrepreneurial marketing: A conceptualisation from qualitative research. Qual. Mark. Res. An Int. J. 2000, 3, 47–54. [Google Scholar] [CrossRef]
- Pan, W. Akaike’s Information Criterion in Generalized Estimating Equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]
- Takashima, Y.; Hiraoka, Y.; Matsushita, M.; Takahashi, M. Evaluation of responsivity to drought stress using infrared thermography and chlorophyll fluorescence in potted clones of cryptomeria japonica. Forests 2021, 12, 55. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.M.; Adams, H.D.; Yu, K.; Wilson, L.A.; Will, R.E.; Anderegg, W.R.L. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol. 2019, 223, 1834–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovovic, M.; Govedarica-lucic, A.; Tesanovic, D.; Tunguz, V. Influence of Salt and Osmotic Stress on Germination of Different Wheat Cultivars. Int. J. Crops Sci. Technol. 2015, 1. [Google Scholar]
- dos Santos, C.L.; de Borja Reis, A.F.; Mazzafera, P.; Favarin, J.L. Determination of the water potential threshold at which rice growth is impacted. Plants 2018, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallardy, S.G. Chapter 11 Absorption of Water and Ascent of Sap. In Phisiology of Woody Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 287–323. [Google Scholar]
- Ridolfi, M.; Dreyer, E. Responses to water stress in an ABA-unresponsive hybrid poplar (Populus koreana × trichocarpa cv. Peace) III. Consequences for photosynthetic carbon assimilation. New Phytol. 1997, 135, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Moysset, L.; Llambrich, E.; López-Iglesias, C.; Simón, E. Microautoradiographic localisation of [3H]sucrose and [3H]mannitol in Robinia pseudoacacia pulvinar tissues during phytochrome-mediated nyctinastic closure. Protoplasma 2006, 229, 63–73. [Google Scholar] [CrossRef]
- George, E. Plant Propagation by Tissue Culture: Handbook and Directory of Commercial Laboratories; Exegetics Ltd.: Hampshire, UK, 1984; ISBN 9780950932507. [Google Scholar]
- Hazarika, B.N. Acclimatization of tissue cultured plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Kishor, P.B.K.; Dange, V. Sucrose metabolism in callus cultures of cotton during growth. Indian J. Exp. Biol. 1990, 28, 352–355. [Google Scholar]
- Finkelstein, R.R.; Gibson, S.I. ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 2002, 5, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Sulmon, C.; Cabello-Hurtado, F.; Taconnat, L.; Martin-Magniette, M.-L.; Renou, J.-P.; Amrani, A.E.; Couée, I.; Gouesbet, G. Genome-wide interacting effects of sucrose and herbicide-mediated stress in Arabidopsis thaliana: Novel insights into atrazine toxicity and sucrose-induced tolerance. BMC Genom. 2007, 8, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kang, H.; Yang, W. Climate change-induced water stress suppresses the regeneration of the critically endangered forest tree Nyssa yunnanensis. PLoS ONE 2017, 12, e0182012. [Google Scholar] [CrossRef] [PubMed]

| Clone Name | Clone Number |
|---|---|
| 4SO | 9757 |
| 6SO | 9735 |
| 10PT | 9755 |
| No. | Ψ Level (MPa) | Sucrose Concentration (gL−1) | Mannitol Concentration (gL−1) | Abbreviation * |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | Control (MS) |
| 2 | −0.2 | 30 | - | S30 |
| 3 | −0.3 | - | 25 | M25 |
| 4 | −0.4 | 60 | - | S60 |
| 5 | −0.6 | 90 | - | S90 |
| 6 | −1.2 | - | 90 | M90 |
| 7 | −1.5 | - | 120 | M120 |
| Model Effect | DF | Chi-Square | p |
|---|---|---|---|
| Clone | 2 | 28.02 | <0.001 |
| Osmoticum | 6 | 77.12 | <0.001 |
| Time | 4 | 56.19 | <0.001 |
| Clone × Osmoticum | 11 | 68.61 | <0.001 |
| Clone × Time | 8 | 40.43 | <0.001 |
| Osmoticum × Time | 23 | 59.55 | <0.001 |
| Osmoticum | Clone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10PT | 4SO | 6SO | Total for Osmoticum | ||||||||
| control | 0.0 ± 0.0 | a | A | 5.3 ± 1.6 | b | B | 2.0 ± 1.1 | a | A | 2.4 ± 0.7 | b |
| S30 | 0.0 ± 0.0 | a | A | 4.0 ± 4.0 | ab | A | 0.0 ± 0.0 | a | A | 1.3 ± 1.3 | ab |
| M25 | 2.7 ± 1.6 | a | A | 18 ± 4.9 | b | B | 0.0 ± 0.0 | a | A | 6.9 ± 1.9 | bc |
| S60 | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a |
| S90 | 10.0 ± 5.5 | b | A | 24.7 ± 8.7 | c | B | 0.0 ± 0.0 | a | A | 11.6 ± 3.6 | cd |
| M90 | 0.7 ± 0.7 | a | A | 24.7 ± 5.5 | c | B | 3.3 ± 1.4 | a | A | 9.6 ± 2.3 | cd |
| M120 | 12.0 ± 5.4 | b | A | 27.3 ± 4.9 | c | A | 20.0 ± 7.6 | b | A | 19.8 ± 3.6 | d |
| Total for clone | 3.6 ± 1.2 | A | 14.9 ± 2.1 | B | 3.6 ± 1.2 | A | |||||
| Clone | Time (weeks) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||||||||
| 10PT | 0.0 ± 0.0 | a | A | 1.4 ± 0.8 | a | A | 7.6 ± 3.9 | a | B | 9.1 ± 4.1 | a | B |
| 4SO | 3.3 ± 1.6 | a | A | 13.3 ± 3.5 | b | B | 20.5 ± 4.2 | b | C | 37.2 ± 6.7 | b | D |
| 6SO | 0.0 ± 0.0 | a | A | 4.7 ± 3.1 | ab | A | 6.7 ± 3.7 | a | B | 6.7 ± 3.7 | a | B |
| Total for time | 1.1 ± 0.6 | A | 6.5 ± 1.6 | B | 11.6 ± 2.3 | C | 17.6 ± 3.2 | D | ||||
| Osmoticum | Time (weeks) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||||||||
| control | 0.0 ± 0.0 | a | A | 2.2 ± 1.5 | a | A | 4.4 ± 1.9 | ab | A | 5.6 ± 2.1 | a | A |
| S30 | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A | 6.7 ± 6.7 | ab | A |
| M25 | 0.0 ± 0.0 | a | A | 10.0 ± 4.2 | b | B | 10.0 ± 4.24 | b | B | 14.4 ± 7.1 | a | B |
| S60 | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A | 0.0 ± 0.0 | a | A |
| S90 | 0.0 ± 0.0 | a | A | 7.8 ± 6.7 | ab | AB | 15.6 ± 8.9 | bc | B | 34.4 ± 12.4 | bc | B |
| M90 | 3.3 ± 2.4 | a | A | 6.7 ± 2.7 | b | AB | 15.6 ± 5.5 | c | AB | 22.2 ± 8.2 | b | B |
| M120 | 4.4 ± 3.0 | a | A | 18.9 ± 6.9 | c | B | 35.6 ± 9.2 | c | C | 40.0 ± 10.1 | c | C |
| Total for time | 1.1 ± 0.6 | A | 6.5 ± 1.6 | B | 11.6 ± 2.3 | C | 17.6 ± 3.2 | D | ||||
| Model Effect | DF | TSL | FW | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Clone | 2 | 52.05 | <0.001 | 2.35 | 0.096 |
| Osmoticum | 6 | 22.7 | <0.001 | 72.21 | <0.001 |
| Time | 4 | 157.7 | <0.001 | 257.53 | <0.001 |
| Clone × Osmoticum | 12 | 3.87 | <0.001 | 3.91 | <0.001 |
| Clone × Time | 8 | 0.84 | 0.568 | 5.18 | <0.001 |
| Osmoticum × Time | 24 | 15.29 | <0.001 | 51.93 | <0.001 |
| Clone × Osmoticum × Time | 48 | 2.52 | <0.001 | 4.46 | <0.001 |
| Osmoticum | Clone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10PT | 4SO | 6SO | Total for Osmoticum | ||||||||
| control | 2.94 ± 0.08 | b | C | 2.47 ± 0.08 | bc | B | 2.18 ± 0.08 | a | A | 2.53 ± 0.05 | b |
| S30 | 2.92 ± 0.08 | b | B | 2.60 ± 0.09 | c | A | 2.87 ± 0.08 | c | B | 2.79 ± 0.05 | c |
| M25 | 2.51 ± 0.08 | a | B | 1.96 ± 0.10 | a | A | 2.11 ± 0.08 | a | A | 2.19 ± 0.05 | a |
| S60 | 2.79 ± 0.08 | b | C | 2.17 ± 0.08 | b | A | 2.45 ± 0.08 | b | B | 2.47 ± 0.04 | b |
| S90 | 2.54 ± 0.09 | a | B | 1.97 ± 0.17 | a | A | 2.49 ± 0.08 | b | B | 2.33 ± 0.07 | b |
| M90 | 2.47 ± 0.08 | a | C | 1.77 ± 0.12 | a | A | 2.1 ± 0.08 | a | B | 2.12 ± 0.05 | a |
| M120 | 2.43 ± 0.09 | a | B | 2.26 ± 0.11 | b | A | 2.12 ± 0.10 | a | A | 2.27 ± 0.06 | a |
| Total for clone | 2.66 ± 0.03 | C | 2.17 ± 0.04 | A | 2.33 ± 0.03 | B | |||||
| Osmoticum | Time (weeks) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |||||||||||
| control | 2.05 ± 0.05 | a | A | 2.51 ± 0.05 | c | B | 2.64 ± 0.05 | c | C | 2.69 ± 0.05 | c | D | 2.76 ± 0.05 | c | E |
| S30 | 2.18 ± 0.05 | a | A | 2.65 ± 0.05 | d | B | 2.85 ± 0.05 | d | C | 3.07 ± 0.05 | d | D | 3.25 ± 0.05 | d | E |
| M25 | 2.03 ± 0.05 | a | A | 2.13 ± 0.05 | ab | B | 2.26 ± 0.05 | ab | C | 2.27 ± 0.05 | ab | C | 2.28 ± 0.06 | ab | C |
| S60 | 2.13 ± 0.05 | a | A | 2.41 ± 0.05 | c | B | 2.53 ± 0.05 | c | C | 2.60 ± 0.05 | c | D | 2.69 ± 0.05 | c | E |
| S90 | 2.15 ± 0.07 | a | A | 2.30 ± 0.07 | bc | B | 2.36 ± 0.07 | bc | B | 2.41 ± 0.08 | b | B | 2.45 ± 0.08 | b | B |
| M90 | 2.00 ± 0.06 | a | A | 2.08 ± 0.06 | a | B | 2.14 ± 0.06 | a | C | 2.19 ± 0.06 | a | D | 2.21 ± 0.06 | a | D |
| M120 | 2.15 ± 0.06 | a | A | 2.24 ± 0.06 | ab | B | 2.29 ± 0.06 | ab | C | 2.31 ± 0.06 | ab | C | 2.36 ± 0.07 | ab | C |
| Total for time | 2.09 ± 0.02 | A | 2.33 ± 0.02 | B | 2.44 ± 0.02 | C | 2.50 ± 0.02 | D | 2.57 ± 0.02 | E | |||||
| Osmoticum | Clone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10PT | 4SO | 6SO | Total for Sugar | ||||||||
| control | 0.09 ± 0.02 | b | A | 0.089 ± 0.02 | a | A | 0.07 ± 0.02 | a | A | 0.08 ± 0.01 | a |
| S30 | 0.22 ± 0.02 | c | A | 0.30 ± 0.02 | c | B | 0.37 ± 0.02 | c | C | 0.29 ± 0.01 | c |
| M25 | 0.06 ± 0.02 | ab | A | 0.07 ± 0.02 | a | A | 0.05 ± 0.02 | a | A | 0.06 ± 0.01 | a |
| S60 | 0.19 ± 0.02 | c | A | 0.19 ± 0.02 | b | A | 0.25 ± 0.02 | b | B | 0.21 ± 0.01 | b |
| S90 | 0.19 ± 0.02 | c | B | 0.09 ± 0.04 | a | A | 0.20 ± 0.02 | b | B | 0.16 ± 0.02 | b |
| M90 | 0.04 ± 0.02 | a | A | 0.07 ± 0.03 | a | A | 0.03 ± 0.02 | a | A | 0.05 ± 0.01 | a |
| M120 | 0.06 ± 0.02 | ab | A | 0.08 ± 0.03 | a | A | 0.04 ± 0.02 | a | A | 0.06 ± 0.01 | a |
| Total for clone | 0.12 ± 0.01 | A | 0.13 ± 0.01 | A | 0.14 ± 0.01 | A | |||||
| Osmoticum | Time (weeks) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |||||||||||
| control | 0.03 ± 0.01 | a | A | 0.06 ± 0.01 | ab | B | 0.08 ± 0.01 | a | C | 0.11 ± 0.01 | b | D | 0.13 ± 0.01 | b | E |
| S30 | 0.03 ± 0.01 | a | A | 0.09 ± 0.01 | c | B | 0.26 ± 0.01 | c | C | 0.47 ± 0.01 | e | D | 0.63 ± 0.01 | e | E |
| M25 | 0.03 ± 0.01 | a | A | 0.05 ± 0.01 | a | B | 0.06 ± 0.01 | a | C | 0.08 ± 0.01 | ab | C | 0.08 ± 0.01 | a | C |
| S60 | 0.03 ± 0.01 | a | A | 0.08 ± 0.01 | bc | B | 0.23 ± 0.01 | c | C | 0.31 ± 0.01 | d | D | 0.41 ± 0.01 | d | E |
| S90 | 0.03 ± 0.02 | a | A | 0.07 ± 0.02 | bc | B | 0.16 ± 0.02 | b | C | 0.22 ± 0.02 | c | D | 0.32 ± 0.02 | c | E |
| M90 | 0.03 ± 0.01 | a | A | 0.04 ± 0.01 | a | A | 0.06 ± 0.01 | a | B | 0.06 ± 0.01 | a | B | 0.06 ± 0.01 | a | B |
| M120 | 0.04 ± 0.02 | a | A | 0.05 ± 0.02 | ab | A | 0.06 ± 0.02 | a | A | 0.07 ± 0.02 | a | A | 0.07 ± 0.02 | a | A |
| Total for time | 0.03 ± 0.01 | A | 0.06 ± 0.01 | B | 0.13 ± 0.01 | C | 0.19 ± 0.01 | D | 0.25 ± 0.01 | E | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyp-Borowska, I.; Ukalska, J.; Niemczyk, M.; Wojda, T.; Thomas, B.R. Effects of Water Deficit Stress on Growth Parameters of Robinia pseudoacacia L. Selected Clones under In Vitro Conditions. Forests 2022, 13, 1979. https://doi.org/10.3390/f13121979
Szyp-Borowska I, Ukalska J, Niemczyk M, Wojda T, Thomas BR. Effects of Water Deficit Stress on Growth Parameters of Robinia pseudoacacia L. Selected Clones under In Vitro Conditions. Forests. 2022; 13(12):1979. https://doi.org/10.3390/f13121979
Chicago/Turabian StyleSzyp-Borowska, Iwona, Joanna Ukalska, Marzena Niemczyk, Tomasz Wojda, and Barb R. Thomas. 2022. "Effects of Water Deficit Stress on Growth Parameters of Robinia pseudoacacia L. Selected Clones under In Vitro Conditions" Forests 13, no. 12: 1979. https://doi.org/10.3390/f13121979
APA StyleSzyp-Borowska, I., Ukalska, J., Niemczyk, M., Wojda, T., & Thomas, B. R. (2022). Effects of Water Deficit Stress on Growth Parameters of Robinia pseudoacacia L. Selected Clones under In Vitro Conditions. Forests, 13(12), 1979. https://doi.org/10.3390/f13121979





