Abstract
Water acquisition via the root system of woody species is a key factor governing plant physiology. In order to compare the impact of water acquisition on the hydraulic and photosynthetic characteristics of different-sized Populus euphratic, which is a desert riparian tree species, we quantified leaf hydraulic conductance (KL), stomatal conductance (gs), net photosynthetic rate (PN), predawn and midday leaf water potential (Ψ), and the stem δ18O of the saplings and mature trees. The results showed that the saplings had a lower predawn leaf water potential (Ψpd) and soil-to-leaf water potential gradient (ΔΨ) and a higher KL than the mature trees but had a similar gs and PN to the mature trees. In arid zones, probably due to root limitation, the saplings were more likely to use unreliable topsoil water (<80 cm), whereas the mature trees typically uptake reliable deep soil water (>80 cm) and groundwater due to having deeper root systems. The unreliability of the water supply might make saplings hold a higher hydraulic conductance to ensure that the water is transported efficiently to the leaves and to satisfy their transpiration need. In contrast, the mature trees, which uptake the more reliable deeper water resources, had a relatively low leaf-specific hydraulic conductance because of the increased path length versus the saplings. However, adult trees can maintain stomatal conductance by upregulating ΔΨ, thereby facilitating their ability to maintain a carbon assimilation rate similar to that of the saplings. This regulating behavior benefits mature trees’ net carbon gain, but it comes at the expense of an increased risk of hydraulic failure. These results imply that the top priority for saplings should be to maintain hydraulic system functioning, whereas, for mature trees, the priority is to assure stable net carbon gain for their growth.
1. Introduction
Recognizing the mechanisms of leaf physiological changes as trees grow is important for extending individual leaf observations to stand scales and estimating carbon fluxes in forest ecosystems [1,2]. Photosynthesis, stomatal conductance [3,4,5,6], and hydraulic conductance [7,8] are crucial physiological processes that vary with plant size and age. According to the hydraulic limitation hypothesis, the lengthened hydraulic channel causes the leaf-specific hydraulic conductance to decrease as a tree’s height increases [9,10]. The primary factor limiting tall trees’ ability to conduct stomatal conductance is a size-related decrease in leaf-specific hydraulic conductivity [9,11]. Thus, declining leaf-specific hydraulic conductance with increasing height would reduce stomatal conductance, which may result in lower intercellular CO2 concentrations in the leaves and less assimilation [12,13]. Moreover, as plants grow, the morphology and anatomical structure of leaves, including leaf thickness, leaf-specific mass, and stomatal density, may change significantly, which, in turn, affects the photosynthesis of plants [14,15]. It has been discovered that many species’ stomatal conductance and photosynthetic rates decrease with height [16,17,18]. However, certain study findings contradict these fundamental patterns [19,20]. In addition, some studies found that there was no change in either stomatal conductance or photosynthetic rate with tree height or age [1,21]. In summary, plant physiological processes are related to plant sizes or ages, although the correlations vary widely depending on species and site conditions.
As trees grow in size, physiological and structural alterations may compensate for the gravitational effect and mitigate potential hydraulic limitations to leaf-specific gas exchange in old/tall trees [22,23]. These compensatory mechanisms could improve water delivery to tall tree leaves [24], although these are not usually sufficient to prevent the depression of photosynthesis [11,25]. A lower minimum leaf water potential and increased access to deep water resources were discovered to be compensatory mechanisms in the taller trees of diverse species [7,10,26]. The decline in minimum leaf water potential with tree height maintains the driving force between the roots and leaves [18] and allows for liquid-phase water flux maintenance in taller trees, thereby minimizing the reductions in leaf-specific hydraulic conductance and stomatal conductance as trees grow larger [22].
In general, old/big trees are likely to have developed deeper or more extensive root systems than young/small trees [27,28]. Differences in root development between big and small trees lead to differences in water resources [29], which may regulate stomatal conductance and subsequent net carbon assimilation [18]. In addition, deeper roots in big trees can uptake deeper water sources [30,31], allowing them to have a greater photosynthetic rate and transpiration rate than small trees with limited root systems [7] while sustaining a stomatal conductance that is equivalent to small trees [23].
Populus euphratica is a desert riparian tree species in the lower reaches of the Heihe River in western China that plays a crucial part in supporting natural systems. [32,33]. Many researchers have explored the effects of drought on the gas exchange, hydraulic properties, and water uptake of P. euphratica [31,34,35,36,37,38]. However, only a handful of these studies have evaluated the photosynthetic capability, hydraulic conductance, and water uptake of saplings and mature specimens of P. euphratica. Consequently, the relationships between hydraulic conductance and gas exchange in P. euphratica are still not well understood. Moreover, there is little information on how hydraulic traits and water uptake change during the maturation from saplings to trees. We hypothesized that, as P. euphratica grows, the mature trees have better access to deeper water sources than the seedlings; hydraulic conductivity varies between seedlings and mature trees, and the water sources of P. euphratica have a certain link with its hydraulic and photosynthetic characteristics. The objectives of this study were to (1) compare the differences between leaf gas exchange and hydraulic conductance among saplings and mature trees and to investigate the relationship between hydraulic conductance and gas exchange, (2) identify whether soil water and groundwater contribute differently to water uptake between the saplings and trees, (3) and to investigate whether the differences in gas exchange and hydraulic conductance are reflected in changes in water sources.
2. Materials and Methods
2.1. Study Area and Plant Material
The study area is located in the lower reaches of the Heihe River (40°20′–42°41′ N, 97°36′–102°08′ E) in the county of Ejina, Inner Mongolia, northwest China. The study area belongs to arid desert regions and has the obvious characteristic of a warm temperate zone and arid continental climate, with little rainfall and strong evaporation. In the research region, the mean annual precipitation was less than 42 mm, the potential evaporation was greater than 3200 mm, and the mean annual temperature was around 8.0 °C [39]. The study location is representative of a desert riparian woodland habitat. The trees mainly consist of Populus euphratica Oliv., the shrubs are mainly Tamarix chinensis Lour., followed by Lycium ruthenicum Murr., and Alhagi sparsifolia Shap., and the grass is mainly made up of Sophora alopecuroides L., Peganum Linn., etc.
We set up a 50 × 50 m plot in the study region and took all measurements within it. According to the diameter at breast height (DBH), we selected 10 P. euphratica trees in two size classes (sapling and mature tree) for measuring and sampling at the study site. The DBH of the saplings was 2.1 ± 0.1 cm, and for mature trees, it was 82.8 ± 6.9 cm. The saplings had a mean height of 3.2 m and were 4 years old, whereas the mature trees had a mean height of 11.4 m and were 55 years old at the study site. All measurements were taken from the same trees.
2.2. Leaf Gas Wxchange
In early August 2014, the gas exchange for the sapling and mature P. euphratica trees (n = 5 plants per size class) was measured on clear days. At midday (11:00–13:00 h), the gas exchange on at least three leaves per plant was observed using the Li 6400 portable gas exchange instrument (LiCOR Inc., Lincoln, NE, USA). In order to maintain a constant external environment for each measurement, the PAR was set to 2000 µmol m−2 s−1 using the 6400-02B LED light source, the cuvette CO2 concentration to 400 µmol mol−1 using the CO2 injection system, the flow rate to 400 µmol s−1, and the block temperature to 28.0 °C, respectively. In the middle and upper canopy, fully opened, normally growing, and mature leaves were chosen for in situ measurements with the help of a herringbone ladder. The photosynthetic rate (PN), transpiration rate (E), and stomatal conductance (gs) were recorded after the data had stabilized, and each measurement was carried out three times.
2.3. Leaf Water Potential
The leaf water potential (Ψleaf) was determined using a portable plant water status console (Model 3115). Predawn (04:00–06:00 h) leaf water potentials (Ψpd) were recorded from three leaves from every plant (n = 5 plants each size). The midday (11:00–13:00 h) leaf water potentials (Ψmd) were measured in the same individuals immediately following the end of each gas exchange measurement.
2.4. Hydraulic Conductivity
The leaf-specific apparent hydraulic conductance (KL) was estimated based on Ohm’s law [40]:
where E is transpiration rate as determined by the gas exchange system. Ψsoil and Ψleaf are the soil and leaf water potentials, respectively. ρgh is the potential energy at a given height ((h) height of the measured leaf from the ground surface), g is the gravity acceleration, and ρ is the water density. Instead of Ψsoil, the predawn leaf water potential (Ψpd) was used as a proxy of bulk soil water potential under zero sap flow in the trees.
2.5. Xylem, Soil, and Ground Water Sampling
Approximately 5 cm-long suberized twigs (n = 5 plants for saplings and mature trees) were clipped from sunlit branches. The xylem samples were quickly removed from the bark and packed into sampling bottles. These bottles were then sealed with Parafilm, stored in ice boxes, and brought back to the laboratory for frozen storage prior to testing.
At intervals of 10 cm for each layer from 0 to 40 cm and 20 cm for each layer below 40 cm up to the groundwater, the soil samples were taken using a manual soil auger. For each layer, two soil samples were collected: one in an aluminum box for measuring soil water content and the other in a sampling bottle for measuring soil water isotopes, which were handled in the same manner as the plant samples. Each soil sample was taken three times for each layer. The gravimetric water content (θg) of soil was determined by the drying and weighing methods.
Groundwater was gathered from a monitoring well in the experimental location built in 2010. In the field, the groundwater samples were handled in the same manner as the xylem samples, but in the lab, they were kept chilled until testing.
2.6. Isotopic Analyses
Using a cryogenic vacuum extraction technique developed by West et al. (2006) [41], the water from soil and plant xylem was extracted in the State Key Laboratory of Desert and Oasis Ecology, China. The stable oxygen isotope ratios of all the water samples were then determined using a Finnigan MAT Delta V advantage mass spectrometer.
where Rsam and Rstd are the 18O/16O ratios of the sample water and the standard mean ocean water, respectively [42].
According to the similarity and trend of soil water δ18O values at different depths, the soil profile was divided into two layers: surface soil (0–80 cm) and subsurface soil (80–320 cm). By the weighted averaging of the soil water content and δ18O values in the different sampling layers, the average isotope values of the surface and subsurface soils were determined. More details can be found in Chen et al. [31].
The proportional contribution of various latent water sources to the P. euphratica trees and saplings was estimated by the IsoSource model [43]. Because P. euphratica is a halophyte and might fractionate against hydrogen isotopes [44], we only used δ18O data for the model calculations.
2.7. Statistical Analyses
Statistical analyses were conducted with SPSS version 20.0 software package (IBM Corp., Armonk, NY, USA). The tests for the differences between the saplings and mature trees for parameters measured were performed using analysis of variance. The effects of KL on PN, gs, E, and leaf water potential were described by least square regressions.
3. Results
3.1. Soil Moisture
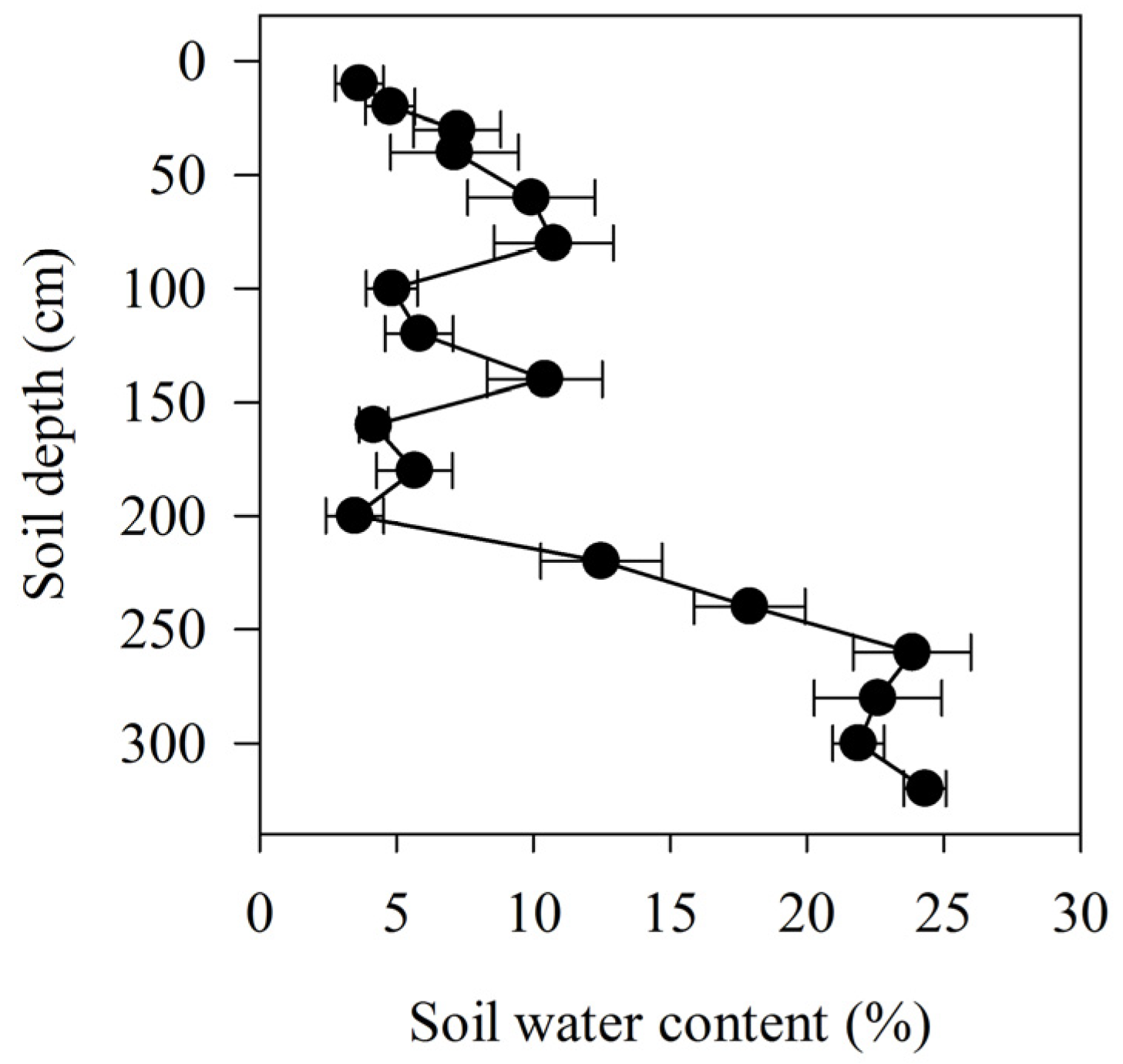
The average water content in the soil profile was 3.6 ± 0.9% at a depth of 10 cm; this increased to 10.7 ± 2.1% at 80 cm, decreased to 4.8 ± 0.9% at 100 cm, increased again to 10.4 ± 2.1% at 140 cm, and decreased again to 3.5 ± 1.1% at 200 cm (Figure 1). The water content of the deeper soil profile increased with depth, from 12.5 ± 2.2% at 220 cm to 24.3 ± 0.8% at 320 cm.
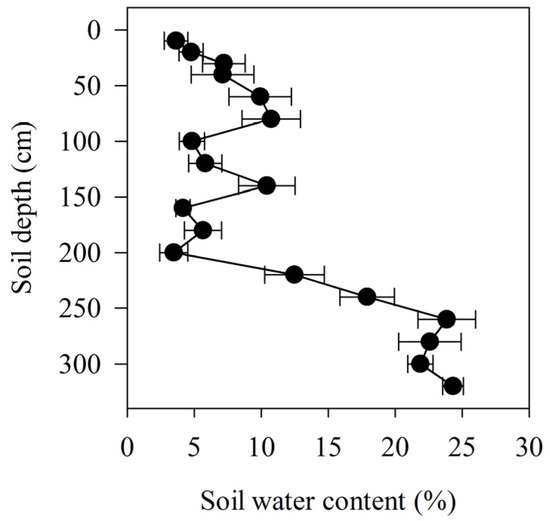
Figure 1.
Soil water content relative to soil depth, collected in the lower reaches of the Heihe River.
3.2. Isotopic Composition of Water
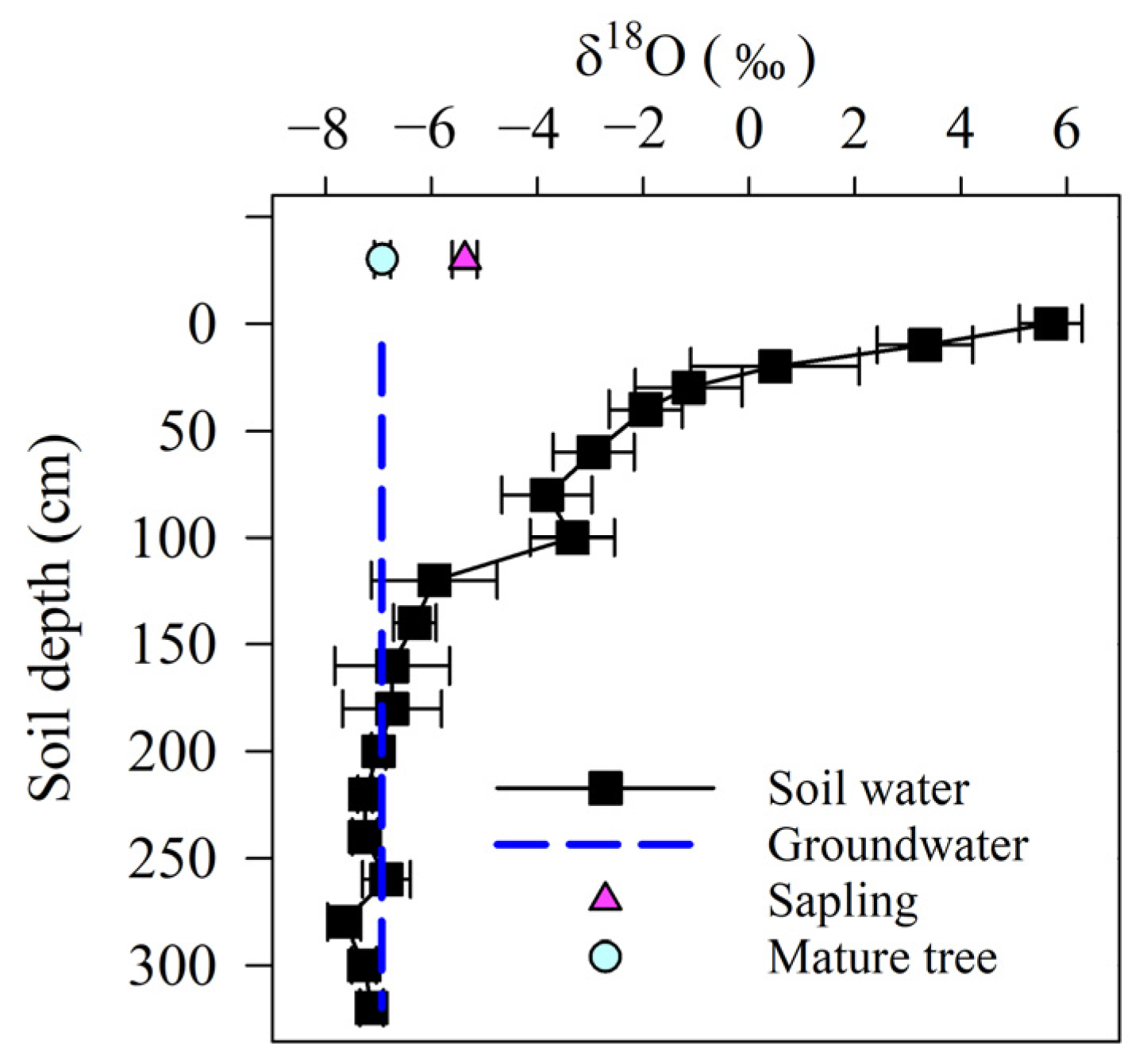
The δ18O soil water values exhibited evaporative enrichment in the heavier isotopes close to the surface but decreased markedly with increasing depth (Figure 2). The δ18O soil water values dropped suddenly from 5.70‰ at 0 cm to −3.82‰ at 80 cm. Soil water became progressively depleted in 18O between 120 and 320 cm. Overall, the δ18O of the subsurface soil water was more stable than that of surface soil water. The groundwater δ18O was −6.94‰, which was close to the δ18O of the soil water stored at 160–320 cm. The δ18O of P. euphratica of different size classes showed a marked difference (p < 0.01). The δ18O of the xylem water in the mature trees was most similar to the groundwater or deep soil water and was more negative than in the saplings (p < 0.01)
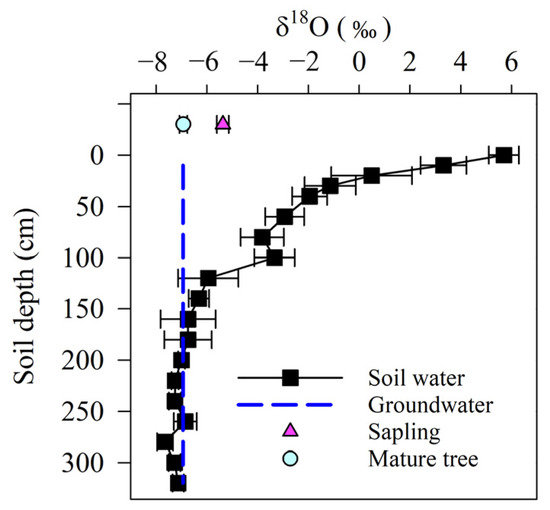
Figure 2.
Stable oxygen isotopic signature of plant xylem water, soil water, and groundwater.
3.3. Water Uptake
The contribution ratios of various water sources for the two sizes of P. euphratica calculated by the IsoSource model are shown in Table 1. The results showed that between 68% and 71% of the water uptake by the saplings was taken from the surface soil, and the subsurface soil water and groundwater were less used. However, the mature trees used little surface soil water (2%–6%) and mainly used subsurface soil (58.4% on average) and groundwater (37.6% on average) (Table 1). Due to the similar δ18O between the subsurface soil water and groundwater, it is difficult to distinguish which source (groundwater or subsurface soil) was utilized by the mature trees.

Table 1.
Estimated proportion of water source use (%) in Populus euphratica.
3.4. Leaf Water Potential
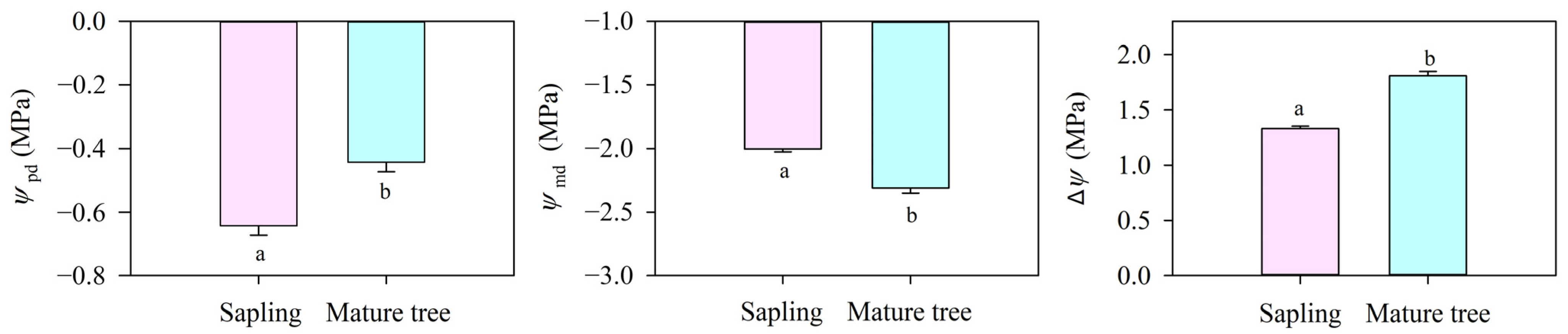
Predawn leaf water potential (Ψpd) in the mature trees (−0.44 MPa) was significantly higher than that in the saplings (−0.64 MPa, p < 0.05, Figure 3) because of deeper water resource uptake. Midday leaf water potential (Ψmd) in the mature trees was obviously lower than that in the saplings (p < 0.05). As a consequence, the water potential difference between the soil and the leaf (ΔΨ) did vary between the saplings and the mature trees and generated a different driving force (about 1.33 MPa in the saplings and about 1.81 MPa in the mature trees) for the water flow from the soil to the leaves.
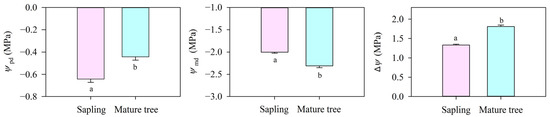
Figure 3.
Predawn water potential (Ψpd), midday water potential (Ψmd), and soil-to-leaf water potential gradient (ΔΨ, calculated as Ψsoil−Ψleaf-ρgh) for P. euphratica at different sizes. Vertical bars represent the standard error of the means (n = 5). Different letters at each column indicate significant differences between the saplings and the mature trees (p < 0.05).
3.5. Hydraulic Conductivity and Gas Exchange
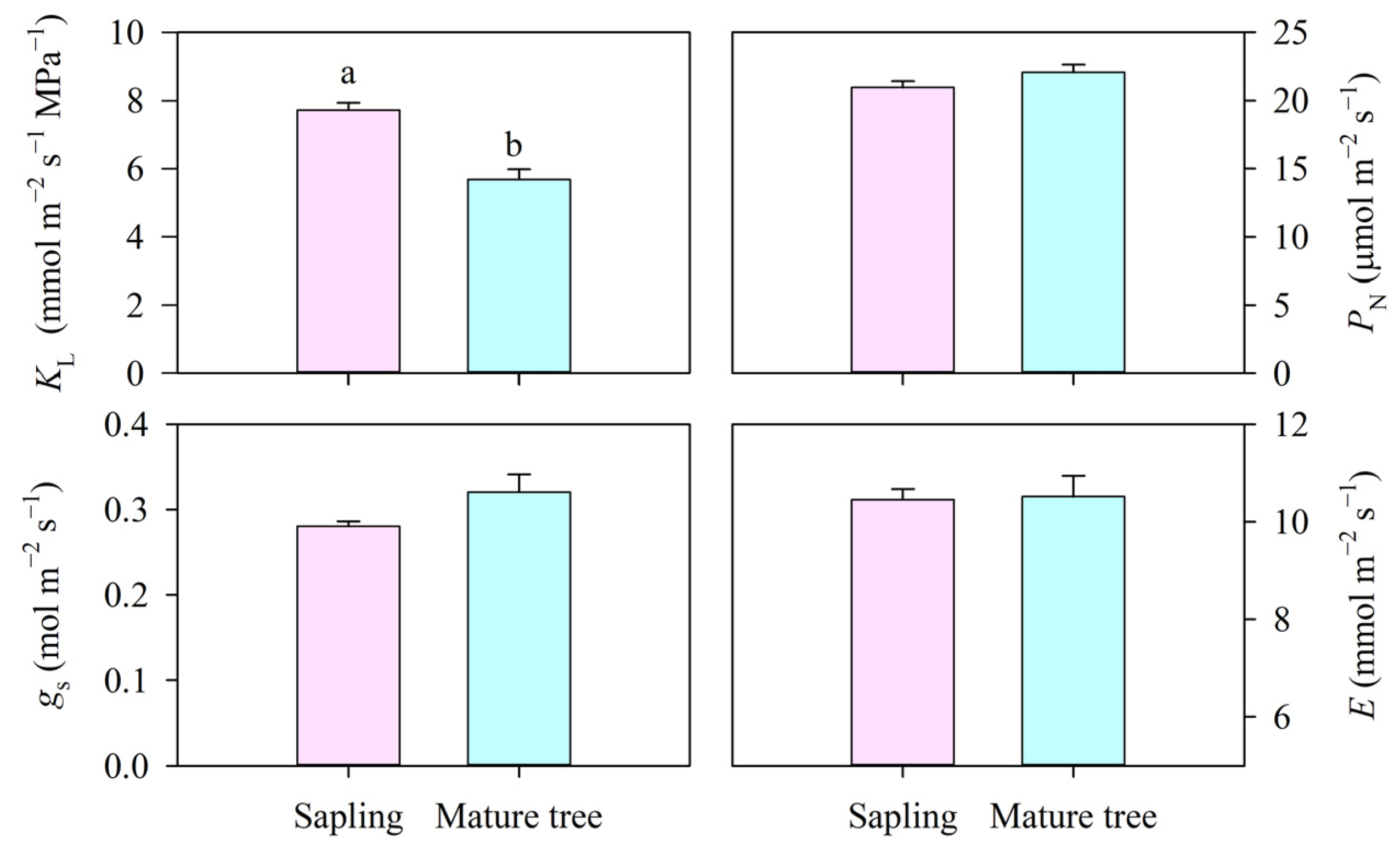
The leaf-specific apparent hydraulic conductance (KL) significantly differed between the size classes (p < 0.01, Figure 4). The KL in the mature trees was 5.67 mmol m−2 s−1 MPa−1, which was 26.5% lower than that in the saplings (p < 0.01) because of the similar E and higher water potential difference between the soil and leaves. Furthermore, the PN was about 21 μmol m−2 s−1 and was not found to be significantly different between the size classes (p > 0.05). Likewise, no statistical difference was found in gs or E between the saplings and the mature trees (p > 0.05, Figure 4).
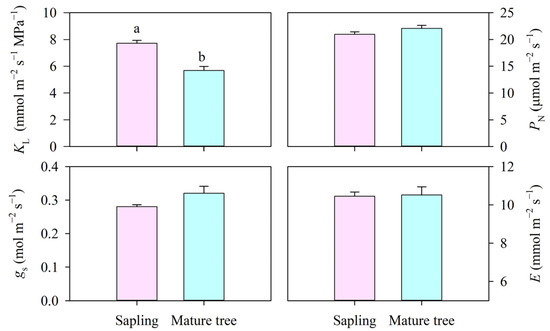
Figure 4.
The leaf-specific apparent hydraulic conductance (KL), net photosynthesis rate (PN), stomatal conductance (gs), and transpiration rate (E) of P. euphratica of different sizes. Vertical bars represent the standard error of the means (n = 5). Different letters at each column denote significant differences between the saplings and the mature trees (p < 0.05).
3.6. The Relationship between KL and Gas Exchange, Ψ
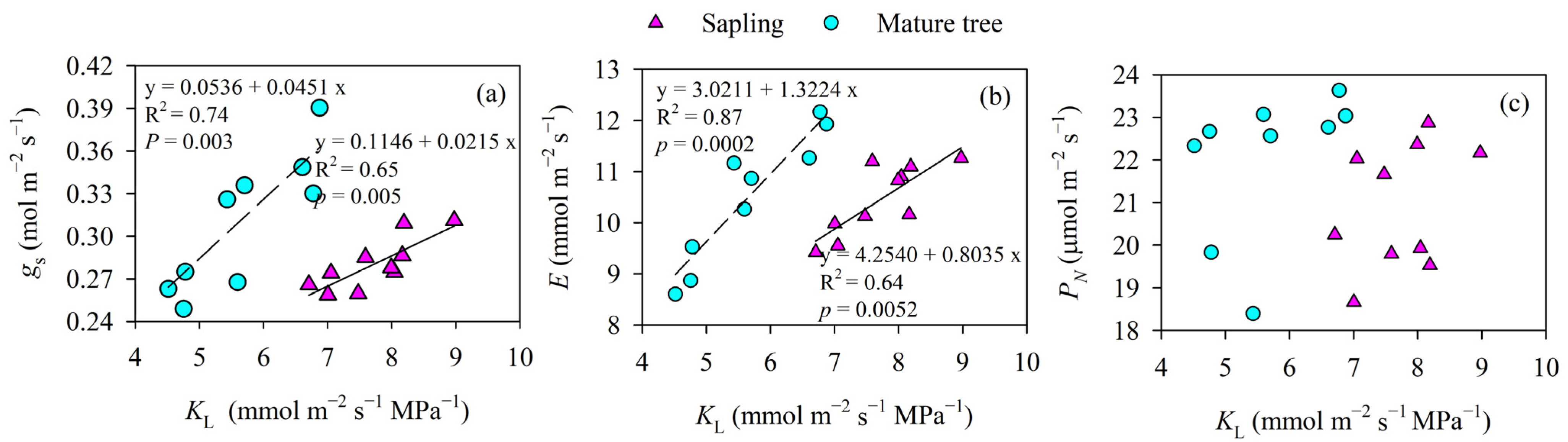
When all the data for KL and gs for the saplings and mature trees were pooled, KL was not positively correlated with the gs measured at midday in P. euphratica (p > 0.05), but a positive correlation relationship was observed for saplings or mature trees (p < 0.01). However, the effect of KL on gs for different tree heights was different. For example, the mature trees showed greater increases in gs for the same KL increase when compared to the saplings (p < 0.05, Figure 5a). Moreover, the mature trees tended to show a higher gs and E than the saplings for the same KL, although the difference was not significant in gs and E between the saplings and the mature trees (p > 0.05).
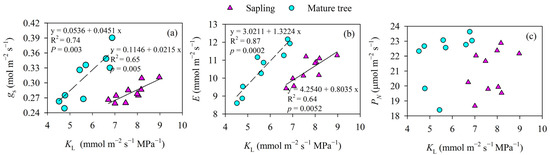
Figure 5.
The relationship between leaf-specific apparent hydraulic conductance (KL) and stomatal conductance (gs) and the transpiration rate (E) and photosynthesis rate (PN) of P. euphratica at different sizes. Lines indicate significant linear regressions fit at a p value of <0.05. Asterisks indicate a significant difference in the slopes of gs versus KL between the size classes (p < 0.05). (a) stomatal conductance; (b) transpiration rate; (c) photosynthesis rate.
The mature trees showed a significant linear increase in E with increasing KL (p < 0.01). Again, the saplings also showed a similar linear relationship between the two variables, but the slope of E versus KL in the mature trees was marginally higher than that in the saplings (p = 0.09, Figure 5b). There were no significant correlations between PN and KL in either the trees or the saplings (R2 = 0.14, p = 0.28 in saplings, and R2 = 0.19, p = 0.24 in mature trees, Figure 5c).
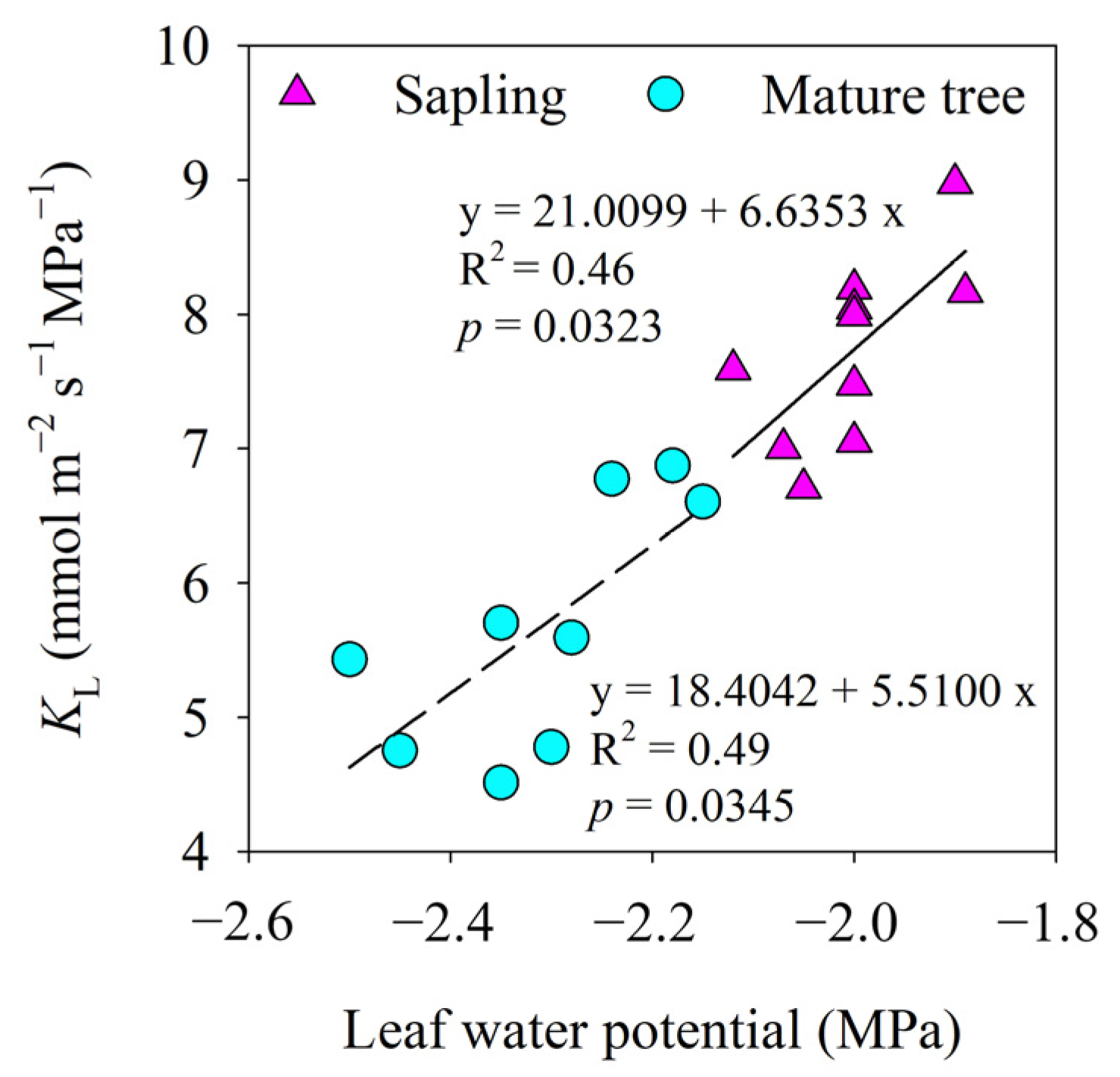
At different stages of development, there was a clear link between leaf water potential and the KL of P. euphratica (p < 0.01). Over the midday water potential range, the KL steadily declined with decreasing leaf water potential, indicating anisohydric regulation. The rate of increase in KL with leaf water potential in the saplings and mature trees was similar (Figure 6).
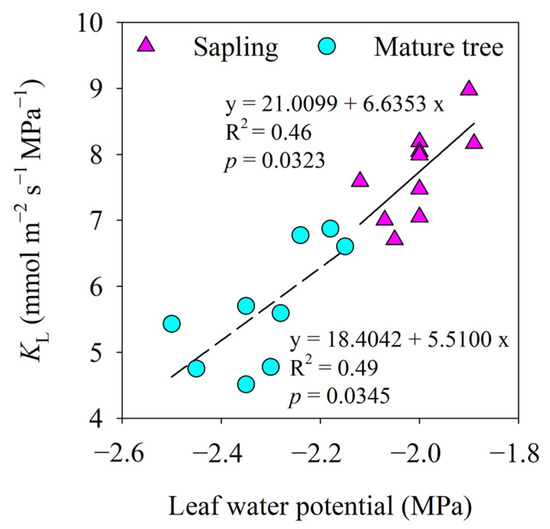
Figure 6.
The relationship between leaf water potential and leaf-specific apparent hydraulic conductance (KL). Lines indicate significant linear regressions fitted at a p value of <0.05.
4. Discussion
4.1. The Effect of Leaf Hydraulic Conductance on Stomatal Conductance
Some studies suggest that manipulating leaf-specific hydraulic conductance would alter gs, but not in every case [45,46]. The mechanisms by which the hydraulic signals control stomatal closure have not been clear yet [47]. In this study of both saplings and mature trees, leaf stomatal conductance had a strong positive correlation with KL (Figure 5a), suggesting that a decrease in leaf-specific apparent hydraulic conductance exercises a great influence on stomatal closure, reducing transpiration. This is in agreement with previous studies that documented the coordination of gs with plant hydraulic conductance [48,49]. Meanwhile, we found that the effect of KL on gs was different between saplings and mature trees via the slope rate of gs and KL (Figure 5a). Stomatal conductance (gs) is primarily a function of hydraulic conductance between soil and leaf (KL), the leaf-to-air vapor pressure gradient (D), and the soil-to-leaf water potential difference (ΔΨ): gs = KL (Ψsoil−Ψleaf)/D [22]. At one measurement time, Ψsoil and D are constant, and the response of stomata to KL depends only on Ψleaf, according to the formula mentioned above. Mature trees had a lower Ψleaf during midday (Figure 3) so the decrease in gs of the mature trees was larger than that of the saplings under the same decrease in KL.
4.2. The Relationship between Hydraulic Properties and Gas Exchange
According to the hydraulic limitation hypothesis, increased path length with height can diminish leaf-specific hydraulic conductance [25], and plants with high hydraulic conductance can efficiently transport water from their roots to their leaves, maximizing gs [50]. Our result is not completely consistent with the hydraulic limitation hypothesis. In this study, the KL in mature P. euphratica was much lower than in saplings, but the lower KL did not result in a lower gs in the mature trees (Figure 4). If ΔΨ remained constant as the trees increased in height, the gs would reduce and increasingly limit photosynthetic assimilation [10,51]. The results above may be caused by the difference in the water potential gradient from the soil to the leaf between the saplings and the mature trees, which may generate a variation in gs [52]. The mature trees had a high Ψpd due to the uptake of deeper water resources and low Ψmd, so the ΔΨ in the mature trees was higher than that in the saplings (Figure 3). The increase in ΔΨ offset the negative effect of the decreasing KL on gs with increasing tree height. Consequently, great leaf water potential regulation in the mature trees resulted in a similar gs in the saplings and mature trees.
Plants generally display either isohydric and anhydrous properties or both, depending on the species [53]. Interestingly, the slope of the linear fit between the predawn and midday leaf water potential is higher for anisohydric species [53]. Anisohydric plants were able to sustain high photosynthetic rates while their leaf water potential decreased [52]. In the present research, we also discovered that although the leaf water potential of the P. euphratica trees declines with their development, they may still sustain a certain photosynthetic rate (Figure 3 and Figure 4). The PN of anisohydric species may show less response to sequential decreases in leaf-specific hydraulic conductance [51]. In this study, the PN did not respond to a decrease in KL in the mature trees or saplings (Figure 5), but the leaf water potential obviously decreased with decreasing KL (Figure 6), indicating that P. euphratica is an anisohydric plant. This result coincides with the study by Gries et al. (2003) [54]. The linkage between gs and PN changes with environments but is approximately linear [55]. The saplings had a similar value for gs to the mature trees (Figure 4), and thus PN did not respond to a reduction in KL as P. euphratica grew older.
4.3. Comparison of Water Sources between Saplings and Mature Trees
Plant predawn water potential (Ψpd) is a close proxy for the water potential of the soil, where plants extract water [56]. Therefore, Ψpd is correlated with plant water sources [57]. The differences in Ψpd between the mature trees and the saplings (Figure 3) suggest differences in their water acquisition strategies. Furthermore, the isotopic 18O signatures of the trees and saplings were different (Figure 2), showing that they took up different water resources. Mature trees with a high Ψpd may uptake deeper water resources, and saplings were likely to utilize the water sources closer to the soil surface because of the drop in Ψpd. Predawn plant water potential does not always correspond with the available soil water potential according to investigations of plant nocturnal sap flow, which have identified an imbalance between the predawn leaf water potential and soil water potential in several species [58,59]. However, field studies have also shown that soil water potential and Ψpd are closely related under various water treatments, even when there is little transpiration at night, and Ψpd can be used as a rough substitute for the water potential of soil, from which plants extract water [60]. This suggests that there is still a great deal of uncertainty in the results above. The extrapolation of water resources made above using Ψpd was consistent with the calculation results (of contributions of different water sources for plants) of the IsoSource model (Table 1), indicating the validity of using predawn leaf water potential to extrapolate the water source of P. euphratica.
In general, larger trees had access to deep water resources using their deep roots, whereas smaller trees did not [61,62]. However, this does not always hold true. For example, Meinzer et al. (2001) observed that during dry periods, smaller trees were more likely than larger trees to tap deeper soil water [47]. In addition, Stahl et al. (2013) showed that there is no strong relationship between tree size and the depth of water uptake [57]. In this study, we found different patterns of water uptake by the saplings and mature trees. The saplings mostly depend on 0–80 cm of surface soil water, but the mature trees mainly uptook deep soil water stored below 80 cm and groundwater. By not using the surface soil water, the mature trees may be able to avoid water competition with the saplings that inhabit the same sites. This could increase the likelihood of surviving a drought.
4.4. The Effect of Water Source Changes on Plant Physiology
Water acquisition via the root system of woody species is a key factor governing plant physiology [63,64]. Mature trees can tap deeper soil water and groundwater (Table 1, Figure 2), supplying the canopy with water more steadily. However, saplings mostly rely on surface soil water (Table 1, Figure 2), which is an unstable water resource for plant transpiration in arid regions. The difference in the water acquisition patterns of the saplings and mature trees may influence their water transport strategy. The unreliability of the water supply could make saplings hold high hydraulic conductance to ensure efficient water transport from soil to leaf and to satisfy their transpiration need. In contrast, mature trees that uptake reliable, deeper water resources had a relatively low leaf-specific hydraulic conductance because of the increased path length (versus the saplings). However, mature trees can maintain stomatal conductance by upregulating ΔΨ (decreasing midday Ψleaf) and thus maintain a carbon assimilation rate similar to that of the saplings (Figure 3 and Figure 4). This regulation behavior benefits mature trees’ net carbon gain, but it is at the cost of an increased risk of hydraulic failure [65,66]. These results implied that the top priority for saplings should be to maintain hydraulic system functioning, but for mature trees, the priority is to assure stable net carbon gain for growth.
Our earlier studies have also shown that the anatomical characteristics of P. euphratica leaves vary according to the stage of development, with mature trees showing a noticeably higher leaf epidermal thickness, palisade tissue thickness, and stomatal density than saplings [67,68], all of which will have an effect on the physiological processes in plants [15,69]. Therefore, more research into the link between anatomical structure and physiological processes is required to fully understand the impact of shifting water resources on the physiological processes in mature trees and saplings.
5. Conclusions
In the present study, we found that the net photosynthetic rate and stomatal conductance in P. euphratica did not consistently decline with increasing size; we did not observe coordination between the hydraulic and leaf gas exchange properties among the different tree sizes. This study also suggests that water source partitioning is coordinated with the hydraulic properties of P. euphratica of different sizes. The saplings mostly uptook unstable water resources but had higher water transport efficiency and safety. In contrast, the mature trees mostly rely on reliable deeper water resources and therefore had a larger risk of losing the conducting system. With global warming, drought may become more severe in arid areas, and in response to this, mature trees will continuously downregulate water potential to maintain their growth, making them likely to die due to hydraulic failure, while saplings will die of “thirst” because there is insufficient water in the topsoil moisture deficit. In order to provide additional information and references for the conservation and regeneration of desert riparian vegetation in arid areas, additional comparative investigations of the hydraulic characteristics and water uptake of P. euphratica under different drought situations are needed.
Author Contributions
Conceptualization, Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, X.H. and C.Z.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number U1903114.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank A.H. Fu and S.B. Liu for their assistance with fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steppe, K.; Niinemets, Ü.; Teskey, R.O. Tree Size- and Age-Related Changes in Leaf Physiology and Their Influence on Carbon Gain. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 235–253. [Google Scholar]
- Anderegg, W.R.L.; Venturas, M.D. Plant hydraulics play a critical role in Earth system fluxes. New Phytol. 2020, 226, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Vanderklein, D.; Martínez-Vilalta, J.; Lee, S.M.; Encuccini, M. Plant size, not age, regulates growth and gas exchange in grafted Scots pine trees. Tree Physiol. 2007, 27, 71–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bielczynski, L.W.; Łącki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and Plant Age Affects Photosynthetic Performance and Photoprotective Capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, D.; Barbour, M.M.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T. Effects of leaf age and tree size on stomatal and mesophyll limitations to photosynthesis in mountain beech (Nothofagus solandrii var. cliffortiodes). Tree Physiol. 2011, 31, 985–996. [Google Scholar] [CrossRef]
- Bao, J.T.; Wang, J.; Li, X.R.; Zhang, Z.S.; Su, J.Q. Age-related changes in photosynthesis and water relations of revegetated Caragana korshinskii in the Tengger desert, Northern China. Trees 2015, 29, 1749–1760. [Google Scholar] [CrossRef]
- Bond, B.J. Age-related changes in photosynthesis of woody plants. Trends Plant Sci. 2000, 5, 349–353. [Google Scholar] [CrossRef]
- Hölttä, T.; Kurppa, M.; Nikinmaa, E. Scaling of xylem and phloem transport capacity and resource usage with tree size. Front. Plant Sci. 2013, 4, 496. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic Limits to tree height and tree Growth. BioScience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef]
- Yoder, B.J.; Ryan, M.G.; Waring, R.H.; Schoettle, A.W.; Kaufmann, M.R. Evidence of reduced photosynthetic rates in old trees. For. Sci. 1994, 40, 513–527. [Google Scholar]
- Sendall, K.M.; Reich, P.B. Variation in leaf and twig CO2 flux as a function of plant size: A comparison of seedlings, saplings and trees. Tree Physiol. 2013, 33, 713–729. [Google Scholar] [CrossRef]
- Xiong, D.; Nadal, M. Linking water relations and hydraulics with photosynthesis. Plant J. 2020, 101, 800–815. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Kröber, W.; Heklau, H.; Bruelheide, H. Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol. 2015, 17, 373–383. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C.; Lachenbruch, B.; Johnson, D.M. Coordination of leaf structure and gas exchange along a height gradient in a tall conifer. Tree Physiol. 2009, 29, 261–272. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Liu, W.; Xu, H.; Sun, O.J. Changes in water use with growth in Ulmus pumila in semiarid sandy land of northern China. Trees 2014, 28, 41–52. [Google Scholar] [CrossRef]
- Pangle, R.; Kavanagh, K.; Duursma, R. Decline in canopy gas exchange with increasing tree height, atmospheric evaporative demand, and seasonal drought in co-occurring inland Pacific Northwest conifer species. Can. J. For. Res. 2015, 45, 1086–1101. [Google Scholar] [CrossRef]
- Kenzo, T.; Inoue, Y.; Yoshimura, M.; Yamashita, M.; Tanaka-Oda, A.; Ichie, T. Height-related changes in leaf photosynthetic traits in diverse Bornean tropical rain forest trees. Oecologia 2015, 177, 191–202. [Google Scholar] [CrossRef]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between saplings and adult trees: An integration of field results by meta-analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef]
- McDowell, N.G.; Licata, J.; Bond, B.J. Environmental sensitivity of gas exchange in different-sized trees. Oecologia 2005, 145, 9–20. [Google Scholar] [CrossRef]
- McDowell, N.G.; Phillips, N.; Lunch, C.; Bond, B.J.; Ryan, M.G. An investigation of hydraulic limitation and compensation in large, old Douglas-fir trees. Tree Physiol. 2002, 22, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.; Bond, B.J.; McDowell, N.G.; Ryan, M.G.; Schauer, A. Leaf area compounds height-related hydraulic costs of water transport in Oregon White Oak trees. Funct. Ecol. 2003, 17, 832–840. [Google Scholar] [CrossRef]
- Koch, G.W.; Sillett, S.C.; Antoine, M.E.; Williams, C.B. Growth maximization trumps maintenance of leaf conductance in the tallest angiosperm. Oecologia 2015, 177, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Barnard, H.R.; Ryan, M.G. A test of the hydraulic limitation hypothesis in fast-growing Eucalyptus saligna. Plant Cell Environ. 2003, 26, 1235–1245. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Mueller, R.C.; Scudder, C.M.; Porter, M.E.; Trotter, R.T.; Gehring, C.A.; Whitham, T.G. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J. Ecol. 2005, 93, 1085–1093. [Google Scholar] [CrossRef]
- Madsen, C.; Potvin, C.; Hall, J.; Sinacore, K.; Turner, B.L.; Schnabel, F. Coarse root architecture: Neighbourhood and abiotic environmental effects on five tropical tree species growing in mixtures and monocultures. For. Ecol. Manag. 2020, 460, 117851. [Google Scholar] [CrossRef]
- Song, L.; Yang, B.; Liu, L.L.; Mo, Y.X.; Liu, W.J.; Meng, X.J.; Lu, H.Z.; Li, Y.; Zakari, S.; Tan, Z.H.; et al. Spatial-temporal differentiations in water use of coexisting trees from a subtropical evergreen broadleaved forest in Southwest China. Agric. For. Meteorol. 2022, 316, 108862. [Google Scholar] [CrossRef]
- Jackson, R.B.; Moore, L.A.; Hoffmann, W.A.; Pockman, W.T.; Linder, C.R. Ecosystem rooting depth determined with caves and DNA. Proc. Natl. Acad. Sci. USA 1999, 96, 11387–11392. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xu, C.; Li, W. The effects of groundwater depth on water uptake of Populus euphratica and Tamarix ramosissima in the hyperarid region of Northwestern China. Environ. Sci. Pollut. Res. 2016, 23, 17404–17412. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Zhou, H.; Chen, Y.; Hao, X.; Fu, A.; Ma, J. Experimental study on water transport observations of desert riparian forests in the lower reaches of the Tarim River in China. Int. J. Biometeorol. 2017, 61, 1055–1062. [Google Scholar] [CrossRef]
- Si, J.H.; Feng, Q.; Cao, S.K.; Yu, T.F.; Zhao, C.Y. Water use sources of desert riparian Populus euphratica forests. Environ. Monit. Assess. 2014, 186, 5469–5477. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xu, C.; Li, W. Groundwater depth affects the daily course of gas exchange parameters of Populus euphratica in arid areas. Environ. Earth Sci. 2012, 66, 433–440. [Google Scholar] [CrossRef]
- Li, D.; Si, J.; Zhang, X.; Gao, Y.; Luo, H.; Qin, J.; Gao, G. The Mechanism of Changes in Hydraulic Properties of Populus euphratica in Response to Drought Stress. Forests 2019, 10, 904. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Chen, Y.; Wang, R.; Ren, Z. Impact of groundwater depth on leaf hydraulic properties and drought vulnerability of Populus euphratica in the Northwest of China. Trees 2016, 30, 2029–2039. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhou, H.; Hao, X.; Zhu, C.; Fu, A.; Yang, Y.; Li, W. Research Advances in Plant Physiology and Ecology of Desert Riparian Forests under Drought Stress. Forests 2022, 13, 619. [Google Scholar] [CrossRef]
- Wan, Y.; Shi, Q.; Dai, Y.; Marhaba, N.; Peng, L.; Peng, L.; Shi, H. Water Use Characteristics of Populus euphratica Oliv. and Tamarix chinensis Lour. at Different Growth Stages in a Desert Oasis. Forests 2022, 13, 236. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W. Characterizing the spatial structures of riparian plant communities in the lower reaches of the Heihe River in China using geostatistical techniques. Environ. Res. 2006, 21, 551–559. [Google Scholar] [CrossRef]
- Vandenhonert, T.H. Water transport in plants as a catenary process. Discuss. Faraday Soc. 1948, 3, 146–153. [Google Scholar] [CrossRef]
- West, A.G.; Patrickson, S.J.; Ehleringer, J.R. Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun. Mass Spectrom. 2006, 20, 1317–1321. [Google Scholar] [CrossRef]
- Dawson, T.E. Water Sources of Plants as Determined from Xylem-Water Isotopic Composition: Perspectives on Plant Competition, Distribution, and Water Relations. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 465–496. [Google Scholar]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.H.; Sternberg, L.D.L. Utilization of surface water by red mangrove (Rhizophora Mangle L.): An isotopic study. Bull. Mar. Sci. 1994, 54, 94–102. [Google Scholar]
- Liu, X.R.; Liu, H.; Gleason, S.M.; Goldstein, G.; Zhu, S.D.; He, P.C.; Hou, H.; Li, R.H.; Ye, Q. Water transport from stem to stomata: The coordination of hydraulic and gas exchange traits across 33 subtropical woody species. Tree Physiol. 2019, 39, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.; Bond, B.J.; Ryan, M.G. Gas exchange and hydraulic properties in the crowns of two tree species in a Panamanian moist forest. Trees 2001, 15, 123–130. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Clearwater, M.J.; Goldstein, G. Water transport in trees: Current perspectives, new insights and some controversies. Environ. Exp. Bot. 2001, 45, 239–262. [Google Scholar] [CrossRef]
- Woodruff, D.R.; McCulloh, K.A.; Warren, J.M.; Meinzer, F.C.; Lachenbruch, B. Impacts of tree height on leaf hydraulic architecture and stomatal control in Douglas-fir. Plant Cell Environ. 2007, 30, 559–569. [Google Scholar] [CrossRef]
- Beikircher, B.; Sack, L.; Ganthaler, A.; Losso, A.; Mayr, S. Hydraulic-stomatal coordination in tree seedlings: Tight correlation across environments and ontogeny in Acer pseudoplatanus. New Phytol. 2021, 232, 1297–1310. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Hubbard, R.M.; Ryan, M.G.; Stiller, V.; Sperry, J.S. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 2001, 24, 113–121. [Google Scholar] [CrossRef]
- Attia, Z.; Domec, J.C.; Oren, R.; Way, D.A.; Moshelion, M. Growth and physiological responses of isohydric and anisohydric poplars to drought. J. Exp. Bot. 2015, 66, 4373–4381. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, R.A.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Gries, D.; Zeng, F.; Foetzki, A.; Arndt, S.K.; Bruelheide, H.; Thomas, F.M.; Zhang, X.; Runge, M. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ. 2003, 26, 725–736. [Google Scholar] [CrossRef]
- Leuning, R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Ratzmann, G.; Zakharova, L.; Tietjen, B. Optimal leaf water status regulation of plants in drylands. Sci. Rep. 2019, 9, 3768. [Google Scholar] [CrossRef]
- Stahl, C.; Herault, B.; Rossi, V.; Burban, B.; Brechet, C.; Bonal, D. Depth of soil water uptake by tropical rainforest trees during dry periods: Does tree dimension matter? Oecologia 2013, 173, 1191–1201. [Google Scholar] [CrossRef]
- Kangur, O.; Tullus, A.; Sellin, A. Night-time transpiration, predawn hydraulic conductance and water potential disequilibrium in hybrid aspen coppice. Trees 2020, 34, 133–141. [Google Scholar] [CrossRef]
- Forster, M.A. How significant is nocturnal sap flow? Tree Physiol. 2014, 34, 757–765. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Romero-Saltos, H.; Sternberg, L.; Moreira, M.Z.; Nepstad, D.C. Rainfall exclusion in an eastern Amazonian forest alters soil water movement and depth of water uptake. Am. J. Bot. 2005, 92, 443–455. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, H.W.; Shi, Q.D. Contrasting plant water-use responses to groundwater depth from seedlings to mature trees in the Gurbantunggut Desert. J. Hydrol. 2022, 610, 127986. [Google Scholar] [CrossRef]
- Dawson, T.E.; Pate, J.S. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of di-morphic root morphology: A stable isotope investigation. Oecologia 1996, 107, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and Root Water Uptake. In Plant Aquaporins: From Transport to Signaling; Chaumont, F., Tyerman, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 133–153. [Google Scholar]
- Scoffoni, C.; Sack, L. The causes and consequences of leaf hydraulic decline with dehydration. J. Exp. Bot. 2017, 68, 4479–4496. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Moura, C.F.; Caldeira, M.C.; Willson, C.J.; Jackson, R.B. Functional coordination between leaf gas exchange and vulnerability to xylem cavitation in temperate forest trees. Plant Cell Environ. 2006, 29, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q. The Response of the Anatomical Structure and Hydraulic Characteristics in Xylem of Populus euphratica Oliv.to Drought Stress. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2012. [Google Scholar]
- Pan, Y.P.; Chen, Y.P.; Wang, H.J.; Ren, Z.G. Leaf structure and functional traits of Populus euphratica. J. For. Res. 2018, 38, 765–771. [Google Scholar]
- Li, S.; Hamani, A.K.M.; Zhang, Y.; Liang, Y.; Gao, Y.; Duan, A. Coordination of leaf hydraulic, anatomical, and economical traits in tomato seedlings acclimation to long-term drought. BMC Plant Biol. 2021, 21, 536. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).