Unraveling the Effects of Pruning Frequency on Biomass Productivity, Nonstructural Carbohydrates and Nitrogen Fixation Rates of Sesbania sesban
Abstract
:1. Introduction
2. Materials and Method
2.1. Experimental Site and Plant Culture
2.2. Nodulation
2.3. Pruning Treatments
2.4. Plant Sampling and Processing
2.5. Carbohydrate Analyses
2.6. Analysis of Isotopic Composition
2.7. Statistical Analysis
3. Results
3.1. Above- and Belowground Biomass Productivity
3.2. Reserve Carbohydrate Concentrations
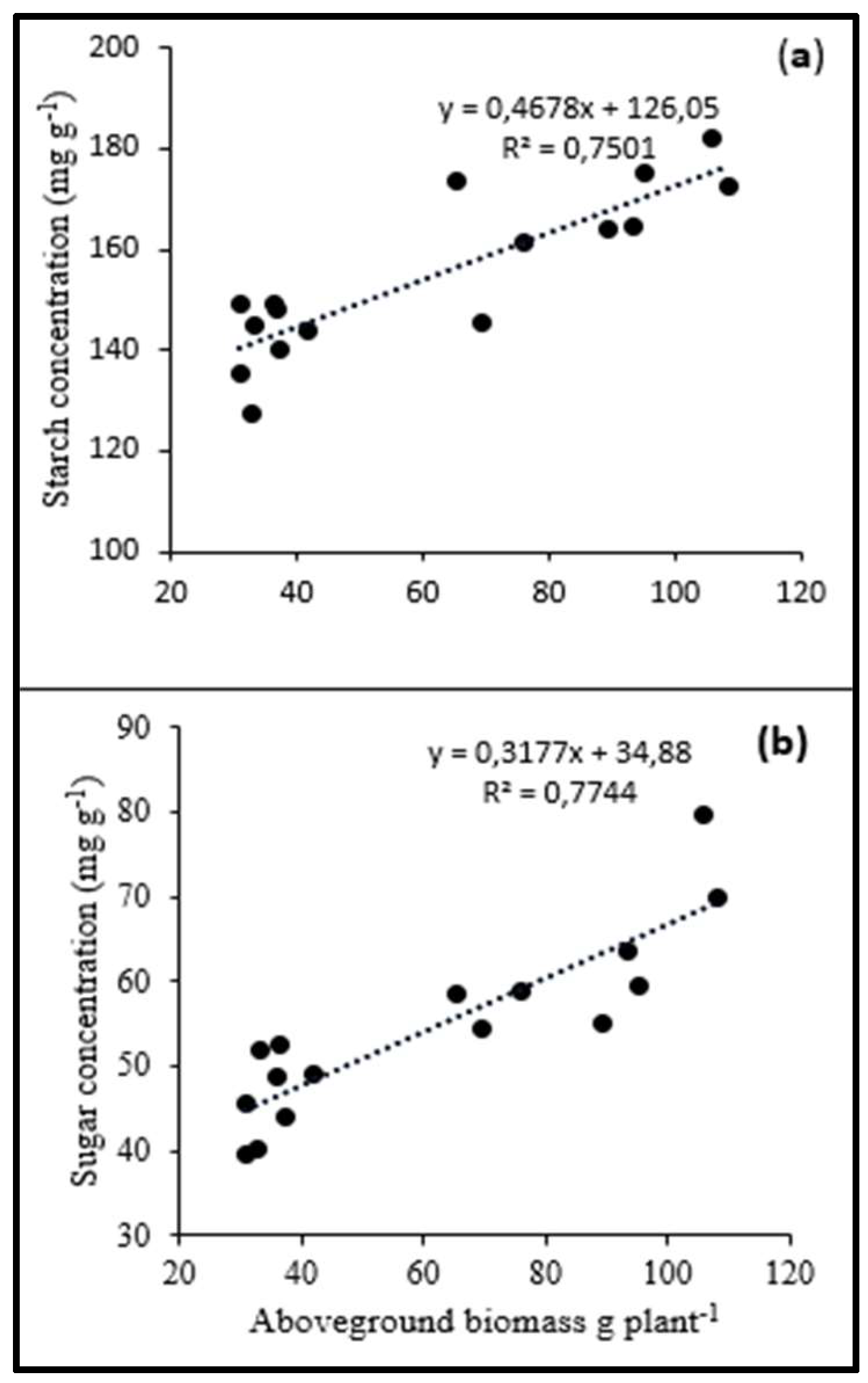
3.3. Relationship between Aboveground DM Yield and Carbohydrate Concentrations
3.4. Nodulation and Symbiotic Performance
4. Discussion
4.1. Above- and Belowground Biomass Productivity
4.2. Reserve Carbohydrate Concentrations
4.3. Relationship between Aboveground DM Yield and Carbohydrate Concentrations
4.4. Nodulation and Symbiotic Performance
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chikowo, R.; Mapfumo, P.; Nyamugafata, P.; Giller, K.E. Woody legume fallow productivity, biological N2-fixation and residual benefits to two successive maize crops in Zimbabwe. Plant Soil. 2004, 262, 303–315. [Google Scholar] [CrossRef]
- Ståhl, L.; Nyberg, G.; Högberg, P.; Buresh, R.J. Effects of planted tree fallows on soil nitrogen dynamics, above-ground and root biomass, N2-fixation and subsequent maize crop productivity in Kenya. Plant Soil. 2002, 243, 103–117. [Google Scholar] [CrossRef]
- Bala, A.; Giller, K.E. Symbiotic specificity of tropical tree rhizobia for host legumes. New Phytol. 2001, 149, 495–507. [Google Scholar] [CrossRef]
- Franzel, S.; Carsan, S.; Lukuyu, B.; Sinja, J.; Wambugu, C. Fodder trees for improving livestock productivity and smallholder livelihoods in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Roothaert, R.; Franzel, S.; Kiura, M. On-farm evaluation of fodder trees and shrubs preferred by farmers in central Kenya. Exp. Agric. 2003, 39, 423–440. [Google Scholar] [CrossRef]
- Cuni-Sanchez, A.; Pfeifer, M.; Marchant, R.; Pompeu, P.V.; Burgess, N.D. Harvesting fodder trees in montane forests in Kenya: Species, techniques used and impacts. New For. 2018, 49, 511–528. [Google Scholar] [CrossRef] [Green Version]
- Duguma, B.; Kang, B.T.; Okali, D.U.U. Effect of pruning intensities of three woody leguminous species grown in alley cropping with maize and cowpea on an alfisol. Agrofor. Syst. 1988, 6, 19–35. [Google Scholar] [CrossRef]
- Ezenwa, I.V.; Atta-Krah, A.N. Early growth and nodulation in Leucaena and Gliricidia and the effects of pruning on biomass productivity. In Biological Nitrogen Fixation and Sustainability of Tropical Agriculture; Mulongoy, K., Gueye, M., Spencer, D.S.C., Eds.; Wiley & Sons: Chichester, UK, 1992; pp. 171–178. [Google Scholar]
- Garcia, H.; Nygren, P.; Desfontaines, L. Dynamics of nonstructural carbohydrates and biomass yield in a fodder legume tree at different harvest intensities. Tree Physiol. 2001, 21, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Moyo, H.; Scholes, M.C.; Twine, W. The effects of repeated cutting on coppice response of Terminalia sericea. Trees 2015, 29, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Chesney, P.; Vasquez, N. Dynamics of non-structural carbohydrate reserves in pruned Erythrina poeppigiana and Gliricidia sepium trees. Agrofor. Syst. 2007, 69, 89–105. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol. Evol. 2015, 5, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Partey, S.T. Effect of pruning frequency and pruning height on the biomass production of Tithonia diversifolia (Hemsl) A. Gray. Agrofor. Syst. 2011, 83, 181–187. [Google Scholar] [CrossRef]
- Latt, C.R.; Nair, P.K.R.; Kang, B.T. Interactions among cutting frequency, reserve carbohydrates, and post-cutting biomass production in Gliricidia sepium and Leucaena leucocephala. Agrofor. Syst. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Molero, G.; Erice, G.; Aldasoro, J.; Arrese-Igor, C.; Nogués, S. Effect of shoot removal on remobilization of carbon and nitrogen during regrowth of nitrogen-fixing alfalfa. Physiol. Plant 2014, 153, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesney, P.; Nygren, P. Fine root and nodule dynamics of Erythrina poeppigiana in an alley cropping system in Costa Rica. Agrofor. Syst. 2002, 56, 259–269. [Google Scholar] [CrossRef]
- Ruess, R.W.; Anderson, M.D.; Mitchell, J.S.; McFarland, J.W. Effects of defoliation on growth and N fixation in Alnus tenuifolia: Consequences for changing disturbance regimes at high latitudes. Ecoscience 2006, 13, 404–412. [Google Scholar] [CrossRef]
- Ståhl, L.; Högberg, P.; Sellstedt, A.; Buresh, R.J. Measuring nitrogen fixation by Sesbania sesban planted fallows using 15N tracer technique in Kenya. Agrofor. Syst. 2005, 65, 67–79. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; van Langevelde, F.; Ward, D.; Bongers, F.; da Silva, D.A.; Prins, H.H.; de Bie, S.; Sterck, F.J. Deciduous and evergreen trees differ in juvenile biomass allometries because of differences in allocation to root storage. Ann. Bot. 2013, 112, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, A.; Germon, J.C.; Hubert, P.; Kaiser, P.; Letolle, R.; Tardieux, A.; Tardieux, P. Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes. Plant Soil. 1981, 62, 413–430. [Google Scholar] [CrossRef]
- Chalk, P.M.; Peoples, M.B.; McNeill, A.M.; Boddey, R.M.; Unkovich, M.J.; Gardener, M.J.; Silva, C.F.; Chen, D. Methodologies for estimating nitrogen transfer between legumes and companion species in agro-ecosystems: A review of 15N-enriched techniques. Soil Biol. Biochem. 2014, 73, 10–21. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2-fixation in field settings: Estimations based on natural 15N abundance. Funct. Plant Biol. 1986, 13, 699–756. [Google Scholar]
- Gathumbi, S.M.; Cadisch, G.; Giller, K.E. 15N natural abundance as a tool for assessing N2-fixation of herbaceous, shrub and tree legumes in improved fallows. Soil Biol. Biochem. 2002, 34, 1059–1071. [Google Scholar] [CrossRef]
- Büchi, L.; Gebhard, C.A.; Liebisch, F.; Sinaj, S.; Ramseier, H.; Charles, R. Accumulation of biologically fixed nitrogen by legumes cultivated as cover crops in Switzerland. Plant Soil. 2015, 393, 163–175. [Google Scholar] [CrossRef]
- Galang, M.C.; Gutteridge, R.C.; Shelton, H.M. The effect of cutting height and frequency on the productivity of Sesbania sesban var. nubica in a sub-tropical environment. Nitrogen Fixing Tree Res. Rep. 1990, 8, 161–164. [Google Scholar]
- Kadiata, B.D.; Mulongoy, K.; Isirimah, N.O. Influence of pruning frequency of Albizia lebbeck, Gliricidia sepium and Leucaena leucocephala on nodulation and potential nitrogen fixation. Biol. Fertil. Soils 1997, 24, 255–260. [Google Scholar] [CrossRef]
- Peter, I.; Lehmann, J. Pruning effects on root distribution and nutrient dynamics in an acacia hedgerow planting in northern Kenya. Agrofor. Syst. 2000, 50, 59–75. [Google Scholar] [CrossRef]
- Demirtas, N.M.; Bolat, I.; Ercisli, S.; Ikinci, A.; Olmez, H.; Sahin, M.; Celik, B. The effects of different pruning treatments on seasonal variation of carbohydrates in ‘Hacihaliloglu’ apricot cultivar. Not. Bot. Hort. Agrobot. Cluj. 2010, 38, 223–225. [Google Scholar]
- Carpenter, L.T.; Pezeshki, S.R.; Shields, F.D., Jr. Responses of nonstructural carbohydrates to shoot removal and soil moisture treatments in Salix nigra. Trees 2008, 22, 737–748. [Google Scholar] [CrossRef]
- Nygren, P.; Cruz, P. Biomass allocation and nodulation of Gliricidia sepium under two cut-and-carry forage production regimes. Agrofor. Syst. 1998, 41, 277–292. [Google Scholar] [CrossRef]
- Nygren, P.; Vaillant, V.; Desfontaines, L.; Cruz, P.; Domenach, A.M. Effects of nitrogen source and defoliation on growth and biological dinitrogen fixation of Gliricidia sepium seedlings. Tree Physiol. 2000, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- SANBI. Senna siamea (Lam.) H.S. Irwin & Barneby. National Assessment: Red List of South African Plants Version 2020.1. 2020. Available online: http://redlist.sanbi.org/species.php?species=388-14 (accessed on 13 September 2022).
| Treatment 1 | Aboveground DM Yield (g plant−1) | ||||
|---|---|---|---|---|---|
| Stem | Branch | Twig | Leaf | Total | |
| PF0 | 52.82 ± 2.26a | 33.40 ± 2.91b | 1.82 ± 0.11a | 11.70 ± 0.49a | 99.73 ± 4.48a |
| PF1 | 16.56 ± 1.37b | 49.50 ± 4.77a | 0.70 ± 0.11c | 9.33 ± 0.31b | 76.10 ± 6.18b |
| PF2 | 13.20 ± 1.19bc | 15.17 ± 1.25c | 1.83 ± 0.22a | 6.50 ± 0.54c | 36.70 ± 2.25c |
| PF3 | 17.88 ± 0.78c | 8.00 ± 0.54c | 1.67 ± 0.16b | 5.83 ± 0.66c | 33.38 ± 1.32c |
| Probability | ≤0.001 | ≤0.001 | ≤0.01 | ≤0.001 | ≤0.001 |
| Treatment 1 | DM Yield | Length |
|---|---|---|
| g plant−1 | cm | |
| PF0 | 77.35 ± 3.74a | 71.50 ± 3.49a |
| PF1 | 65.45 ± 3.72b | 58.75 ± 4.57ab |
| PF2 | 47.02 ± 2.97c | 47.85 ± 4.24bc |
| PF3 | 42.78 ± 3.81c | 44.20 ± 3.17c |
| Probability | ≤0.001 | ≤0.001 |
| Treatment 1 | Nonstructural Carbohydrates (mg g−1) | ||
|---|---|---|---|
| Starch | Sugar | TNC | |
| PF0 | 173.52 ± 3.74a | 66.02 ± 5.49a | 239.54 ± 8.88a |
| PF1 | 161.27 ± 5.84ab | 58.82 ± 1.87ab | 220.09 ± 7.17a |
| PF2 | 144.09 ± 3.08bc | 48.91 ± 1.41b | 193.01 ± 4.31b |
| PF3 | 140.34 ± 4.71c | 43.89 ± 2.87c | 184.23 ± 6.19b |
| Probability | ≤0.001 | ≤0.01 | ≤0.001 |
| Treatment 1 | Nodule DW | Nodule Number |
|---|---|---|
| g Plant−1 | No. Plant−1 | |
| PF0 | 3.20 ± 0.10a | 185 ± 10a |
| PF1 | 2.82 ± 0.09ab | 163 ± 15ab |
| PF2 | 2.71 ± 0.10b | 140 ± 9b |
| PF3 | 1.97 ± 0.12c | 110 ± 9c |
| Probability | ≤0.001 | ≤0.01 |
| Treatment 1 | N | N Content | δ15N | Ndfa | N2 Fixed |
|---|---|---|---|---|---|
| % | g Plant−1 | ‰ | % | g Plant−1 | |
| PF0 | 3.36 ± 0.29a | 3.35 ± 0.12a | −1.45 ± 0.04b | 90.72 ± 1.19a | 3.04 ± 0.11a |
| PF1 | 3.39 ± 0.27a | 2.59 ± 0.34b | −1.24 ± 0.04ab | 84.80 ± 1.23a | 2.17 ± 0.26ab |
| PF2 | 3.48 ± 0.31a | 1.29 ± 0.18c | −1.16 ± 0.10a | 81.99 ± 3.06b | 1.05 ± 0.11b |
| PF3 | 4.00 ± 0.21a | 1.33 ± 0.08c | −1.03 ± 0.08a | 78.28 ± 2.46b | 1.04 ± 0.05b |
| Probability | ≤0.25 | ≤0.001 | ≤0.01 | ≤0.01 | ≤0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhubedu, T.I.; Letty, B.A.; Mafongoya, P.L.; Scogings, P.F. Unraveling the Effects of Pruning Frequency on Biomass Productivity, Nonstructural Carbohydrates and Nitrogen Fixation Rates of Sesbania sesban. Forests 2022, 13, 2035. https://doi.org/10.3390/f13122035
Makhubedu TI, Letty BA, Mafongoya PL, Scogings PF. Unraveling the Effects of Pruning Frequency on Biomass Productivity, Nonstructural Carbohydrates and Nitrogen Fixation Rates of Sesbania sesban. Forests. 2022; 13(12):2035. https://doi.org/10.3390/f13122035
Chicago/Turabian StyleMakhubedu, Thabo I., Brigid A. Letty, Paramu L. Mafongoya, and Peter F. Scogings. 2022. "Unraveling the Effects of Pruning Frequency on Biomass Productivity, Nonstructural Carbohydrates and Nitrogen Fixation Rates of Sesbania sesban" Forests 13, no. 12: 2035. https://doi.org/10.3390/f13122035




