Impregnation Properties of Nigerian-Grown Gmelina arborea Roxb. Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Procurement
2.2. Sample Preparation
2.3. Sample Impregnation
2.4. Penetration Measurement
2.5. Data Analysis
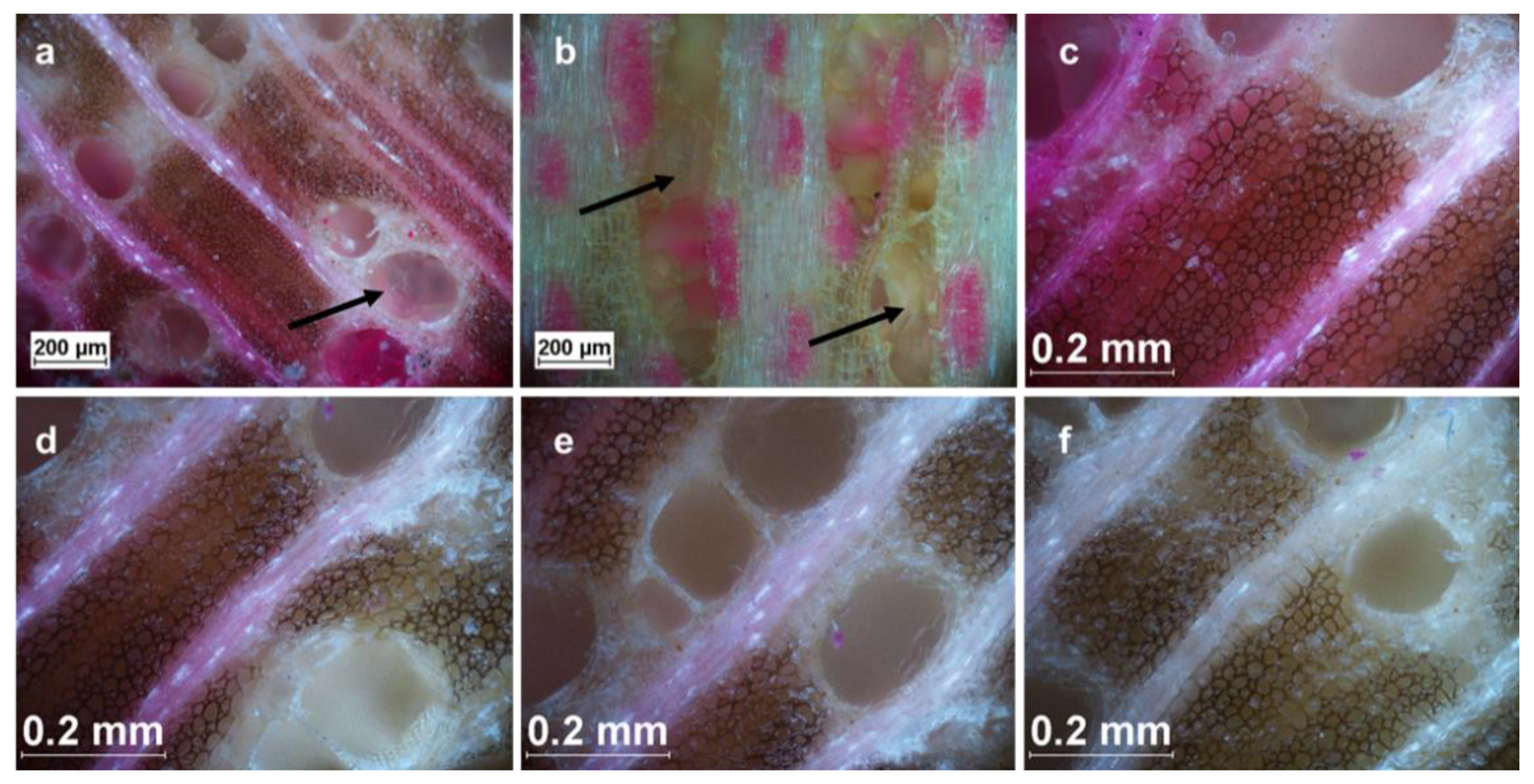
2.6. Light Microscopy
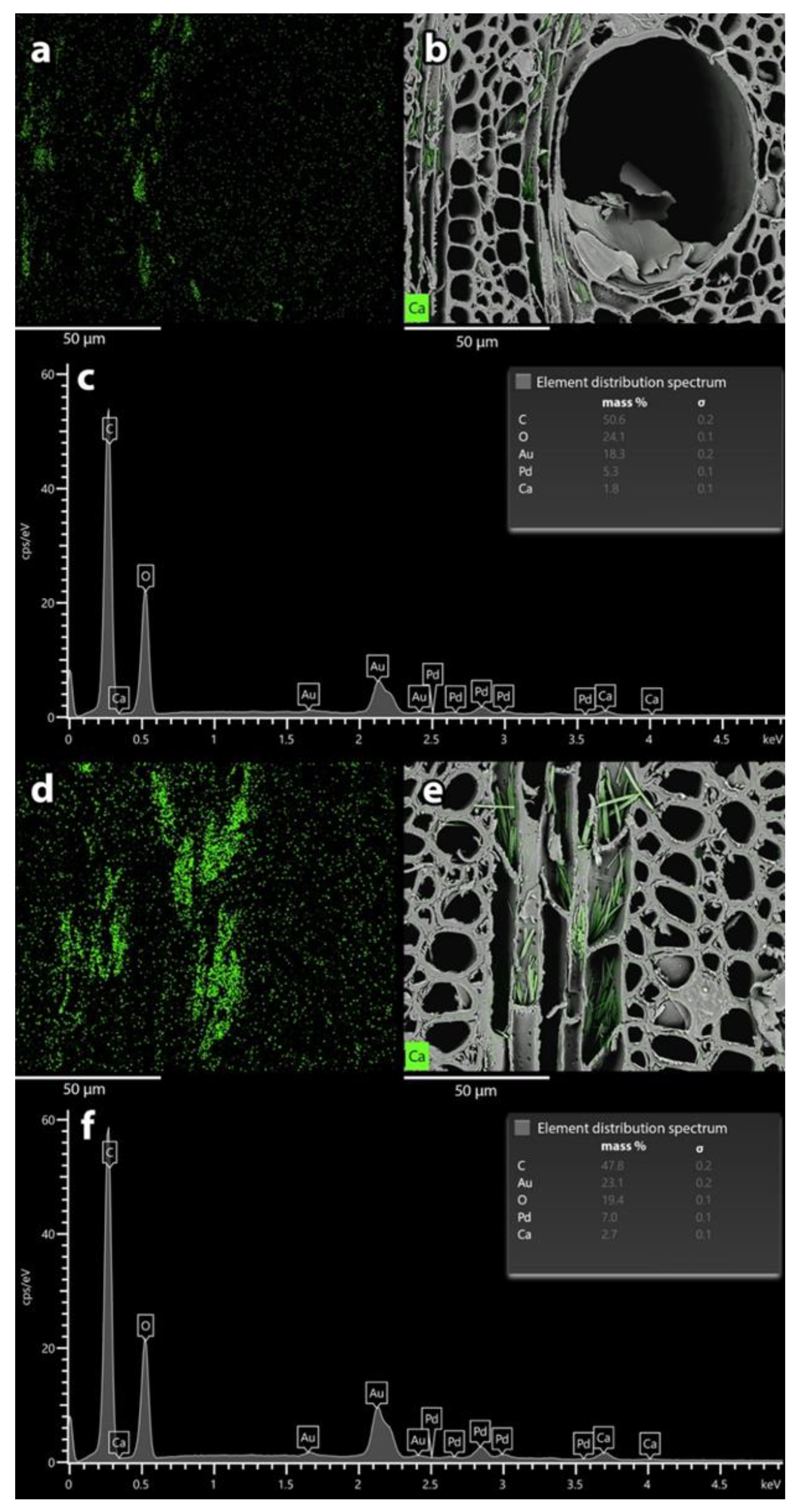
2.7. SEM/EDX Analysis
3. Results and Discussion
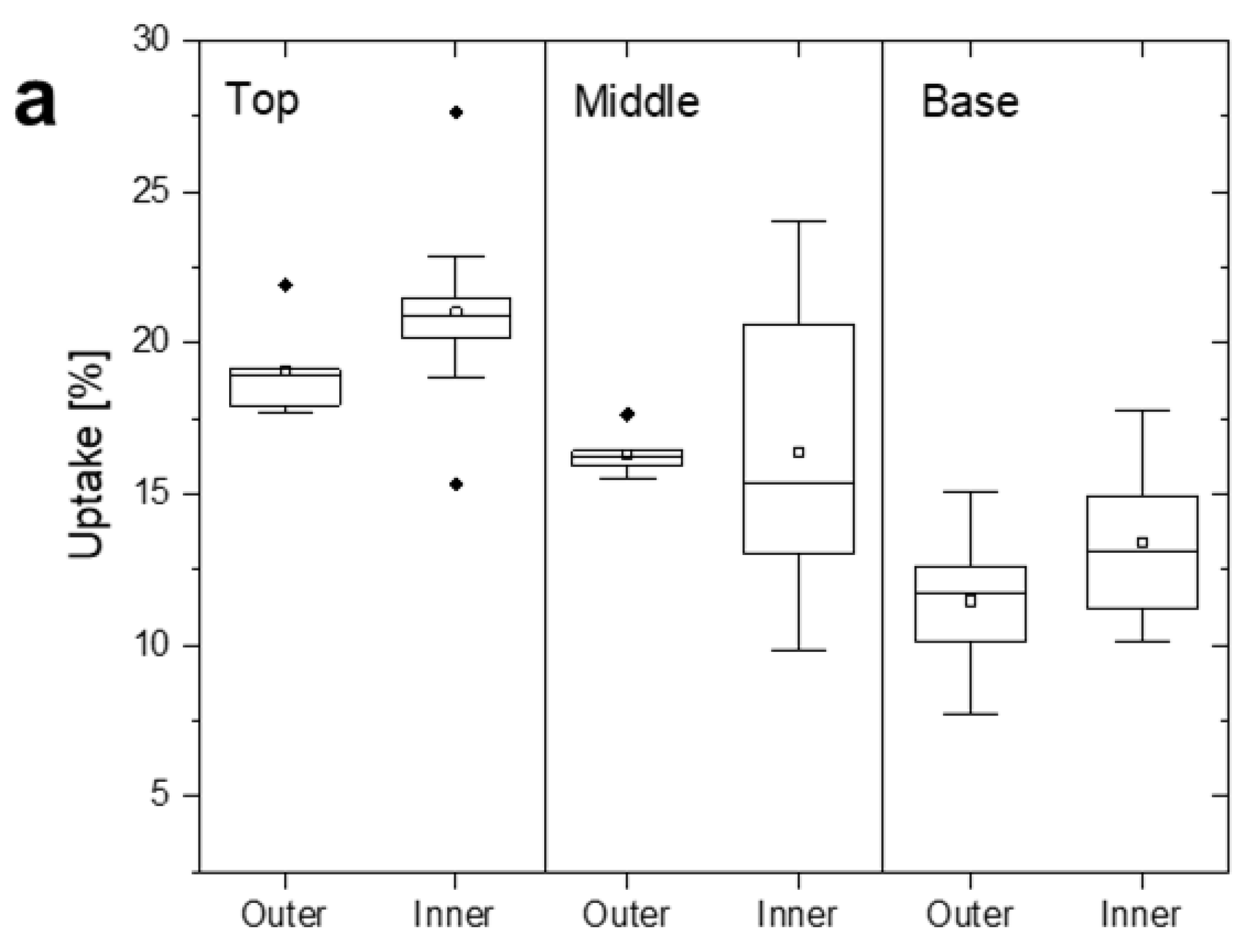
3.1. Variation in Uptake within Stems and Stands of Gmelina Wood
3.2. Influence of Anatomy on Impregnation Properties of Gmelina Wood
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onyekwelu, J.C.; Biber, P.; Stimm, B. Thinning Scenarios for Gmelina arborea Plantations in South-Western Nigeria Using Density Management Diagrams. Food Agric. Environ. 2003, 1, 320–325. [Google Scholar]
- Dvorak, W.S. World View of Gmelina arborea: Opportunities and Challenges. New For. 2004, 28, 111–126. [Google Scholar] [CrossRef]
- Kojima, M.; Yamamoto, H.; Marsoem, S.N.; Okuyama, T.; Yoshida, M.; Nakai, T.; Yamashita, S.; Saegusa, K.; Matsune, K.; Nakamura, K.; et al. Effects of the Lateral Growth Rate on Wood Quality of Gmelina arborea from 3.5-, 7- and 12-Year-Old Plantations. Ann. For. Sci. 2009, 66, 507. [Google Scholar] [CrossRef]
- Iwuoha, S.E.; Seim, W.; Onyekwelu, J.C. Mechanical Properties of Gmelina arborea for Engineering Design. Constr. Build. Mater. 2021, 288, 123123. [Google Scholar] [CrossRef]
- Haslett, A.N.; Zoung, G.D.; Britton, R.A.J. Plantation Grown Tropical Timbers. 2. Properties, Processing and Uses. J. Trop. For. Sci. 1991, 3, 229–237. [Google Scholar]
- Tarmian, A.; Zahedi Tajrishi, I.; Oladi, R.; Efhamisisi, D. Treatability of Wood for Pressure Treatment Processes: A Literature Review. Eur. J. Wood Wood Prod. 2020, 78, 635–660. [Google Scholar] [CrossRef]
- EN 350:2016; Durability of Wood and Wood-Based Products-Testing and Classification of the Durability to Biological Agents of Wood and Wood-Based Materials. Deutsches Institut für Normung, Beuth Verlag GmbH: Berlin, Germany, 2016.
- Lehringer, C.; Richter, K.; Schwarze, F.W.M.R.; Militz, H. A Review on Promising Approaches for Liquid Permeability Improvement in Softwoods. Wood Fiber Sci. 2009, 41, 373–385. [Google Scholar]
- Matsumura, J.; Booker, R.E.; Ridoutt, B.G.; Donaldson, L.A.; Mikajiri, N.; Matsunaga, H.; Oda, K. Impregnation of Radiata Pine Wood by Vacuum Treatment II: Effect of Pre-Steaming on Wood Structure and Resin Content. J. Wood Sci. 1999, 45, 456–462. [Google Scholar] [CrossRef]
- Tarmian, A.; Mastouri, A. Water-Repellent Efficiency of Thermally Modified Wood as Affected by Its Permeability. J. For. Res. 2018, 29, 859–867. [Google Scholar] [CrossRef]
- Esmailpour, A.; Taghiyari, H.R.; Golchin, M.; Avramidis, S. On the Fluid Permeability of Heat-treated Paulownia Wood. Int. Wood Prod. J. 2019, 10, 55–63. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Sehlstedt-Persson, M.; Hansson, L.; Moren, T. Evaluation of Preservative Distribution in Thermally Modified European Aspen and Birch Board Using Computed Tomography and Scanning Electron Microscopy. J. Wood Sci. 2012, 57, 57–66. [Google Scholar] [CrossRef]
- Winandy, J.E. Effect of Treatment, Incising and Drying on Mechanical Properties of Timber; Forest Products Laboratory: Madison, WI, USA, 1996. [Google Scholar]
- Olaniran, S.O.; Olufemi, B. Flexural Strength of Incised Rubber-Wood (Hevea Brasiliensis Muell. Arg.) with Creosote Oil and Liquefied Biomass. Appl. Trop. Agric. 2015, 20, 57–61. [Google Scholar]
- Civardi, C.; van den Bulcke, J.; Schubert, M.; Michel, E.; Butron, M.I.; Boone, M.N.; Dierick, M.; van Acker, J.; Wick, P.; Schwarze, F.W.M.R. Penetration and Effectiveness of Micronized Copper in Refractory Wood Species. PLoS ONE 2016, 11, e0163124. [Google Scholar] [CrossRef]
- Ross, A.S.; Clawson, R.W., Jr. New Method for Pretreatment of Railroad Crossties. In Proceedings of the 45th Annual Meeting of the International Research Group on Wood Protection, IRG/WP 14-40675, George, UT, USA, 11–15 May 2014. [Google Scholar]
- Owoyemi, J.M. The Influence of Preservative Viscosity on Fluid Absorption by Gmelina arborea Wood. For. For. Prod. J. 2010, 3, 32–39. [Google Scholar]
- Maclean, J.D. Preservative Treatment of Wood by Pressure Methods; US Department of Agriculture, Forest Service: Washington, DC, USA, 1952. [Google Scholar]
- Freeman, M.H.; McIntyre, C.R.; Jackson, D. A Critical and Comprehensive Review of Boron in Wood Preservation. In Proceedings of the Annual Meeting of the American Wood Protection Association, San Antonio, TX, USA, 19–21 April 2009. [Google Scholar]
- He, X.; Xiong, X.; Xie, J.; Li, Y.; Wei, Y.; Quan, P.; Mou, Q.; Li, X. Effect of Microwave Pretreatment on Permeability and Drying Properties of Wood. BioResources 2017, 12, 3850–3863. [Google Scholar] [CrossRef]
- Messner, K.; Bruce, A.; Bongers, H.P.M. Treatability of Refractory Wood Species after Fungal Pre-Treatment. In Proceedings of the Second European Conference on Wood Modification, Goettingen, Germany, 3 April 2003; pp. 389–401. [Google Scholar]
- Ahmed, S.A.; Chun, S.K. Descriptions of the Wood Anatomy and Safranine Impregnation in Gmelina arborea Roxb. from Bangladesh. J. Korea Furnit. Soc. 2007, 18, 100–105. [Google Scholar]
- Sujatha, M.; Venmalar, D. Anatomical Approach to Evaluating the Treatability Class of Five Species of Plantation Timbers. J. Indian Acad. Wood Sci. 2019, 16, 15–21. [Google Scholar] [CrossRef]
- Sint, K.M.; Militz, H.; Hapla, F. Treatability and Penetration Indices of Four Lesser Used Myanmar Hardwoods. Wood Res. 2011, 56, 13–22. [Google Scholar]
- Militz, H. Die Verbesserung der Imprägnierbarkeit von Fichtenholz Mittels Chemischer und Enzymatischer Vorbehandlung; Universität Wageningen: Wageningen, The Netherlands, 1990. [Google Scholar]
- Milota, M.R.; Tschernitz, J.L.; Verrill, S.P.; Mianowski, T. Gas Permeability of Plantation Loblolly Pine. Wood Fiber Sci. 1995, 27, 23–40. [Google Scholar]
- Larnøy, E.; Lande, S.; Vestøl, G. Variations of Furfuryl Alcohol and Wolmanit CX-8 Treatability of Pine Sapwood within and between Trees. In The International Research Group on Wood Protection Document No. IRG.WP/08-40421, Proceedings of the 39th Annual Meeting, Istanbul, Turkey, 25–29 May 2008; IRGWP: Stockholm, Sweden, 2008. [Google Scholar]
- Siau, J.F. Transport Processes in Wood; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Ahmed, S.A.; Chun, S.K.; Miller, R.B.; Chong, S.H.; Kim, A.J. Liquid Penetration in Different Cells of Two Hardwood Species. J. Wood Sci. 2011, 57, 179–188. [Google Scholar] [CrossRef]
- Halverson, S.; Lebow, S. Observed relationships between wood density and solution uptake during pressure treatment. In Proceedings of the One Hundred Seventh Annual Meeting of the American Wood Protection Association, Fort Lauderdale, FL, USA, 15–17 May 2011; American Wood Protection Association: Hoover, AL, USA; Birmingham Ala, Alabama, 2011; Volume 107, pp. 93–98. [Google Scholar]
- Sulaiman, A.; Lim, S.C. Some Timber Characteristics of Gmelina arborea Grown in a Plantation in Peninsular Malaysia. J. Trop. For. Sci. 1989, 2, 135–141. [Google Scholar]
- Moya, R.; Fo, M.T. Variation in the Wood Anatomical Structure of Gmelina arborea (Verbenaceae) Trees at Different Ecological Conditions in Costa Rica. Rev. Biol. Trop. 2008, 56, 689–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kozlowski, T.T.; Pallardy, S.G. Growth Control in Woody Plants; Academic Press: San Diego, CA, USA; London, UK; Boston, MA, USA; New York, NY, USA; Sydney, Australia; Tokyo, Japan; Toronto, ON, Canada, 1997; ISBN 0-12-424210-3. [Google Scholar]
- Zabel, R.A.; Morrell, J.J. Chemical protection of wood (wood preservation). In Wood Microbiology, Decay and Its Prevention; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Kumar, S.; Dobriyal, P.B. Penetration Indices of Hardwoods: A Quantitative Approach to Define Treatability. Wood Fiber Sci. 1993, 2, 192–197. [Google Scholar]
- Winandy, J.E.; Morrell, J.J. Effects of Incising on Lumber Strength and Stiffness: Relationships between Incision Density and Depth, Species, and MSR Grade. Wood Fiber Sci. 1998, 2, 185–197. [Google Scholar]
- Rautkari, L.; Honkanen, J.; Hill, C.A.S.; Ridley-Ellis, D.; Hughes, M. Mechanical and Physical Properties of Thermally Modified Scots Pine Wood in High Pressure Reactor under Saturated Steam at 120, 150 and 180 °C. Eur. J. Wood Wood Prod. 2014, 72, 33–41. [Google Scholar] [CrossRef]


| Parameter | ||||
|---|---|---|---|---|
| EHT [kV] | I Probe [pA] | WD [mm] | OptiBeam Mode [-] | Mag [-] |
| 10 | 700 | 8.5 | Depth/Resolution | variable |
| Density | Solution Uptake | Penetration Depth | |||
|---|---|---|---|---|---|
| kgm−3 | (%) | (mm) | |||
| Large Sized Samples | Small-Sized Samples | Axial | Lateral | ||
| Along the stem height | |||||
| Top | 455 | 20.41(2.54) a | 19.03(6.04) | 2.65(1.23) b | 0.48(0.13) a |
| Middle | 429 | 16.37(3.35) b | 18.68(4.51) | 3.81(1.07) a | 0.48(0.10) a |
| Base | 436 | 12.12(2.58) c | 14.55(2.57) | 1.31(0.56) c | 0.53(0.13) a |
| Across the stem diameter | |||||
| Outer wood | n/a | 14.93(3.66) a | n/a | n/a | n/a |
| Inner wood | n/a | 17.82(4.43) a | n/a | n/a | n/a |
| Sampled Trees | Density (kgm−3) | Solution Uptake (%) |
|---|---|---|
| Tree 1 | 440 | 16.61(4.33) b |
| Tree 2 | 452 | 16.23(5.43) b |
| Tree 3 | 457 | 23.32(12.37) a |
| Tree 4 | 420 | 17.69(1.76) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaniran, S.O.; Löning, S.; Buschalsky, A.; Militz, H. Impregnation Properties of Nigerian-Grown Gmelina arborea Roxb. Wood. Forests 2022, 13, 2036. https://doi.org/10.3390/f13122036
Olaniran SO, Löning S, Buschalsky A, Militz H. Impregnation Properties of Nigerian-Grown Gmelina arborea Roxb. Wood. Forests. 2022; 13(12):2036. https://doi.org/10.3390/f13122036
Chicago/Turabian StyleOlaniran, Samuel Oluyinka, Sophie Löning, Andreas Buschalsky, and Holger Militz. 2022. "Impregnation Properties of Nigerian-Grown Gmelina arborea Roxb. Wood" Forests 13, no. 12: 2036. https://doi.org/10.3390/f13122036
APA StyleOlaniran, S. O., Löning, S., Buschalsky, A., & Militz, H. (2022). Impregnation Properties of Nigerian-Grown Gmelina arborea Roxb. Wood. Forests, 13(12), 2036. https://doi.org/10.3390/f13122036









