Response of Soil Organic Carbon Stock to Bryophyte Removal Is Regulated by Forest Types in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
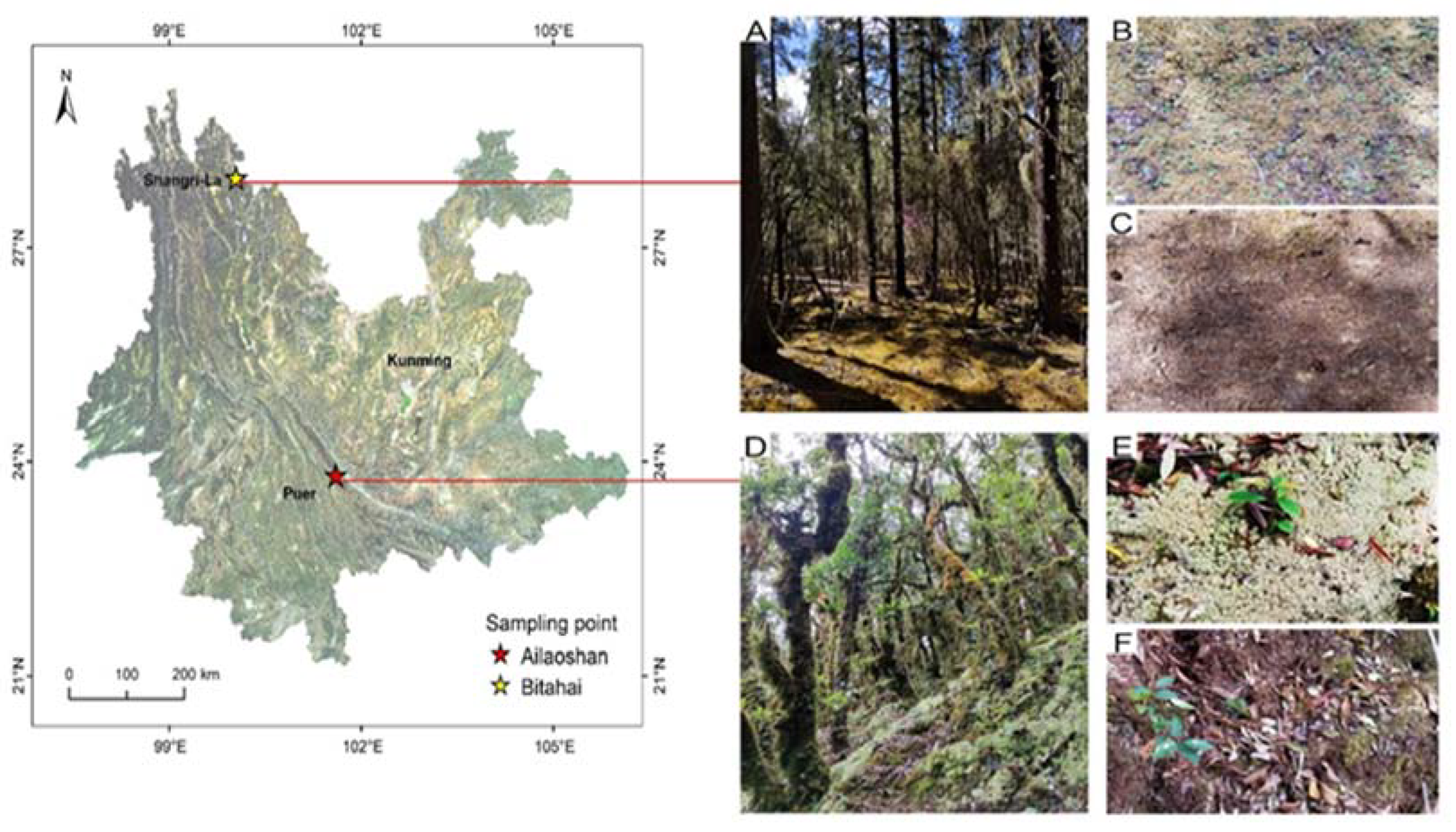
2.1. Study Area
2.2. Experimental Design and Sample Collection
2.3. Soil Measurements
2.4. Statistical Analyses
3. Results
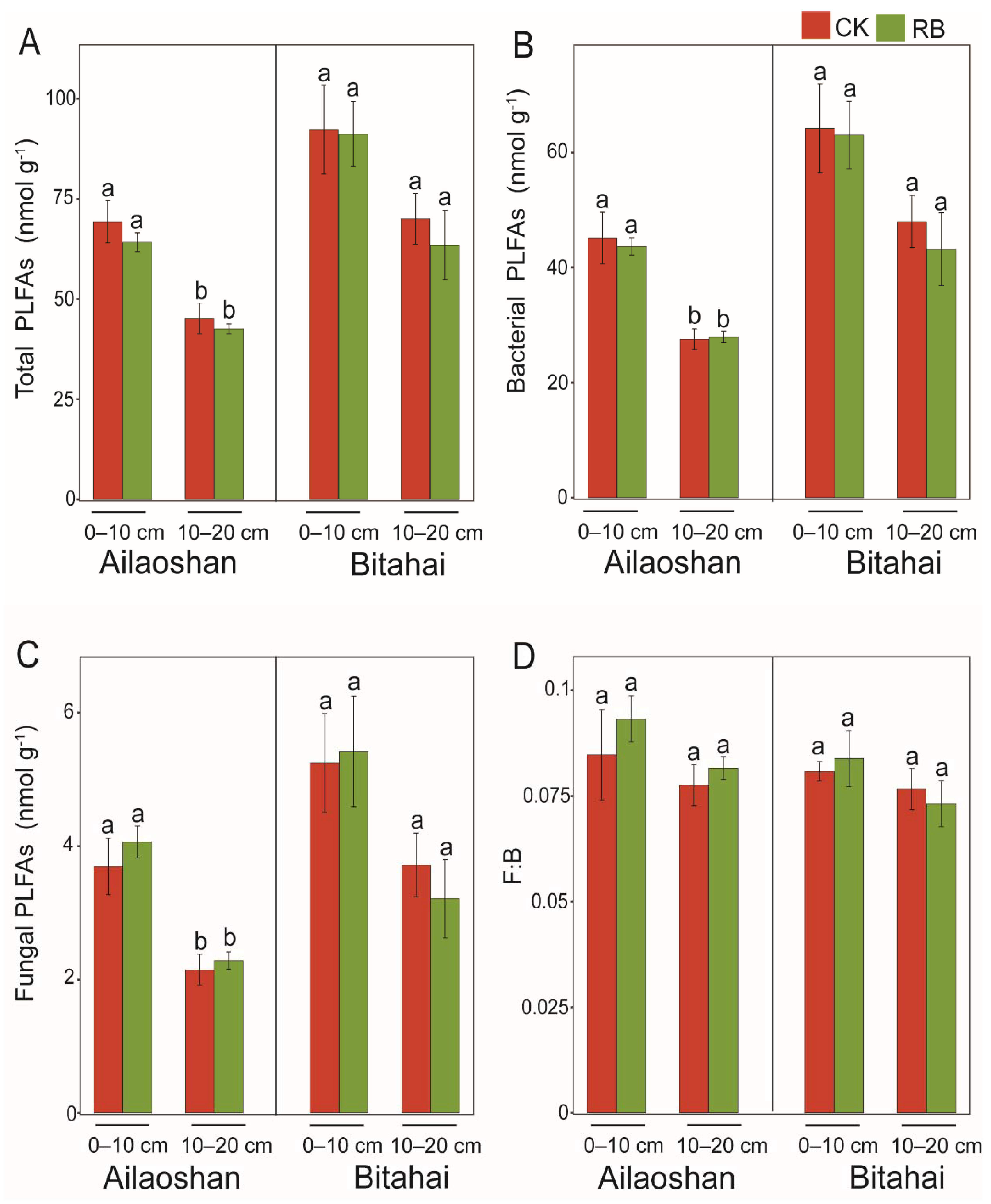
3.1. Responses of Soil Physicochemical Properties and PLFAs
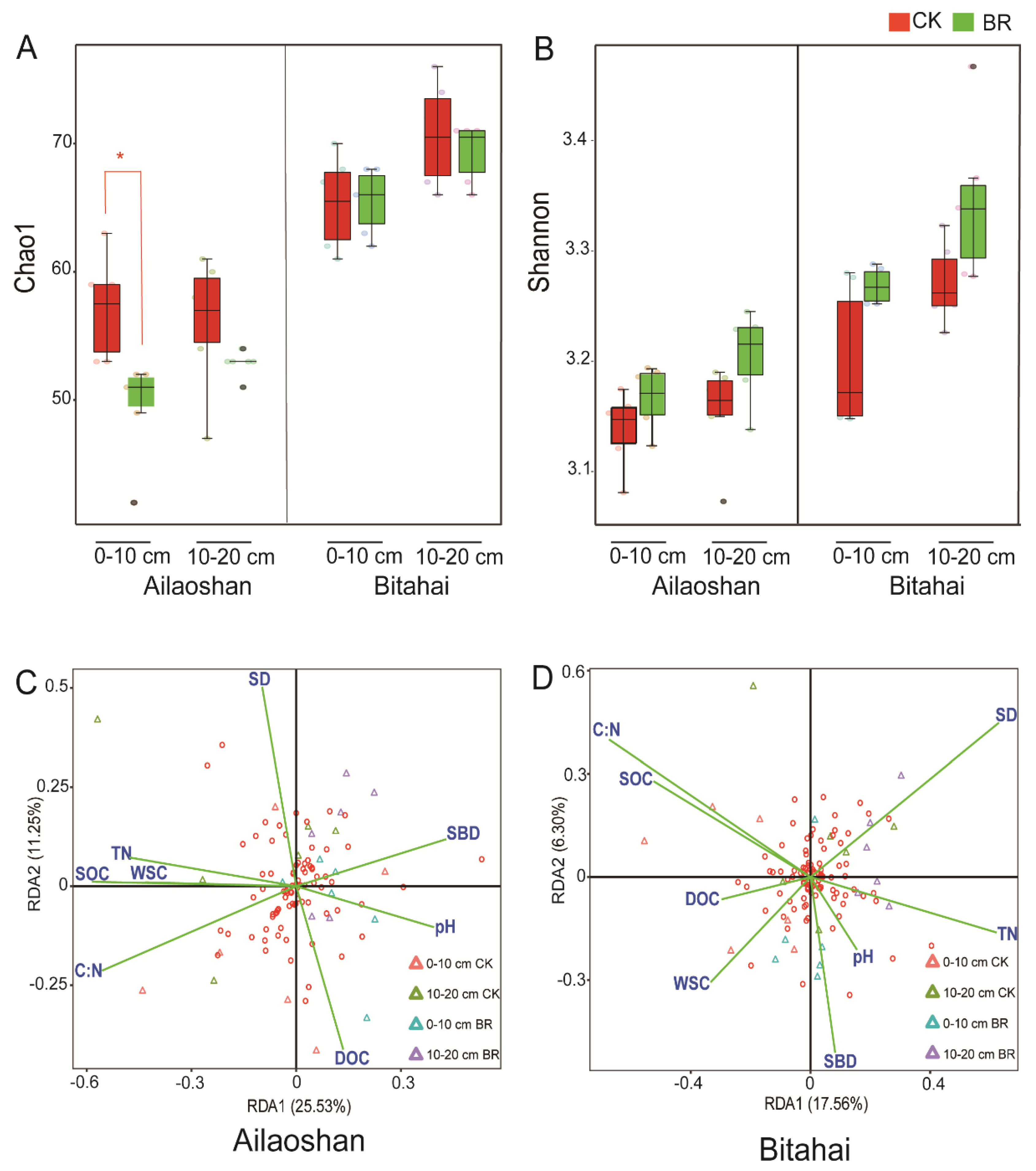
3.2. Effect of Bryophyte Removal on Soil Microbial Communities
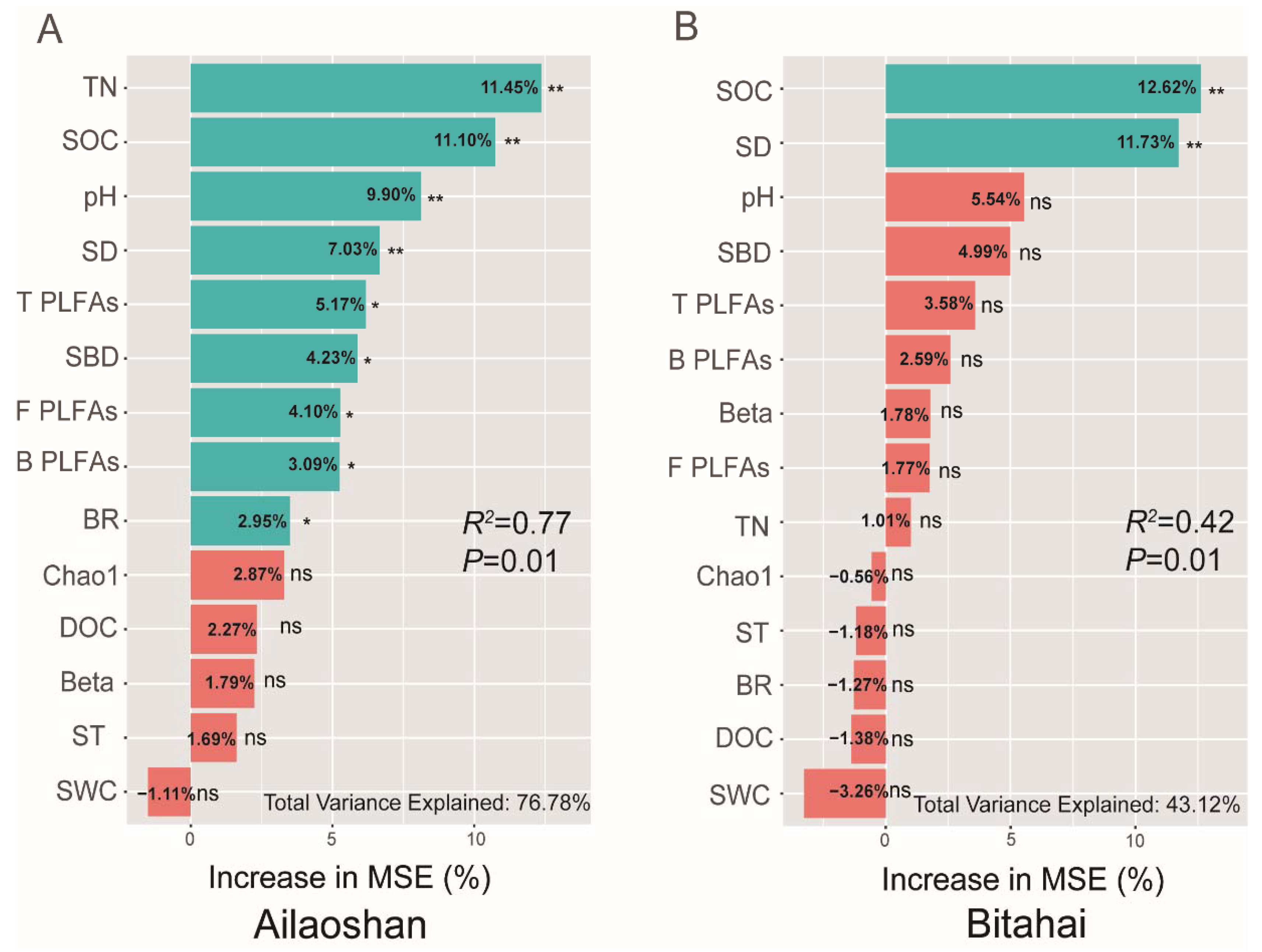
3.3. Soil Organic Carbon Stock and Regulation Factors
4. Discussion
4.1. Effect of Bryophyte Removal on Soil Organic Carbon Stock in Evergreen Broad-Leaved Forest
4.2. Effect of Bryophyte Removal on Soil Organic Carbon Stock in Cold Temperate Coniferous Forest
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Bryophyte Removal (BR) | Forest Type (FT) | Soil Depth (SD) | BR × FT | BR × SD | FT × SD | BR × FT × SD | ||
|---|---|---|---|---|---|---|---|---|
| SOC | F | 4.30 | 9.94 | 129.15 | 1.73 | 1.15 | 0.03 | 0.06 |
| P | 0.045 | 0.003 | 0.001 | 0.20 | 0.29 | 0.86 | 0.80 | |
| SBD | F | 5.62 | 7.003 | 10.40 | 0.29 | 0.03 | 0.33 | 0.51 |
| P | 0.02 | 0.012 | 0.003 | 0.60 | 0.86 | 0.57 | 0.48 | |
| SOCs | F | 0.09 | 29.07 | 80.95 | 5.68 | 0.03 | 1.03 | 0.91 |
| P | 0.77 | 0.001 | 0.001 | 0.02 | 0.87 | 0.32 | 0.35 | |
| DOC | F | 2.18 | 0.24 | 3.87 | 0.23 | 0.14 | 0.01 | 1.21 |
| P | 0.15 | 0.63 | 0.06 | 0.64 | 0.71 | 0.93 | 0.28 | |
| TN | F | 0.73 | 2.27 | 14.27 | 2.34 | 3.99 | 53.40 | 6.43 |
| P | 0.40 | 0.14 | 0.001 | 0.13 | 0.15 | <0.001 | 0.02 | |
| pH | F | 0.41 | 84.31 | 24.94 | 0.72 | 2.04 | 0.02 | 0.67 |
| P | 0.53 | 0.001 | <0.001 | 0.40 | 0.16 | 0.89 | 0.42 | |
| SWC | F | 0.07 | 12.89 | 2.94 | 1.30 | 0.03 | 1.71 | 0.24 |
| P | 0.80 | <0.001 | 0.09 | 0.26 | 0.86 | 0.20 | 0.63 | |
| T PLFAs | F | 0.67 | 26.01 | 25.95 | <0.001 | 0.03 | 0.05 | 0.18 |
| P | 0.42 | <0.001 | <0.001 | 0.99 | 0.87 | 0.82 | 0.68 | |
| B PLFAs | F | 0.27 | 30.22 | 26.52 | 0.13 | 0.02 | 0.04 | 0.17 |
| P | 0.61 | <0.001 | <0.001 | 0.72 | 0.90 | 0.84 | 0.68 | |
| F PLFAs | F | 0.01 | 13.89 | 23.68 | 0.33 | 0.39 | 0.08 | 0.09 |
| P | 0.91 | <0.001 | <0.001 | 0.57 | 0.53 | 0.78 | 0.76 | |
| Chao1 | F | 7.95 | 189.52 | 8.91 | 3.03 | 0.56 | 5.78 | 2.23 |
| P | 0.007 | <0.001 | 0.005 | 0.09 | 0.46 | 0.02 | 0.14 | |
| Beta | F | 0.56 | 0.61 | 0.02 | 3.24 | 2.07 | 0.06 | 0.61 |
| P | 0.46 | 0.44 | 0.87 | 0.08 | 0.16 | 0.81 | 0.44 |
| Indication | ALS | BTH | ||||||
|---|---|---|---|---|---|---|---|---|
| RDA1 | RDA2 | r2 | P | RDA1 | RDA2 | r2 | P | |
| SOC | −0.95 | −0.31 | 0.32 | 0.01 | −0.65 | −0.76 | 0.49 | 0.001 |
| DOC | −0.09 | 0.10 | 0.09 | 0.40 | −0.76 | −0.65 | 0.10 | 0.34 |
| TN | −0.94 | −0.35 | 0.23 | 0.08 | 0.82 | 0.57 | 0.45 | 0.002 |
| C:N | −0.99 | −0.14 | 0.30 | 0.03 | −0.69 | −0.73 | 0.64 | 0.001 |
| pH | 0.89 | 0.45 | 0.19 | 0.11 | 0.27 | 0.96 | 0.23 | 0.07 |
| SWC | −0.94 | −0.34 | 0.15 | 0.18 | −0.99 | 0.07 | 0.09 | 0.39 |
| SBD | 0.98 | 0.19 | 0.18 | 0.12 | 0.14 | 0.99 | 0.41 | 0.003 |
| SD | 0.05 | −0.99 | 0.23 | 0.02 | 0.66 | −0.75 | 0.56 | 0.002 |

References
- IPCC. Climate Change 2022: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Sun, P.; Li, Z.; Song, K.; Su, W.; Wang, B.; Liu, Y.; Zhao, J. A surface-display biohybrid approach to light-driven hydrogen production in air. Sci. Adv. 2018, 4, eaap9253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlstrom, A.; Canadell, J.G.; Schurgers, G.; Wu, M.; Berry, J.A.; Guan, K.; Jackson, R.B. Hydrologic resilience and Amazon productivity. Nat. Commun. 2017, 8, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommerfeld, A.; Senf, C.; Buma, B.; D’Amato, A.W.; Despres, T.; Diaz-Hormazabal, I.; Fraver, S.; Frelich, L.E.; Gutierrez, A.G.; Hart, S.J.; et al. Patterns and drivers of recent disturbances across the temperate forest biome. Nat. Commun. 2018, 9, 4355–4363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Feng, W.; Xu, J.; Kuzyakov, Y. Agroforestry systems: Meta-analysis of soil carbon stocks, sequestration processes, and future potentials. Land Degrad. Dev. 2018, 29, 3886–3897. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Bouskill, N.J.; Riley, W.J.; Zhu, Q.; Mekonnen, Z.A.; Grant, R.F. Alaskan carbon-climate feedbacks will be weaker than inferred from short-term experiments. Nat. Commun. 2020, 11, 5798. [Google Scholar] [CrossRef]
- Wang, N.; Quesada, B.; Xia, L.; Butterbach-Bahl, K.; Goodale, C.L.; Kiese, R. Effects of climate warming on carbon fluxes in grasslands- A global meta-analysis. Glob. Chang. Biol. 2019, 25, 1839–1851. [Google Scholar] [CrossRef]
- Collalti, A.; Ibrom, A.; Stockmarr, A.; Cescatti, A.; Alkama, R.; Fernandez-Martinez, M.; Matteucci, G.; Sitch, S.; Friedlingstein, P.; Ciais, P.; et al. Forest production efficiency increases with growth temperature. Nat. Commun. 2020, 11, 5322. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ferreras, J.; Hartmann, M. Thinning Partially Mitigates the Impact of Atlantic Forest Replacement by Pine Monocultures on the Soil Microbiome. Front. Microbiol. 2020, 11, 1491. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Wang, X.; Sun, Y.; Zhou, L.; Lin, Y.; Fu, S. Effects of understory removal and tree girdling on soil microbial community composition and litter decomposition in two Eucalyptus plantations in South China. Funct. Ecol. 2011, 25, 921–931. [Google Scholar] [CrossRef]
- Matsushima, M.; Chang, S. Effects of understory removal, N fertilization, and litter layer removal on soil N cycling in a 13-year-old white spruce plantation infested with Canada bluejoint grass. Plant Soil 2007, 292, 243–258. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Li, D.; Li, S.; Chen, Z.; Wu, J. A meta-analysis of understory plant removal impacts on soil properties in forest ecosystems. Geoderma 2022, 426, e116116. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chang, S.X.; Jiang, P.; Zhou, G.; Liu, J.; Wu, J.; Shen, Z. Understory vegetation management affected greenhouse gas emissions and labile organic carbon pools in an intensively managed Chinese chestnut plantation. Plant Soil 2013, 376, 363–375. [Google Scholar] [CrossRef]
- Koranda, M.; Kerschbaum, S.; Wanek, W.; Zechmeister, H.; Richter, A. Physiological responses of bryophytes Thuidium tamariscinum and Hylocomium splendens to increased nitrogen deposition. Ann. Bot. 2007, 99, 161–169. [Google Scholar] [CrossRef]
- Rola, K.; Plášek, V.; Rożek, K.; Zubek, S. Effect of tree species identity and related habitat parameters on understorey bryophytes-interrelationships between bryophyte, soil and tree factors in a 50-year-old experimental forest. Plant Soil 2021, 466, 613–630. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Bjorn, L.O.; Chernov, Y.; Chapin, T.; Christensen, T.R.; Huntley, B.; Ims, R.A.; Johansson, M.; Jolly, D.; Jonasson, S.; et al. Responses to projected changes in climate and UV-B at the species level. Ambio 2004, 33, 418–435. [Google Scholar] [CrossRef]
- Dorrepaal, E.; Aerts, R.; Cornelissen, J.H.C.; Callaghan, T.V.; Van Logtestijn, R.S.P. Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Glob. Chang. Biol. 2004, 10, 93–104. [Google Scholar] [CrossRef]
- Gerdol, R.; Petraglia, A.; Bragazza, L.; Iacumin, P.; Brancaleoni, L. Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Glob. Chang. Biol. 2007, 13, 1810–1821. [Google Scholar] [CrossRef]
- Wolf, L.; Rizzini, L.; Stracke, R.; Ulm, R.; Rensing, S.A. The molecular and physiological responses of Physcomitrella patens to ultraviolet-B radiation. Plant Physiol. 2010, 153, 1123–1134. [Google Scholar] [CrossRef]
- Van Der Heijden, E.; Verbeek, S.K.; Kuiper, P.J.C. Elevated atmospheric CO2 and increased nitrogen deposition: Effects on C and N metabolism and growth of the peat moss Sphagnum recurvum P. Beauv. var.mucronatum (Russ.) Warnst. Glob. Chang. Biol. 2000, 6, 201–212. [Google Scholar] [CrossRef]
- Morén, A.S.; Lindroth, A. CO2 exchange at the floor of a boreal forest. Agric. For. Meteorol. 2000, 101, 1–14. [Google Scholar] [CrossRef]
- Goulden, M.L.; Crill, P.M. Automated measurements of CO2 exchange at the moss surface of a black spruce forest. Tree Physiol. 1997, 17, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrbáček, F.; Cannone, N.; Kňažková, M.; Malfasi, F.; Convey, P.; Guglielmin, M. Effect of climate and moss vegetation on ground surface temperature and the active layer among different biogeographical regions in Antarctica. Catena 2020, 190, 1683–1693. [Google Scholar] [CrossRef]
- Cao, W.; Xiong, Y.; Zhao, D.; Tan, H.; Qu, J. Bryophytes and the symbiotic microorganisms, the pioneers of vegetation restoration in karst rocky desertification areas in southwestern China. Appl. Microb. Biotechnol. 2020, 104, 873–891. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, D.J.; Reed, S.; Travers, S.K.; Bowker, M.A.; Maestre, F.T.; Ding, J.; Havrilla, C.; Rodriguez-Caballero, E.; Barger, N.; Weber, B.; et al. The pervasive and multifaceted influence of biocrusts on water in the world’s drylands. Glob. Chang. Biol. 2020, 26, 6003–6014. [Google Scholar] [CrossRef]
- Zanatta, F.; Engler, R.; Collart, F.; Broennimann, O.; Mateo, R.G.; Papp, B.; Munoz, J.; Baurain, D.; Guisan, A.; Vanderpoorten, A. Bryophytes are predicted to lag behind future climate change despite their high dispersal capacities. Nat. Commun. 2020, 11, 5601. [Google Scholar] [CrossRef]
- Arroniz-Crespo, M.; Gwynn-Jones, D.; Callaghan, T.V.; Nunez-Olivera, E.; Martinez-Abaigar, J.; Horton, P.; Phoenix, G.K. Impacts of long-term enhanced UV-B radiation on bryophytes in two sub-Arctic heathland sites of contrasting water availability. Ann. Bot. 2011, 108, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Bastida, F.; Garcia, C.; Fierer, N.; Eldridge, D.J.; Bowker, M.A.; Abades, S.; Alfaro, F.D.; Asefaw Berhe, A.; Cutler, N.A.; Gallardo, A.; et al. Global ecological predictors of the soil priming effect. Nat. Commun. 2019, 10, 3481. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, G.; Robinson, D.; Yang, Z.; Guo, J.; Xie, J.; Fu, S.; Zhou, L.; Yang, Y. Large amounts of easily decomposable carbon stored in subtropical forest subsoil are associated with r-strategy-dominated soil microbes. Soil Biol. Biochem. 2016, 95, 233–242. [Google Scholar] [CrossRef]
- Lai, L.; Huang, X.; Yang, H.; Chuai, X.; Zhang, M.; Zhong, T.; Chen, Z.; Chen, Y.; Wang, X.; Thompson, J.R. Carbon emissions from land-use change and management in China between 1990 and 2010. Sci. Adv. 2016, 2, e1601063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Kimberley, M.O.; Zhou, G.; Wang, H. Soil carbon dynamics in successional and plantation forests in subtropical China. J. Soils Sedim. 2016, 17, 2250–2256. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, S.; Zhao, J.; Zhang, K.; Jiang, J.; Guan, Z.; Chen, S.; Chen, F.; Fang, W. Deep tillage combined with biofertilizer following soil fumigation improved chrysanthemum growth by regulating the soil microbiome. Microbiology 2020, 9, e1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semchenko, M.; Leff, J.W.; Lozano, Y.M.; Saar, S.; Davison, J.; Wilkinson, A.; Jackson, B.G.; Pritchard, W.J.; De Long, J.R.; Oakley, S.; et al. Fungal diversity regulates plant-soil feedbacks in temperate grassland. Sci. Adv. 2018, 4, eaau4578. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Wang, X.; Yan, J.; Luo, L. Corrigendum: Characterizing the Intra-Vineyard Variation of Soil Bacterial and Fungal Communities. Front. Microbiol. 2019, 10, 1239–1251. [Google Scholar] [CrossRef] [Green Version]
- Borer, B.; Tecon, R.; Or, D. Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks. Nat. Commun. 2018, 9, 769–780. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, Z.; Chen, D.; Huang, G.; Zhou, L.; Fu, S. Understory plants can make substantial contributions to soil respiration: Evidence from two subtropical plantations. Soil Biol. Biochem. 2011, 43, 2355–2357. [Google Scholar] [CrossRef]
- Shi, S.; Han, P. Estimating the soil carbon sequestration potential of China’s Grain for Green Project. Glob. Biogeoch. Cycl. 2014, 28, 1279–1294. [Google Scholar] [CrossRef]
- Turetsky, M.R. The Role of Bryophytes in Carbon and Nitrogen Cycling. Bryologist 2003, 106, 395–409. [Google Scholar] [CrossRef]
- Oestmann, J.; Dettmann, U.; Düvel, D.; Tiemeyer, B. Experimental warming increased greenhouse gas emissions of a near-natural peatland and Sphagnum farming sites. Plant Soil 2022, 480, 85–104. [Google Scholar] [CrossRef]
- Lindo, Z.; Nilsson, M.C.; Gundale, M.J. Bryophyte-cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Glob. Chang. Biol. 2013, 19, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, E.; Aguilar, M.Á.; Castilla, Y.C.; Chamizo, S.; Aguilar, F.J. Swelling of biocrusts upon wetting induces changes in surface micro-topography. Soil Biol. Biochem. 2015, 82, 107–111. [Google Scholar] [CrossRef]
- Lu, Z.; Song, L.; Wang, X.; Li, Y.W.; Zhang, Y.; Sha, L. Ecological stoichiometry characteristics of the litterfall-humus- soil continuum systems under different successional stages of the subtropical forest in SW China. Mount. Res. 2017, 35, 274–282. [Google Scholar]
- Zhou, Q.; Li, D.; Xia, S.; Chen, Z.; Wang, B.; Wu, J. Plant–rodent interactions after a heavy snowfall decrease plant regeneration and soil carbon emission in an old-growth forest. For. Ecosyst. 2021, 8, 30–39. [Google Scholar] [CrossRef]
- He, S.; Li, W.; Cheng, X.; Tan, R.; Song, W. The effect of trampling disturbance on functional traits, species diversity, and functional diversity of alpine meadows in Bitahai Nature Reserve. Acta Ecol. Sin. 2019, 39, 2063–2070. [Google Scholar]
- Wang, S.; Chen, X.; Li, D.; Wu, J. Effects of soil organism interactions and temperature on carbon use efficiency in three different forest soils. Soil Ecol. Lett. 2021, 3, 156–166. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Su, W.; Xu, Y.; Qian, Q.; Yang, X.; Chen, D.; Chen, Z.; Wu, J. Responses of fungal community to forest fire are species-specific in Yunnan Plateau, southwest China. J. Plant Ecol. 2022, rtac043. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Kakeh, J.; Gorji, M. Effects of biological soil crusts on some physicochemical characteristics of rangeland soils of Alagol, Turkmen Sahra, NE Iran. Soil Till. Res. 2018, 181, 152–159. [Google Scholar] [CrossRef]
- Rousk, K.; Jones, D.L.; Deluca, T.H. Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front. Microbiol 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turetsky, M.R.; Bond-Lamberty, B.; Euskirchen, E.; Talbot, J.; Frolking, S.; McGuire, A.D.; Tuittila, E.S. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol. 2012, 196, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ma, J.; Li, X.D.; Li, Y. Illumina sequencing-based community analysis of bacteria associated with different bryophytes collected from Tibet, China. BMC Microbiol. 2016, 16, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.B.; Piao, S.L.; Chen, A.P.; Liu, Y.W.; Liu, L.L.; Peng, S.S.; Sardans, J.; Sun, Y.; Penuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limpens, J.; Granath, G.; Gunnarsson, U.; Aerts, R.; Bayley, S.; Bragazza, L.; Bubier, J.; Buttler, A.; van den Berg, L.J.L.; Francez, A.J.; et al. Climatic modifiers of the response to nitrogen deposition in peat-forming Sphagnum mosses: A meta-analysis. New Phytol. 2011, 191, 496–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1644–1649. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Or, D. Microscale pH variations during drying of soils and desert biocrusts affect HONO and NH3 emissions. Nat. Commun. 2019, 10, 3944–3956. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Carmona, M.; Arcenegui, V.; Garcia-Orenes, F.; Mataix-Solera, J. The role of mosses in soil stability, fertility and microbiology six years after a post-fire salvage logging management. J. Environ. Manag. 2020, 262, 110287. [Google Scholar] [CrossRef]
- Gundale, M.J.; Nilsson, M.; Bansal, S.; Jaderlund, A. The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol. 2012, 194, 453–463. [Google Scholar] [CrossRef]
- Shi, Z.; Crowell, S.; Luo, Y.; Moore, B. Model structures amplify uncertainty in predicted soil carbon responses to climate change. Nat. Commun. 2018, 9, 2171. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Tobin, B.; Luo, Y.; Osborne, B. Differential responses of soil CO2 and N2O fluxes to experimental warming. Agr. Forest Meteorol. 2018, 259, 11–22. [Google Scholar] [CrossRef]

| Ailaoshan | Bitahai | ||||||
|---|---|---|---|---|---|---|---|
| BR | SD | BR × SD | BR | SD | BR × SD | ||
| SOC | F | 0.38 | 87.87 | 0.44 | 4.62 | 50.40 | 0.71 |
| P | 0.55 | <0.001 | 0.51 | 0.04 | <0.001 | 0.41 | |
| DOC | F | 2.42 | 2.67 | 1.38 | 0.41 | 1.46 | 0.22 |
| P | 0.14 | 0.12 | 0.26 | 0.53 | 0.24 | 0.65 | |
| SOCs | F | 4.19 | 61.20 | 1.20 | 2.42 | 33.88 | 0.21 |
| P | 0.05 | <0.001 | 0.29 | 0.14 | <0.001 | 0.65 | |
| TN | F | 0.20 | 53.91 | 0.13 | 3.31 | 7.24 | 11.94 |
| P | 0.66 | <0.001 | 0.73 | 0.08 | 0.01 | 0.003 | |
| pH | F | 011 | 57.28 | 0.91 | 0.62 | 7.34 | 1.40 |
| P | 0.75 | <0.001 | 0.35 | 0.44 | 0.01 | 0.25 | |
| SWC | F | 1.20 | 5.56 | 0.25 | 0.33 | 0.07 | 0.05 |
| P | 0.29 | 0.03 | 0.62 | 0.57 | 0.79 | 0.83 | |
| SBD | F | 7.57 | 12.91 | 0.71 | 1.17 | 2.44 | 0.10 |
| P | 0.01 | 0.002 | 0.41 | 0.29 | 0.13 | 0.76 | |
| T PLFAs | F | 1.21 | 41.98 | 0.12 | 0.19 | 8.24 | 0.10 |
| P | 0.28 | <0.001 | 0.73 | 0.67 | 0.01 | 0.76 | |
| B PLFAs | F | 0.04 | 41.75 | 0.13 | 0.23 | 8.39 | 0.09 |
| P | 0.84 | <0.001 | 0.72 | 0.64 | 0.01 | 0.77 | |
| F PLFAs | F | 0.82 | 35.97 | 0.18 | 0.06 | 7.76 | 0.26 |
| P | 0.37 | <0.001 | 0.68 | 0.81 | 0.01 | 0.62 | |
| Chao1 | F | 0.49 | 12.35 | 2.13 | 2.64 | 0.21 | 0.34 |
| P | 0.49 | 0.002 | 0.16 | 0.01 | 0.66 | 0.57 | |
| Beta | F | 4.05 | 0.09 | 0.27 | 0.45 | 0.01 | 2.08 |
| P | 0.06 | 0.77 | 0.61 | 0.51 | 0.94 | 0.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Cai, M.; Li, D.; Yang, S.; Wu, J. Response of Soil Organic Carbon Stock to Bryophyte Removal Is Regulated by Forest Types in Southwest China. Forests 2022, 13, 2125. https://doi.org/10.3390/f13122125
Chen D, Cai M, Li D, Yang S, Wu J. Response of Soil Organic Carbon Stock to Bryophyte Removal Is Regulated by Forest Types in Southwest China. Forests. 2022; 13(12):2125. https://doi.org/10.3390/f13122125
Chicago/Turabian StyleChen, Deyun, Mutian Cai, Debao Li, Shiming Yang, and Jianping Wu. 2022. "Response of Soil Organic Carbon Stock to Bryophyte Removal Is Regulated by Forest Types in Southwest China" Forests 13, no. 12: 2125. https://doi.org/10.3390/f13122125





