Analytical Pyrolysis as a Tool to Assess Residual Lignin Content and Structure in Maritime Pine High-Yield Pulp
Abstract
:1. Introduction
2. Material and Methods
2.1. Analytical Pyrolysis (Py-GC/FID)
2.2. Data Treatment
2.3. Lignin Content Determination
3. Results and Discussion
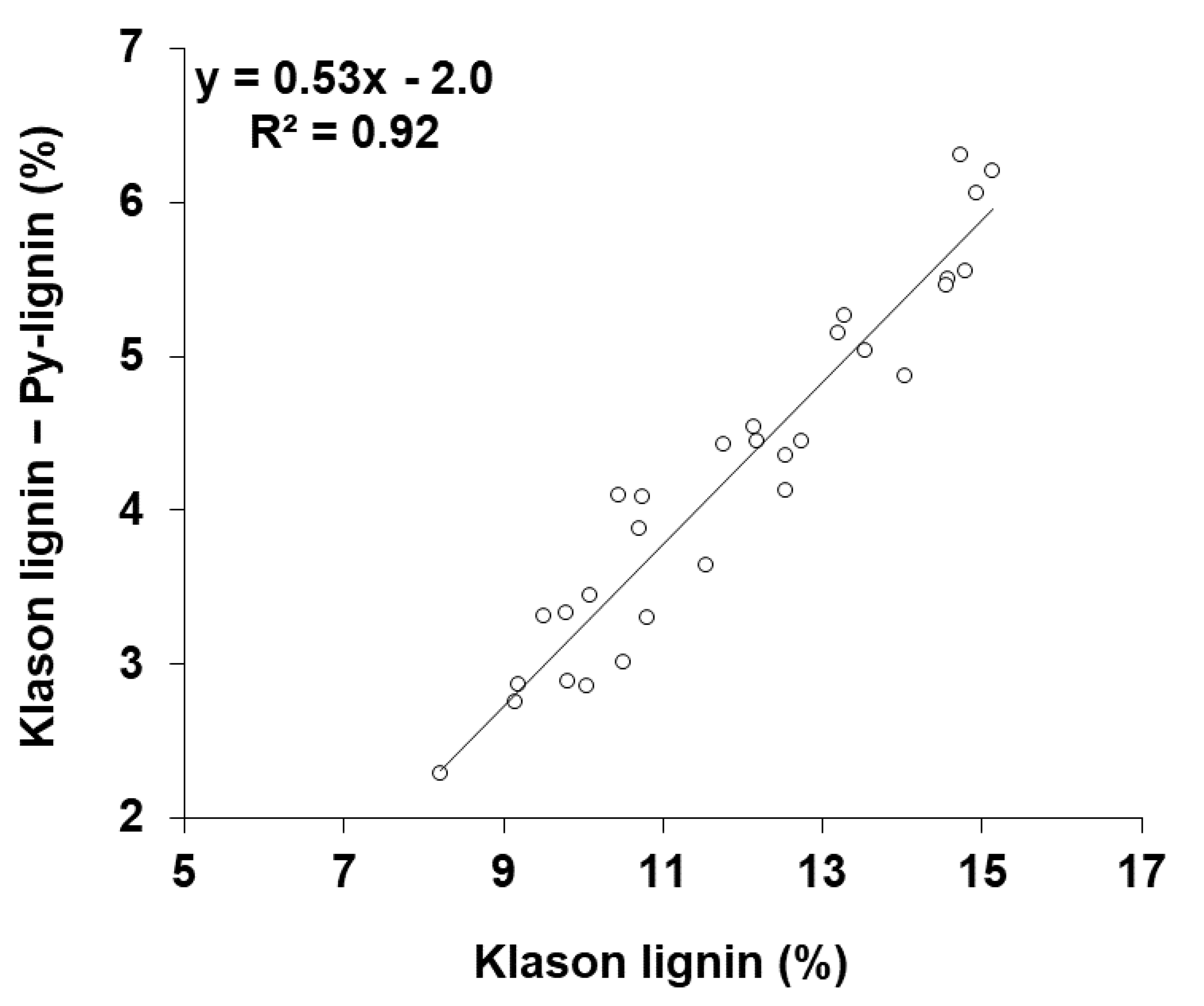
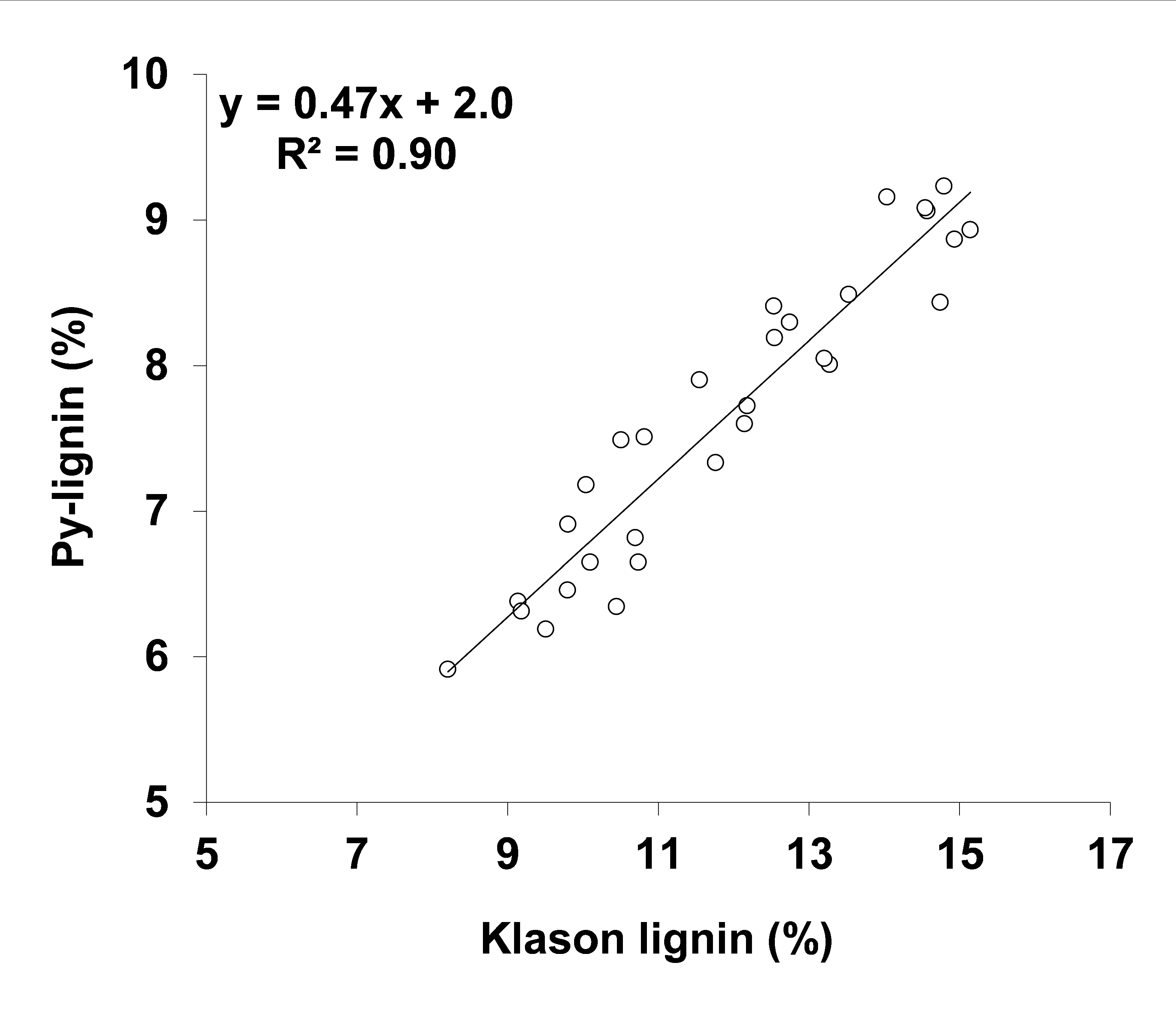
3.1. Klason Lignin in Pulp Can Be Estimated from Py-Lignin
3.2. Pulp Residual Lignin and Wood Have Different H/G Ratios
3.3. Pulp Residual Lignin and Wood Have Different Patterns of Pyrolysis Products
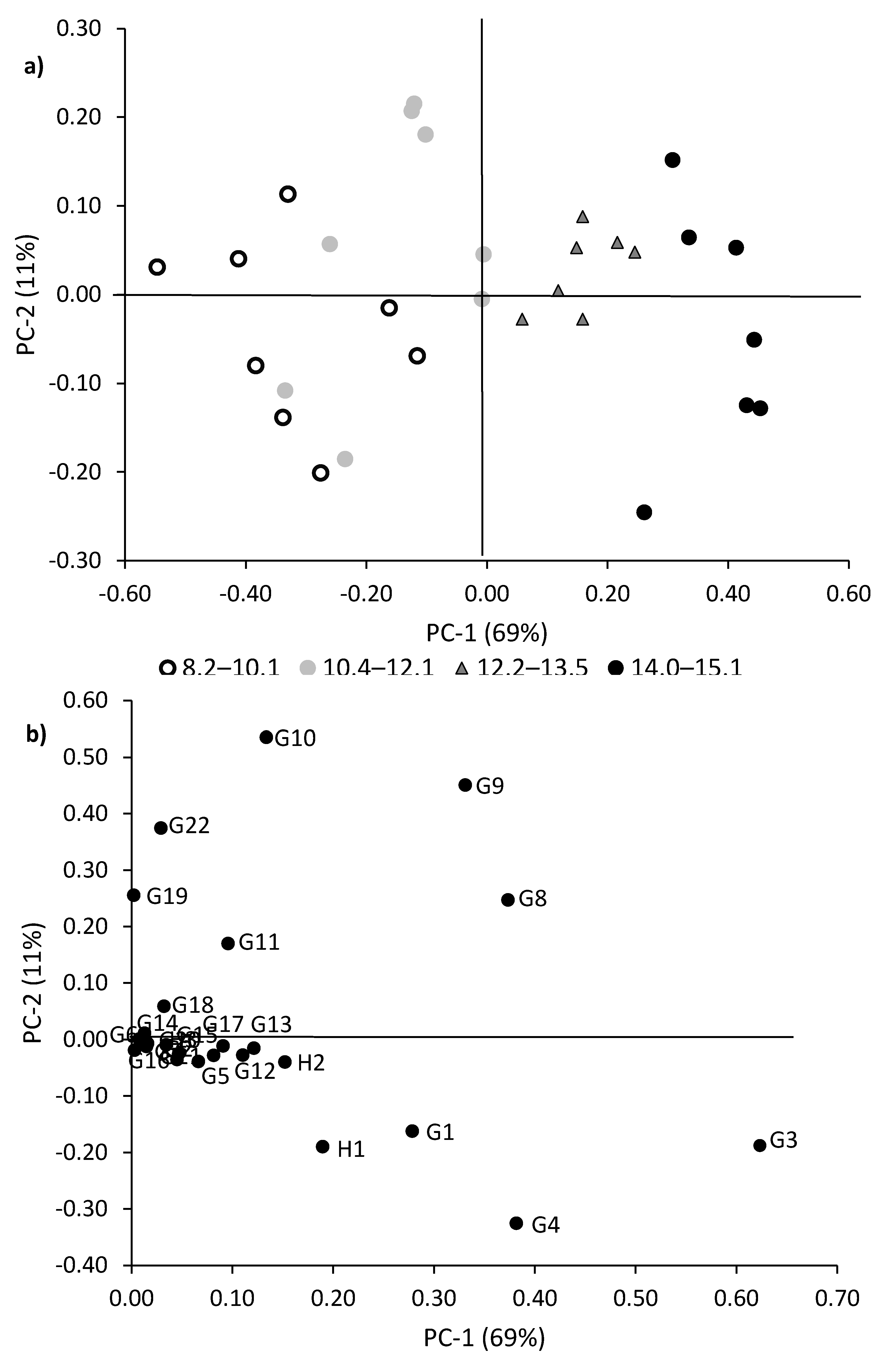
3.4. The PCA of Pyrolysis Products Separates Samples by Their Lignin Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bouffier, L.; Raffin, A.; Alía, R. Maritime pine–Pinus pinaster Ait. In Best Practice for Tree Breeding in Europe; Mullin, T.J., Lee, S., Eds.; Skogforsk: Uppsala, Sweden, 2013; pp. 65–76. ISBN 978-91-977649-6-4. [Google Scholar]
- Baptista, C.; Duarte, A.P.; Belgacem, M.N. Characterization of kraft lignin from Pinus pinaster. Cellul. Chem. Technol. 2002, 36, 137–149. [Google Scholar]
- Baptista, C.; Robert, D.; Duarte, A.P. Effect of pulping conditions on lignin structure from maritime pine kraft pulps. Chem. Eng. J. 2006, 121, 153–158. [Google Scholar] [CrossRef]
- da Silva Perez, D.; Guillemain, A.; Alazard, P.; Plomion, C.; Rozenberg, P.; Carlos Rodrigues, J.; Alves, A.; Chantre, G. Improvement of Pinus pinaster Ait elite trees selection by combining near infrared spectroscopy and genetic tools. Holzforschung 2007, 61, 611–622. [Google Scholar] [CrossRef]
- Shin, S.J.; Schroeder, L.R.; Lai, Y.Z. Impact of residual extractives on lignin determination in Kraft pulps. J. Wood Chem. Technol. 2004, 24, 139–151. [Google Scholar] [CrossRef]
- Letourneau, D.R.; Volmer, D.A. Mass spectrometry-based methods for the advanced characterization and structural analysis of lignin: A review. Mass Spectrom. Rev. 2021, 42, 144–188. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Schwanninger, M.; Pereira, H.; Rodrigues, J. Analytical pyrolysis as a direct method to determine the lignin content in wood-Part 1: Comparison of pyrolysis lignin with Klason lignin. J. Anal. Appl. Pyrolysis 2006, 76, 209–213. [Google Scholar] [CrossRef]
- Alves, A.; Rodrigues, J.; Wimmer, R.; Schwanninger, M. Analytical pyrolysis as a direct method to determine the lignin content in wood. Part 2: Evaluation of the common model and the influence of compression wood. J. Anal. Appl. Pyrolysis 2008, 81, 167–172. [Google Scholar] [CrossRef]
- Alves, A.; Santos, S.; Simoes, R.; Rodrigues, J. Characterization of residual lignin in cellulose isolated by the diglyme method from three Pinus species by IR spectroscopy and analytical pyrolysis. Holzforschung 2018, 72, 91–96. [Google Scholar] [CrossRef]
- Alves, A.; Cisneros, E.F.; Balmelli, G.; Poltri, S.N.M.; Rodrigues, J. Assessment of eucalypts wood lignin content by analytical pyrolysis, comparison with Klason and total lignin contents. J. Wood Chem. Technol. 2021, 41, 229–235. [Google Scholar] [CrossRef]
- Alves, A.; Rodrigues, J.C. Correlation between lignin content and syringyl-to-guaiacyl (S/G) ratio of Eucalyptus globulus wood. Holzforschung 2022, 76, 791–798. [Google Scholar] [CrossRef]
- Choi, J.W.; Faix, O.; Meier, D. Characterization of Residual Lignins from Chemical Pulps of Spruce (Picea abies L ) and Beech (Fagus sylvatica L.) by Analytical PyrolysisGas Chromatography/Mass Spectrometry. Holzforschung 2001, 55, 8. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Thain, S.C.; Donnison, I.S.; Morris, P.M.; Yates, N. Prediction of Klason lignin and lignin thermal degradation products by Py-GC/MS in a collection of Lolium and Festuca grasses. J. Anal. Appl. Pyrolysis 2007, 80, 16–23. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D.; Grobe, I. Studies on Isolated Lignins and Lignins in Woody Materials by Pyrolysis-Gas Chromatography-Mass Spectrometry and Off-Line Pyrolysis-Gas Chromatography with Flame Ionization Detection. J. Anal. Appl. Pyrolysis 1987, 11, 403–416. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D. Pyrolytic and hydrogenolytic degradation studies on lignocellulosics, pulps ans lignins. Holz Als Roh-Und Werkst. 1989, 47, 67–72. [Google Scholar] [CrossRef]
- Lourenco, A.; Gominho, J.; Marques, A.V.; Pereira, H. Comparison of Py-GC/FID and Wet Chemistry Analysis for Lignin Determination in Wood and Pulps from Eucalyptus globulus. Bioresources 2013, 8, 2967–2980. [Google Scholar] [CrossRef] [Green Version]
- Meier, D.; Faix, O. Pyrolysis-Gas Chromatography-Mass Spectrometry. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer-Verlag: Berlin, Germany, 1992; pp. 177–199. [Google Scholar]
- Meier, D.; Fortmann, I.; Odermatt, J.; Faix, O. Discrimination of genetically modified poplar clones by analytical pyrolysis-gas chromatography and principal component analysis. J. Anal. Appl. Pyrolysis 2005, 74, 129–137. [Google Scholar] [CrossRef]
- Ohra-aho, T.; Tenkanen, M.; Tamminen, T. Direct analysis of lignin and lignin-like components from softwood kraft pulp by Py-GC/MS techniques. J. Anal. Appl. Pyrolysis 2005, 74, 123–128. [Google Scholar] [CrossRef]
- van Erven, G.; de Visser, R.; Merkx, D.W.H.; Strolenberg, W.; de Gijsel, P.; Gruppen, H.; Kabel, M.A. Quantification of Lignin and Its Structural Features in Plant Biomass Using C-13 Lignin as Internal Standard for Pyrolysis-GC-SIM-MS. Anal. Chem. 2017, 89, 10907–10916. [Google Scholar] [CrossRef]
- Alves, A.; Simoes, R.; Stackpole, D.J.; Vaillancourt, R.E.; Potts, B.M.; Schwanninger, M.; Rodrigues, J. Determination of the syringyl/guaiacyl ratio of Eucalyptus globulus wood lignin by near infrared-based partial least squares regression models using analytical pyrolysis as the reference method. J. Near Infrared Spectrosc. 2011, 19, 343–348. [Google Scholar] [CrossRef]
- Alves, A.; Schwanninger, M.; Pereira, H.; Rodrigues, J. Calibration of NIR to assess lignin composition (H/G ratio) in maritime pine wood using analytical pyrolysis as the reference method. Holzforschung 2006, 60, 29–31. [Google Scholar] [CrossRef]
- Gaspar, M.J.; Alves, A.; Louzada, J.L.; Morais, J.; Santos, A.; Fernandes, C.; Almeida, M.H.; Rodrigues, J.C. Genetic variation of chemical and mechanical traits of maritime pine (Pinus pinaster Aiton). Correlations with wood density components. Ann. For. Sci. 2011, 68, 255–265. [Google Scholar] [CrossRef]
- Pot, D.; Chantre, G.; Rozenberg, P.; Rodrigues, J.C.; Jones, G.L.; Pereira, H.; Hannrup, B.; Cahalan, C.; Plomion, C. Genetic control of pulp and timber properties in maritime pine (Pinus pinaster Ait.). Ann. For. Sci. 2002, 59, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Pot, D.; Rodrigues, J.C.; Rozenberg, P.; Chantre, G.; Tibbits, J.; Cahalan, C.; Pichavant, F.; Plomion, C. QTLs and candidate genes for wood properties in maritime pine (Pinus pinaster Ait.). Tree Genet. Genomes 2006, 2, 10–24. [Google Scholar] [CrossRef]
- Stackpole, D.J.; Vaillancourt, R.E.; Alves, A.; Rodrigues, J.; Potts, B.M. Genetic Variation in the Chemical Components of Eucalyptus globulus Wood. G3 Bethesda 2011, 1, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Gierlinger, N.; Schwanninger, M.; Rodrigues, J. Analytical pyrolysis as a direct method to determine the lignin content in wood Part 3. Evaluation of species-specific and tissue-specific differences in softwood lignin composition using principal component analysis. J. Anal. Appl. Pyrolysis 2009, 85, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Lapierre, C.; Pollet, B.; Rolando, C. New Insights into the molecular architecture of hardwood lignins by chemical degradative methods. Res. Chem. Intermed. 1995, 21, 397–412. [Google Scholar] [CrossRef]
- Kleen, M.; Gellerstedt, G. Characterization of Chemical and Mechanical Pulps by Pyrolysis-Gas Chromatography Mass-Spectrometry. J. Anal. Appl. Pyrolysis 1991, 19, 139–152. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Klason lignin: Modifications to improve the precision of the standardized determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- TAPPI T 236 om-13; Kappa Number of Pulp. TAPPI T 236 om-13: Atlanta, GA, USA, 2013.
- Alves, A.; Santos, A.; da Silva Perez, D.; Rodrigues, J.; Helena, P.; Simoes, R.; Schwanninger, M. NIR PLSR model selection for Kappa number prediction of maritime pine Kraft pulps. Wood Sci. Technol. 2007, 41, 491–499. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ma, H.; Wu, S.B.; Wei, W.Q. Sequential Fractionation of Lignin-derived Pyrolysis Oil via Extraction with a Combination of Water and Organic Solvents. Bioresources 2019, 14, 2144–2159. [Google Scholar]
- TAPPI T222 om-02; Acid-Insoluble Lignin in Wood and Pulp. TAPPI T222 om-02: Atlanta, GA, USA, 2002.
- Higman, E.B.; Schmeltz, I.; Schlotzh, W.S. Products from Thermal Degradation of Some Naturally Occurring Materials. J. Agric. Food Chem. 1970, 18, 636–639. [Google Scholar] [CrossRef]
- Ziobro, G.C. Origin and Nature of Kraft Color.1. Role of Aromatics. J. Wood Chem. Technol. 1990, 10, 133–149. [Google Scholar] [CrossRef]
- Ohara, S.; Yasuta, Y.; Ohi, H. Structure elucidation of condensed tannins from barks by pyrolysis/gas chromatography. Holzforschung 2003, 57, 145–149. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood, Chemistry, Ultrastructure, Reactions; Fengel, D., Wegener, G., Eds.; Walter de Gruyter: Berlin, Germany, 1984; pp. 28–29. [Google Scholar]
- Amen-Chen, C.; Pakdel, H.; Roy, C. Production of monomeric phenols by thermochemical conversion of biomass: A review. Bioresour. Technol. 2001, 79, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, J.; Jiang, J.A.; Zhang, Y.F.; Song, M.Y.; Zhang, R.; Dong, Z.G.; Yang, H.P.; Yu, H.B. Revealing G-lignin model compounds pyrolysis behavior: Beta-O-4 and 5-5’ dimer and trimer. Fuel 2022, 317, 123531. [Google Scholar] [CrossRef]
- Lei, M.; Wu, S.B.; Liu, C.; Liang, J.J.; Xiao, R. Revealing the pyrolysis behavior of 5-5? biphenyl-type lignin fragment. Part I: A mechanistic study on fragmentation via experiments and theoretical calculation. Fuel Process. Technol. 2021, 217, 106812. [Google Scholar] [CrossRef]
- Kuroda, K.; Nakagawa-izumi, A. Analytical pyrolysis of lignin: Products stemming from beta-5 substructures. Org. Geochem. 2006, 37, 665–673. [Google Scholar] [CrossRef]
| Code | Compound | Wood (%a) | Pulp (%a) | Wood (%b) | Pulp (%b) |
|---|---|---|---|---|---|
| H 1 | Phenol | 0.5 | 0.4 | 2.2 | 6.2 |
| G 1 | Guaiacol | 2.2 | 0.8 | 9.0 | 11.1 |
| H 2 | p-Cresol | 0.4 | 0.3 | 1.5 | 3.5 |
| H 3 | m-Cresol | 0.3 | 0.3 | 1.2 | 4.5 |
| G 2 | 3-Methyl guaiacol | 0.2 | 0.1 | 0.6 | 1.1 |
| G 3 | 4-Methyl guaiacol | 2.5 | 1.1 | 10.3 | 14.7 |
| G 4 | 4-Vinyl guaiacol | 2.9 | 0.9 | 12.0 | 12.6 |
| G 5 | Eugenol | 0.9 | 0.1 | 3.6 | 1.9 |
| G 6 | 4-Propyl guaiacol | 0.1 | 0.1 | 0.6 | 0.7 |
| G 7 | Isoeugenol (cis) | 0.2 | 0.1 | 0.7 | 1.4 |
| G 8 | Isoeugenol (trans) | 2.0 | 0.7 | 8.4 | 9.7 |
| G 9 | Vanillin | 1.9 | 0.5 | 7.9 | 6.4 |
| G 10 | Indene, 6-hydroxy-7-methoxy-, 1H- | 1.2 | 0.5 | 5.2 | 6.6 |
| G 11 | Indene, 6-hydroxy-7-methoxy-, 2H- | 0.6 | 0.2 | 2.3 | 2.5 |
| G 12 | Homovanillin | 1.3 | 0.2 | 5.4 | 2.9 |
| G 13 | Acetoguaiacone | 0.9 | 0.2 | 3.7 | 2.7 |
| G 14 | Guaiacyl acetone | 0.4 | 0.0 | 1.8 | 0.5 |
| G 15 | Propioguaiacone | 0.2 | 0.1 | 0.9 | 0.9 |
| G 16 | Isomer of coniferyl alcohol | 0.8 | 0.1 | 3.3 | 2.0 |
| G 17 | G-CO-CH=CH2 | 0.5 | 0.0 | 2.0 | 0.6 |
| G 18 | G-CO-CO-CH3 | 0.2 | 0.0 | 0.9 | 0.5 |
| G 19 | Dihydroconiferyl alcohol | 0.7 | 0.3 | 3.1 | 4.1 |
| G 20 | Coniferyl alcohol (cis) | 0.4 | 0.0 | 1.5 | 0.2 |
| G 21 | Coniferyl alcohol (trans) | 0.5 | 0.0 | 2.0 | 0.2 |
| G 22 | Coniferylaldehyde | 2.4 | 0.2 | 9.9 | 2.6 |
| Sum | 24 | 7.3 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.; Graça, J.; Rodrigues, J. Analytical Pyrolysis as a Tool to Assess Residual Lignin Content and Structure in Maritime Pine High-Yield Pulp. Forests 2022, 13, 2169. https://doi.org/10.3390/f13122169
Alves A, Graça J, Rodrigues J. Analytical Pyrolysis as a Tool to Assess Residual Lignin Content and Structure in Maritime Pine High-Yield Pulp. Forests. 2022; 13(12):2169. https://doi.org/10.3390/f13122169
Chicago/Turabian StyleAlves, Ana, José Graça, and José Rodrigues. 2022. "Analytical Pyrolysis as a Tool to Assess Residual Lignin Content and Structure in Maritime Pine High-Yield Pulp" Forests 13, no. 12: 2169. https://doi.org/10.3390/f13122169





