Effects of Edaphic Factors at Different Depths on β-Diversity Patterns for Subtropical Plant Communities Based on MS-GDM in Southern China
Abstract
:1. Introduction
2. Material and Methods
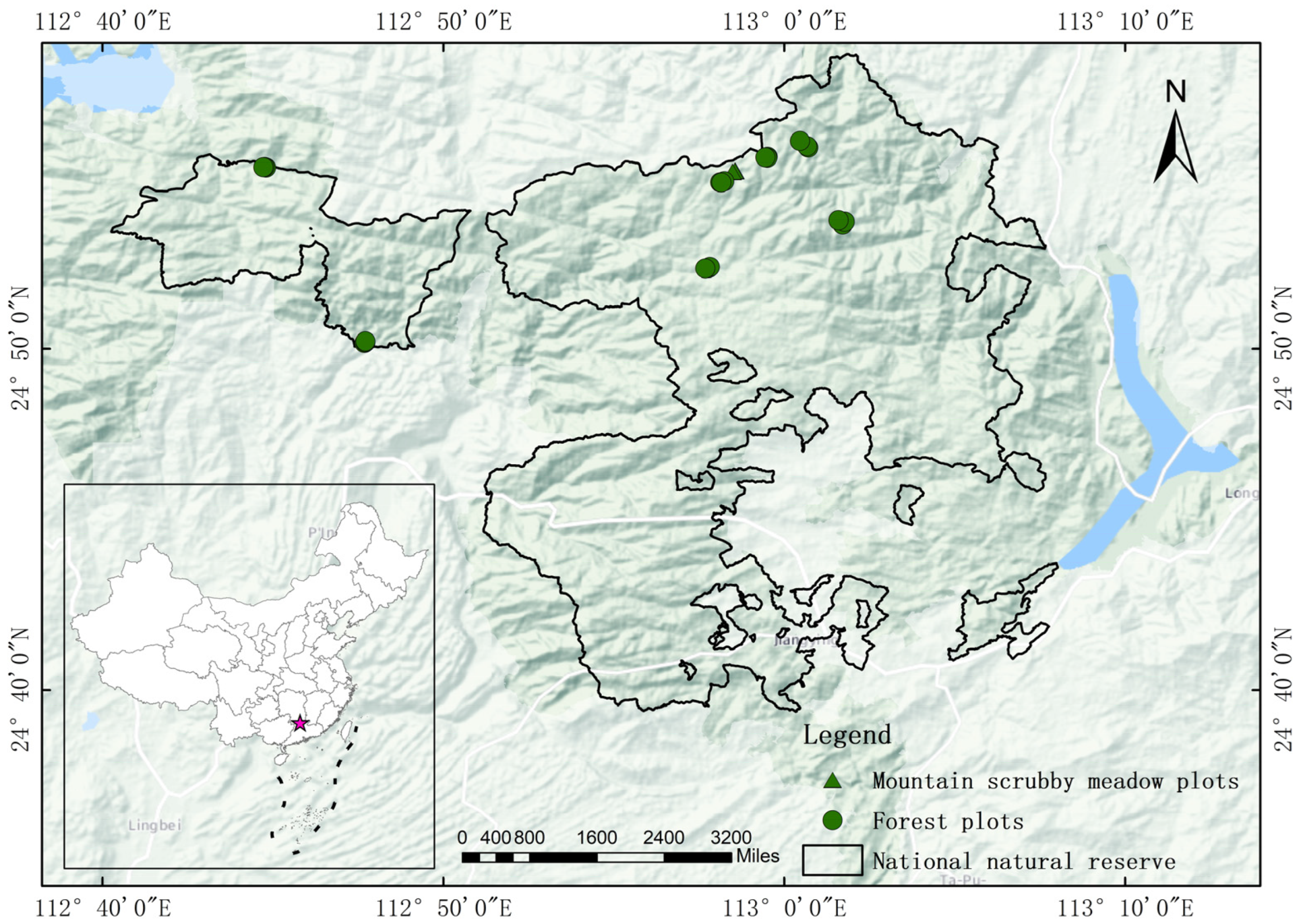
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. β-Diversity Index
2.4. Multi-Site Generalized Dissimilarity Modeling (MS-GDM)
3. Results
3.1. β-Diversity Distribution Pattern of Different Plant Forms
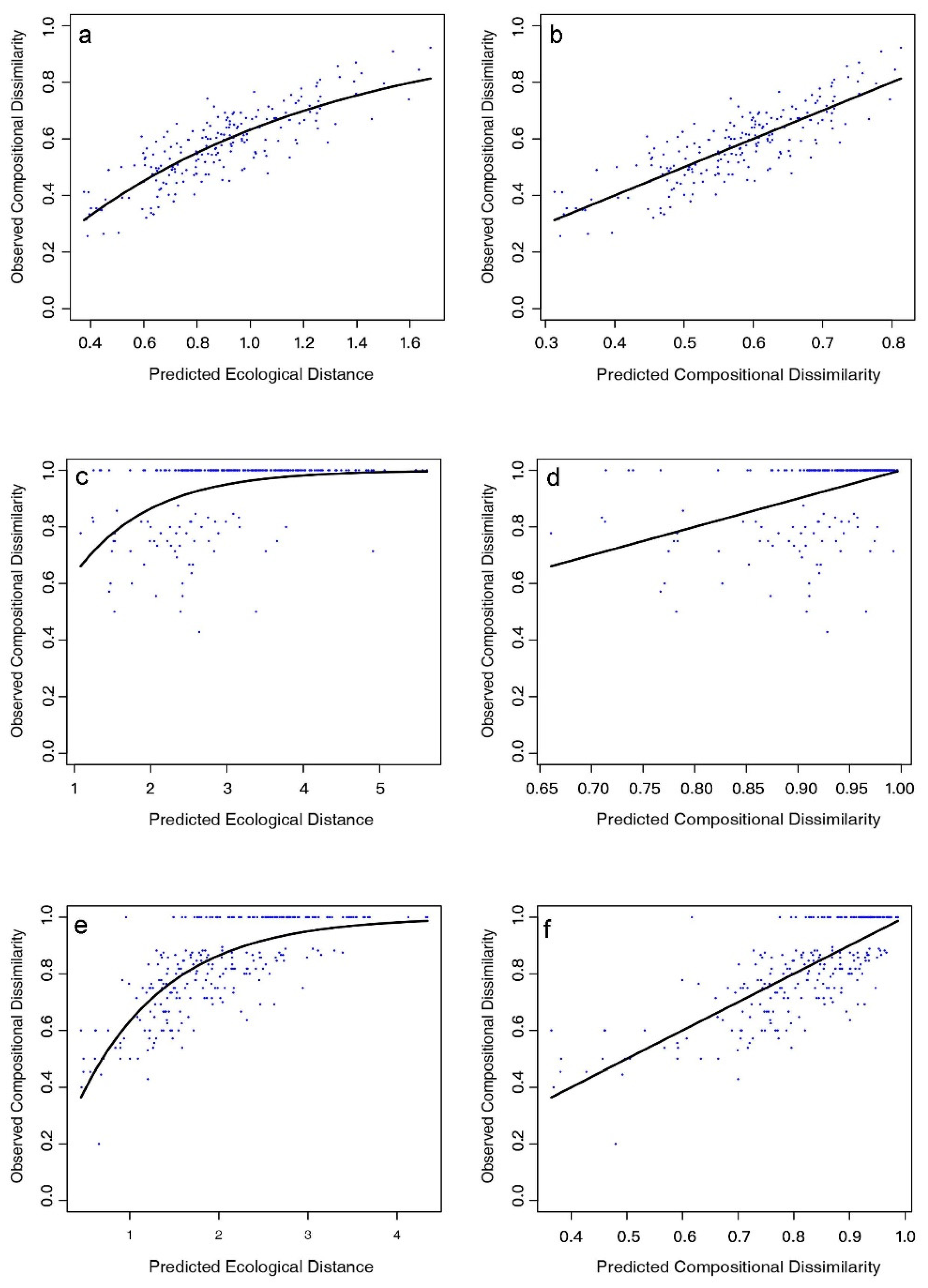
3.2. The Contribution of Environmental Factors to Plant β-Diversity by GDM
3.3. Relationship between Soil Chemical Properties and β-Diversity of Different Plant Forms
4. Discussion
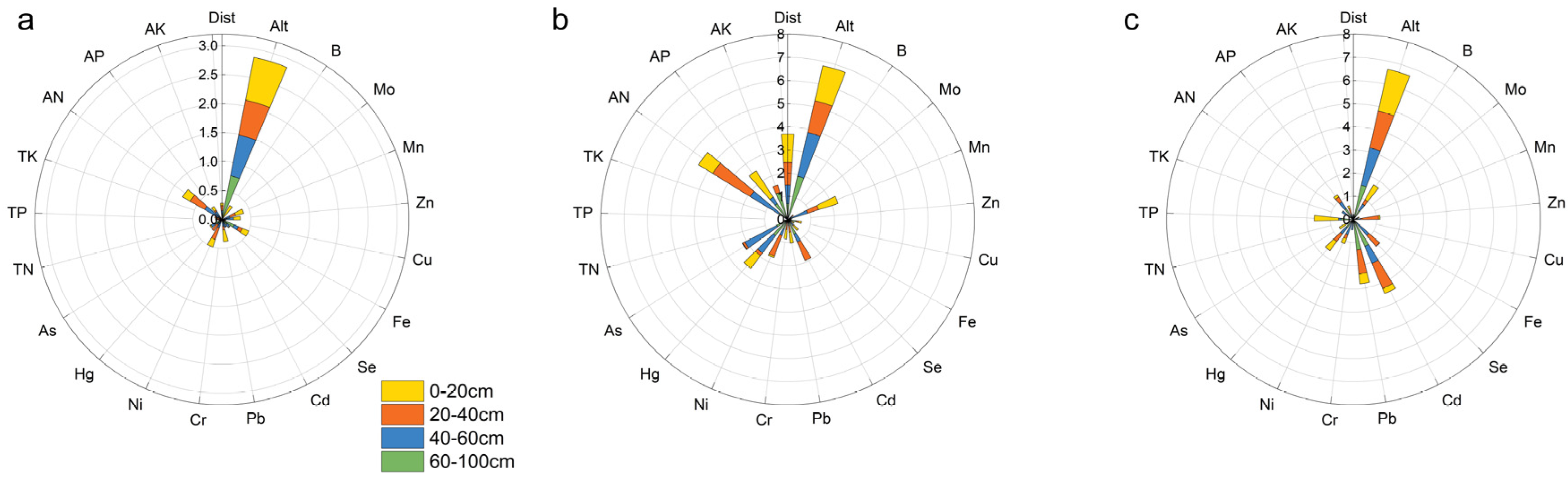
4.1. The Predicted Contribution of Different Soil Depths to the β-Diversity of Nanling Forest Communities
4.2. Relative Effects of Environmental Filtration and Dispersal Limitation on the β-Diversity of Plant Communities
4.3. Effects of Environmental Factors on Different Components of β-Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Pollock, L.J.; O’Connor, L.M.J.; Mokany, K.; Rosauer, D.F.; Talluto, M.V.; Thuiller, W. Protecting Biodiversity (in All Its Complexity): New Models and Methods. Trends Ecol. Evol. 2020, 35, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Magurran, A.E.; Dornelas, M.; Moyes, F.; Henderson, P.A. Temporal β diversity—A macroecological perspective. Glob. Ecol. Biogeogr. 2019, 28, 1949–1960. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Seidl, R. Beta-Diversity, Community Assembly, and Ecosystem Functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef]
- Qian, H.; Jin, Y.; Leprieur, F.; Wang, X.; Deng, T. Geographic patterns and environmental correlates of taxonomic and phylogenetic beta diversity for large-scale angiosperm assemblages in China. Ecography 2020, 43, 1706–1716. [Google Scholar] [CrossRef]
- Mori, A.S.; Shiono, T.; Koide, D.; Kitagawa, R.; Ota, A.T.; Mizumachi, E. Community assembly processes shape an altitudinal gradient of forest biodiversity. Glob. Ecol. Biogeogr. 2013, 22, 878–888. [Google Scholar] [CrossRef]
- Svenning, J.C.; Fløjgaard, C.; Baselga, A. Climate, history and neutrality as drivers of mammal beta diversity in Europe: Insights from multiscale deconstruction. J. Anim. Ecol. 2011, 80, 393–402. [Google Scholar] [CrossRef]
- König, C.; Weigelt, P.; Kreft, H. Dissecting global turnover in vascular plants. Glob. Ecol. Biogeogr. 2017, 26, 228–242. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Martins, P.M.; Poulin, R.; Goncalves-Souza, T. Drivers of parasite beta-diversity among anuran hosts depend on scale, realm and parasite group. Philos. T. R. Soc. B 2021, 376, 20200367. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M. Stochastic community assembly causes higher biodiversity in more productive environments. Science 2010, 328, 1388–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Mi, X.; Harrison, R.D.; Yang, B.; Man, X.; Ren, H.; Ma, K. Resource Heterogeneity, Not Resource Quantity, Plays an Important Role in Determining Tree Species Diversity in Two Species-Rich Forests. Fron. Ecol. Evol. 2020, 8, 224. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.M.; HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef]
- Xue, W.; Bezemer, T.M.; Berendse, F. Soil heterogeneity and plant species diversity in experimental grassland communities: Contrasting effects of soil nutrients and pH at different spatial scales. Plant Soil. 2019, 442, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Kallimanis, A.S.; Mazaris, A.D.; Tzanopoulos, J.; Halley, J.M.; Pantis, J.D.; Sgardelis, S.P. How does habitat diversity affect the species–area relationship? Glob. Ecol. Biogeogr. 2008, 17, 532–538. [Google Scholar] [CrossRef]
- Tukiainen, H.; Bailey, J.J.; Field, R.; Kangas, K.; Hjort, J. Combining geodiversity with climate and topography to account for threatened species richness. Conserv. Biol. 2017, 31, 364–375. [Google Scholar] [CrossRef]
- Bailey, J.J.; Boyd, D.S.; Field, R. Models of upland species’ distributions are improved by accounting for geodiversity. Landscape Ecol. 2018, 33, 2071–2087. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Spasojevic, M.J. The edaphic control of plant diversity. Glob. Ecol. Biogeogr. 2020, 29, 1634–1650. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, X.; Shao, H.; Ma, K. Strong plant-soil associations in a heterogeneous subtropical broad-leaved forest. Plant Soil 2011, 347, 211–220. [Google Scholar] [CrossRef]
- Hall, J.S.; McKenna, J.J.; Ashton, P.M.S.; Gregoire, T.G. Habitat characterizations underestimate the role of edaphic factors controlling the distribution of Entandrophragma. Ecology 2004, 85, 2171–2183. [Google Scholar] [CrossRef]
- Berendse, F. Competition between plant populations with different rooting depths. Oecologia 1979, 43, 19–26. [Google Scholar] [CrossRef]
- Bolte, A.; Villanueva, I. Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur. J. Forest Res. 2006, 125, 15–26. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.; Cavard, X.; Laganiere, J.o.; Reich, P.B.; Bergeron, Y.; Pare, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.; Sala, O.; Schulze, E.-D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Nanjing Institute of Soil Science. Analysis of Soil Physical and Chemical Properties; Shanghai Science and Technology Publishing House: Shanghai, China, 1978. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Ferrier, S.; Manion, G.; Elith, J.; Richardson, K. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 2007, 13, 252–264. [Google Scholar] [CrossRef]
- Overton, J.M.; Barker, G.M.; Price, R. Estimating and conserving patterns of invertebrate diversity: A test case of New Zealand land snails. Divers. Distrib. 2009, 15, 731–741. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; Gollan, J.R.; Faith, D.P.; Carter, G.A.; Lassau, S.A.; Ginn, S.G.; Bulbert, M.W.; Cassis, G. Using generalised dissimilarity models and many small samples to improve the efficiency of regional and landscape scale invertebrate sampling. Ecol. Inform. 2010, 5, 124–132. [Google Scholar] [CrossRef]
- Leaper, R.; Hill, N.A.; Edgar, G.J.; Ellis, N.; Lawrence, E.; Pitcher, C.R.; Barrett, N.S.; Thomson, R. Predictions of beta diversity for reef macroalgae across southeastern Australia. Ecosphere 2011, 2, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Eduard, S.; et al. Package ‘vegan’. Community Ecology Package; R Package Version 2.6-4; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Baselga, A.; Orme, D.; Villeger, S.; Bortoli, J.D.; Leprieur, F.; Logez, M. betapart: Partitioning Beta Diversity into Turnover and Nestedness Components, R package Version 1.5.6; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Zellweger, F.; Roth, T.; Bugmann, H.; Bollmann, K. Beta diversity of plants, birds and butterflies is closely associated with climate and habitat structure. Glob. Ecol. Biogeogr. 2017, 26, 898–906. [Google Scholar] [CrossRef]
- Nanda, S.A.; Haq, M.-u.; Singh, S.; Reshi, Z.A.; Rawal, R.S.; Kumar, D.; Bisht, K.; Upadhyay, S.; Upreti, D.; Pandey, A. Species richness and β-diversity patterns of macrolichens along elevation gradients across the Himalayan Arc. Sci. Rep. 2021, 11, 20155. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fang, J.; Chi, X.; Feng, J.; Liu, Y.; Shen, Z.; Wang, X.; Wang, Z.; Wu, X.; Zheng, C. Patterns of plant beta-diversity along elevational and latitudinal gradients in mountain forests of China. Ecography 2012, 35, 1083–1091. [Google Scholar] [CrossRef]
- Kouba, Y.; Martínez-García, F.; de Frutos, Á.; Alados, C.L. Plant β-diversity in human-altered forest ecosystems: The importance of the structural, spatial, and topographical characteristics of stands in patterning plant species assemblages. Eur. J. Forest Res. 2014, 133, 1057–1072. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Wang, Y.Q.; Xiang, W.S.; Li, X.K.; Cao, K.F. Environmental filtering and dispersal limitation jointly shaped the taxonomic and phylogenetic beta diversity of natural forests in southern China. Ecol. Evol. 2021, 11, 8783–8794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lv, G.; Gong, Y.; Li, Y.; Wang, H.; Wu, D. Characteristics and driving mechanisms of species beta diversity in desert plant communities. PLoS ONE 2021, 16, e0245249. [Google Scholar] [CrossRef] [PubMed]
- Latombe, G.; Hui, C.; McGeoch, M.A. Multi-site generalised dissimilarity modelling: Using zeta diversity to differentiate drivers of turnover in rare and widespread species. Methods Ecol. Evol. 2017, 8, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Krasnov, B.R.; Shenbrot, G.I.; Vinarski, M.M.; Korallo-Vinarskaya, N.P.; Khokhlova, I.S.; McGeoch, M. Multi-site generalized dissimilarity modelling reveals drivers of species turnover in ectoparasite assemblages of small mammals across the northern and central Palaearctic. Glob. Ecol. Biogeogr. 2020, 29, 1579–1594. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Jiménez, I.; Jørgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.-F.; He, F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, J.E.; Ciecka, A.L.; Meyer, N.Y.; Rabenold, K.N. Beta diversity along environmental gradients: Implications of habitat specialization in tropical montane landscapes. J. Anim. Ecol. 2009, 78, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Mazel, F.; Thuiller, W. Global determinants of zoogeographical boundaries. Nat. Ecol. Evol. 2017, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.W.; Archer, S. Influence of an overstorey tree (Prosopis glandulosa) on associated shrubs in a savanna parkland: Implications for patch dynamics. Oecologia 1996, 105, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Li, M.; He, N.; Li, J. Divergent roles of environmental and spatial factors in shaping plant β-diversity of different growth forms in drylands. Glob. Ecol. Conserv. 2021, 26, e01487. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Wang, H.; Wang, Z.; Gu, J. Root tip morphology, anatomy, chemistry and potential hydraulic conductivity vary with soil depth in three temperate hardwood species. Tree Physiol. 2016, 36, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Maeght, J.-L.; Gonkhamdee, S.; Clement, C.; Isarangkool Na Ayutthaya, S.; Stokes, A.; Pierret, A. Seasonal patterns of fine root production and turnover in a mature rubber tree (Hevea brasiliensis Müll. Arg.) stand-differentiation with soil depth and implications for soil carbon stocks. Front. Plant Sci. 2015, 6, 1022. [Google Scholar] [CrossRef] [Green Version]
- Schwinning, S.; Litvak, M.; Pockman, W.; Pangle, R.; Fox, A.; Huang, C.-W.; McIntire, C. A 3-dimensional model of Pinus edulis and Juniperus monosperma root distributions in New Mexico: Implications for soil water dynamics. Plant Soil 2020, 450, 337–355. [Google Scholar] [CrossRef]
- Krišāns, O.; Samariks, V.; Donis, J.; Jansons, Ā. Structural Root-plate characteristics of wind-thrown Norway spruce in hemiboreal forests of Latvia. Forests 2020, 11, 1143. [Google Scholar] [CrossRef]
- Awé, D.V.; Noiha, N.V.; Vroh, B.T.A.; Zapfack, L. Root Distribution of Four Tree Species Planted in Living Hedges according to Two Types of Soil and Three Agroforestry Technologies in the Sudano-Sahelian Zone of Cameroon. Open J. Agric. Res. 2021, 1, 74–83. [Google Scholar]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F.; Duc, C.; Ravet, K.; Gaymard, F. Ferritins and iron storage in plants. BBA–Gen. Subj. 2010, 1800, 806–814. [Google Scholar] [CrossRef] [PubMed]
- López-Millán, A.F.; Duy, D.; Philippar, K. Chloroplast iron transport proteins–function and impact on plant physiology. Front. Plant sci. 2016, 7, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilon, M.; Abdel-Ghany, S.E.; Cohu, C.M.; Gogolin, K.A.; Ye, H. Copper cofactor delivery in plant cells. Curr. Opin. Plant biol. 2006, 9, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Li, J.; He, B.; Chen, Y.; Huang, R.; Tao, J.; Tian, T. Root distribution features of typical herb plants for slope protection and their effects on soil shear strength. TCSAE 2013, 29, 144–152. [Google Scholar]
- Sheoran, I.; Aggarwal, N.; Singh, R. Effects of cadmium and nickel on in vivo carbon dioxide exchange rate of pigeon pea (Cajanus cajan L.). Plant Soil 1990, 129, 243–249. [Google Scholar] [CrossRef]
- Papazoglou, E.G.; Serelis, K.G.; Bouranis, D.L. Impact of high cadmium and nickel soil concentration on selected physiological parameters of Arundo donax L. Eur. J. Soil Biol. 2007, 43, 207–215. [Google Scholar] [CrossRef]


| Element Contents | Soil Depths (cm) | F-Value | p-Value | |||
|---|---|---|---|---|---|---|
| 0–20 | 20–40 | 40–60 | 60–100 | |||
| B(mg/kg) | 23.72(±1.8) | 27.40(±2.0) | 27.92(±2.0) | 27.35(±2.1) | 9.19 | <0.0001 *** |
| Mo(mg/kg) | 3.85(±1.1) | 4.24(±1.2) | 4.20(±1.0) | 4.43(±1.2) | 2.23 | 0.0919 |
| Mn(mg/kg) | 139.15(±13.1) | 155.46(±17.0) | 196.49(±23.2) | 244.63(±44.2) | 7.34 | 0.0002 |
| Zn(mg/kg) | 61.92(±2.9) | 80.71(±10.4) | 72.79(±2.4) | 81.19(±4.4) | 2.41 | 0.0743 |
| Cu(mg/kg) | 6.76(±0.8) | 5.87(±0.7) | 6.43(±0.7) | 6.71(±0.9) | 4.92 | 0.0037 ** |
| Fe(mg/kg) | 23.03(±1.6) | 26.53(±1.8) | 26.43(±1.6) | 26.06(±1.8) | 9.64 | <0.0001 *** |
| Se(mg/kg) | 1.45(±0.1) | 1.39(±0.1) | 1.16(±0.1) | 0.93(±0.1) | 27.55 | <0.0001 *** |
| Cd(mg/kg) | 0.19(±0.01) | 0.09(±0.01) | 0.07(±0.01) | 0.07(±0.01) | 70.40 | <0.0001 *** |
| Pb(mg/kg) | 78.25(±4.3) | 94.88(±10.0) | 90.55(±7.8) | 106.05(±16.5) | 2.16 | 0.1008 |
| Cr(mg/kg) | 26.86(±1.8) | 45.21(±2.9) | 52.88(±3.5) | 51.85(±4.8) | 16.03 | <0.0001 *** |
| Ni(mg/kg) | 10.43(±0.7) | 21.56(±2.1) | 25.47(±2.1) | 27.81(±2.8) | 24.34 | <0.0001 *** |
| Hg(mg/kg) | 0.35(±0.02) | 0.33(±0.02) | 0.31(±0.02) | 0.27(±0.01) | 10.70 | <0.0001 *** |
| As(mg/kg) | 20.11(±1.2) | 20.87(±1.6) | 20.25(±1.4) | 20.56(±1.7) | 0.59 | 0.6237 |
| TN(g/kg) | 3.35(±0.2) | 2.42(±0.3) | 1.87(±0.3) | 1.38(±0.2) | 41.26 | <0.0001 *** |
| TP(g/kg) | 0.24(±0.02) | 0.25(±0.02) | 0.23(±0.02) | 0.19(±0.02) | 11.17 | <0.0001 *** |
| TK(g/kg) | 22.27(±1.1) | 29.78(±1.3) | 31.28(±1.1) | 32.55(±1.4) | 73.28 | <0.0001 *** |
| AN(mg/kg) | 246.39(±15.8) | 264.39(±27.1) | 205.83(±28.7) | 150.79(±21.2) | 17.77 | <0.0001 *** |
| AP(mg/kg) | 1.98(±0.2) | 0.84(±0.2) | 0.58(±0.2) | 0.31(±0.1) | 24.10 | <0.0001 *** |
| AK(mg/kg) | 80.04(±4.8) | 109.88(±7.0) | 101.95(±7.2) | 110.56(±10.3) | 10.59 | <0.0001 *** |
| Soil Properties | β-sor | β-sim | β-nes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | |
| TN | 0.01 | 0.09 ** | 0.11 * | 0.12 | −0.0002 | 0.09 | 0.09 ** | 0.00 | 0.001 | −0.08 | −0.23 | −0.01 |
| TP | 0.06 | 0.04 * | 0.03 * | 0.07 * | 0.01 | 0.03 * | 0.02 | 0.02 | 0.00 | 0.00 | 0.002 | 0.00 |
| TK | 0.02 | 0.02 | −0.001 | 0.02 | 0.01 | 0.01 | −0.004 | 0.01 | 0.001 | 0.00 | 0.01 | −0.01 |
| AN | 0.01 | 0.12 ** | 0.13 ** | 0.003 | 0.01 | 0.11 ** | 0.1 ** | 0.01 | −0.001 | 0.00 | −0.0002 | −0.01 |
| AP | −0.004 | 0.04 | 0.06 | 0.002 | −0.02 | 0.01 | 0.05 | 0.00 | 0.05 * | 0.02 | 0.001 | −0.01 |
| AK | 0.031 * | −0.0001 | 0.03 | 0.02 | 0.05 * | 0.003 | 0.05 * | 0.05 | −0.02 | −0.03 | −0.03 | −0.04 |
| B | 0.05 * | 0.02 | −0.002 | 0.00 | 0.01 | 0.01 | −0.01 | 0.00 | 0.03 | 0.01 | 0.03 | 0.01 |
| Cu | 0.07 ** | 0.08 ** | 0.09 ** | 0.07 * | 0.06 * | 0.06 * | 0.06 * | 0.04 * | 0.00 | 0.00 | 0.00 | 0.01 |
| Fe | 0.05 * | 0.03 * | 0.00 | 0.01 | 0.01 | 0.01 | −0.01 | −0.001 | 0.05 * | 0.02 | 0.08 ** | 0.07 |
| Se | 0.03 * | −0.0004 | 0.002 | −0.01 | 0.05 * | 0.001 | 0.001 | −0.003 | −0.02 | −0.01 | 0.00 | −0.001 |
| Cd | 0.004 | −0.004 | −0.04 | 0.00 | 0.01 | −0.001 | −0.01 | 0.01 | −0.02 | −0.01 | −0.01 | −0.01 |
| Cr | 0.001 | −0.002 | 0.0002 | −0.02 | −0.01 | −0.01 | −0.01 | −0.01 | 0.06 * | 0.01 | 0.03 | −0.001 |
| Ni | 0.003 | 0.01 | 0.004 | −0.01 | −0.01 | 0.001 | −0.003 | −0.01 | 0.1 ** | 0.04 | 0.08 * | 0.00 |
| Hg | 0.01 | 0.08 *** | 0.10 ** | 0.04 * | 0.02 | 0.09 *** | 0.13 ** | 0.04 * | −0.03 | −0.01 | −0.03 | −0.004 |
| Soil Properties | β-sor | β-sim | β-nes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | |
| TN | 0.005 | 0.02 * | 0.02 * | 0.0004 | 0.01 | 0.02 * | 0.02 * | 0.15 | −0.55 | −0.01 | −0.01 | −0.002 |
| TP | 0.002 | 0.02 * | 0.01 | 0.001 | 0.001 | 0.01 | 0.01 | 0.008 | 0.002 | −0.003 | −0.01 | −0.0003 |
| TK | 0.002 | −0.002 | −0.01 | 0-.01 | 0.001 | −0.003 | −0.028 | −0.02 | 0.0001 | 0.004 | 0.02 | 0.01 |
| AN | 0.02 * | 0.04 ** | 0.03 | 0.001 | 0.02 | 0.03 * | 0.03 * | 0.001 | −0.01 | −0.01 | −0.01 | −0.001 |
| AP | 0.01 | 0.004 | 0.02 | 0.002 | 0.01 | 0.01 | 0.02 | 0.002 | −0.003 | −0.005 | −0.01 | −0.001 |
| AK | −0.13 | −0.002 | 0.01 | 0.001 | 0.001 | 0.002 | 0.004 | 0.001 | −0.003 | −0.002 | −0.002 | 0.001 |
| B | −0.002 | −0.0003 | −0.01 | −0.01 | −0.001 | −0.002 | −0.02 | −0.02 | 0.0001 | 0.004 | 0.01 | 0.02 |
| Cu | 0.02 | 0.03 * | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | 0.0006 | −0.0001 | −0.0004 |
| Fe | 0.0001 | 0.0001 | −0.004 | −0.01 | 0.001 | −0.001 | −0.01 | −0.01 | −0.002 | 0.0006 | 0.01 | 0.01 |
| Se | 0.01 | −0.03 | 0.002 | −0.01 | 0.0003 | −0.002 | 0.001 | −0.02 | 0.003 | 0.01 | −0.03 | 0.01 |
| Cd | 0.003 | 0.01 | −0.002 | 0.004 | 0.0001 | 0.004 | −0.001 | −0.01 | 0.002 | −0.002 | −0.002 | 0.004 |
| Cr | 0.001 | 0.02 | −0.004 | −0.003 | −0.001 | 0.01 | 0.001 | −0.001 | 0.004 | −0.006 | −0.01 | −0.002 |
| Ni | 0.01 | 0.03 | 0.001 | 0.0001 | 0.002 | 0.02 | 0.003 | 0.002 | 0.0001 | −0.001 | −0.01 | −0.01 |
| Hg | −0.01 | 0.003 | 0.02 | 0.01 | −0.001 | 0.0002 | 0.01 | 0.02 | 0.001 | 0.001 | 0.001 | −0.02 |
| Soil Properties | β-sor | β-sim | β-nes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–100 cm | |
| TN | 0.69 | 0.04 * | 0.01 | −0.13 | 0.01 | 0.013 | 0.002 | 0.002 | −0.28 | 0.65 | 0.01 | 0.003 |
| TP | 0.14 ** | 0.02 | 0.001 | 0.05 | 0.06 ** | 0.01 | 0 | 0.02 * | −0.12 | −0.03 | 0.002 | −0.01 |
| TK | 0.16 | −0.003 | −0.02 | 0.10 | −0.04 | −0.001 | −0.01 | −0.0001 | 0.0002 | −0.001 | 0 | −0.001 |
| AN | 0.03 * | 0.04 * | 0.02 | 0.07 | 0.01 | 0.01 | 0.002 | 0 | 0.001 | 0.01 | 0.01 | 0 |
| AP | −0.34 | 0.002 | 0.001 | −0.14 | −0.01 | 0 | 0.02 | 0.01 | 0.02 | 0.01 | 0.002 | 0.02 |
| AK | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.01 | 0.03 | 0.02 | −0.03 * | −0.002 | −0.01 | −0.01 |
| B | 0.03 * | 0.04 * | 0.01 | 0.04 * | 0.03 | 0.05 * | 0.02 | 0.05 ** | −0.02 | −0.03 | −0.02 | −0.03 * |
| Cu | 0.02 | 0.02 | 0.02 | 0.01 | 0.001 | 0.002 | 0.002 | 0 | 0.02 | 0.02 | 0.02 | 0.02 |
| Fe | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 * | 0.01 | 0.03 * | −0.01 | −0.02 | −0.004 | −0.01 |
| Se | 0.02 | 0.0004 | 0.01 | 0.01 | 0.01 | 0.001 | 0.003 | 0.01 | 0.002 | −0.1 | −0.001 | −0.01 |
| Cd | 0.15 *** | 0.02 | 0.01 | 0.01 | 0.13 *** | 0.01 | 0.01 | 0.02 | −0.04 * | −0.009 | −0.001 | −0.01 |
| Cr | −0.0002 | 0.03 | −0.003 | 0.002 | −0.01 | 0.04 | −0.0004 | −0.003 | 0.02 | −0.02 | −0.001 | −0.004 |
| Ni | 0.08 ** | 0.12 ** | 0.03 * | 0.02 | 0.09 ** | 0.11 ** | 0.03 | 0.03 | −0.05 ** | −0.03 | −0.02 | −0.02 |
| Hg | 0.01 | 0.06 ** | 0.05 ** | 0.02 | 0.01 | 0.05 ** | 0.002 | 0.004 | −0.0004 | −0.01 | 0.09 ** | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; González-Rodríguez, M.Á.; Li, Z.; Tan, Z.; Yan, P.; Zhou, P. Effects of Edaphic Factors at Different Depths on β-Diversity Patterns for Subtropical Plant Communities Based on MS-GDM in Southern China. Forests 2022, 13, 2184. https://doi.org/10.3390/f13122184
Xu W, González-Rodríguez MÁ, Li Z, Tan Z, Yan P, Zhou P. Effects of Edaphic Factors at Different Depths on β-Diversity Patterns for Subtropical Plant Communities Based on MS-GDM in Southern China. Forests. 2022; 13(12):2184. https://doi.org/10.3390/f13122184
Chicago/Turabian StyleXu, Wei, Miguel Ángel González-Rodríguez, Zehua Li, Zhaowei Tan, Ping Yan, and Ping Zhou. 2022. "Effects of Edaphic Factors at Different Depths on β-Diversity Patterns for Subtropical Plant Communities Based on MS-GDM in Southern China" Forests 13, no. 12: 2184. https://doi.org/10.3390/f13122184





