The Opposite of Biotic Resistance: Herbivory and Competition Suppress Regeneration of Native but Not Introduced Mangroves in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
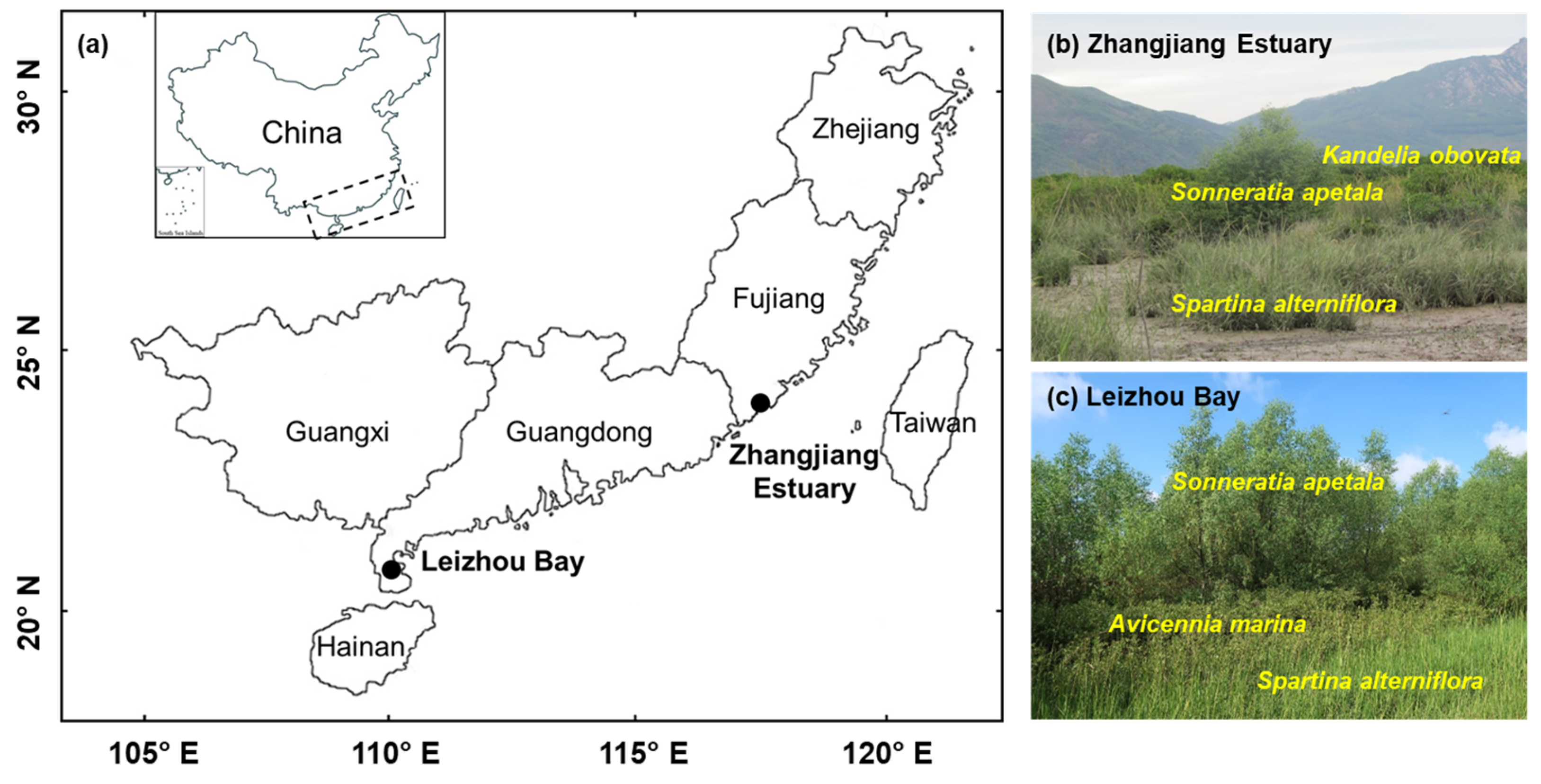
2.2. Study Sites
2.3. Transplant Experiment to Evaluate Early Establishment of Mangroves
2.4. Effects of Herbivory
2.5. Abiotic Factors
2.6. Relative Importance of Biotic and Abiotic Factors
3. Results
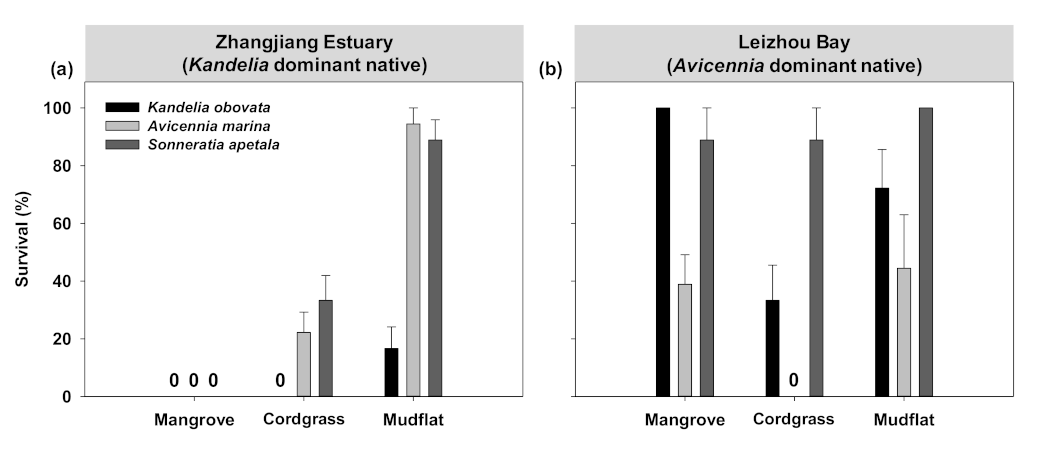
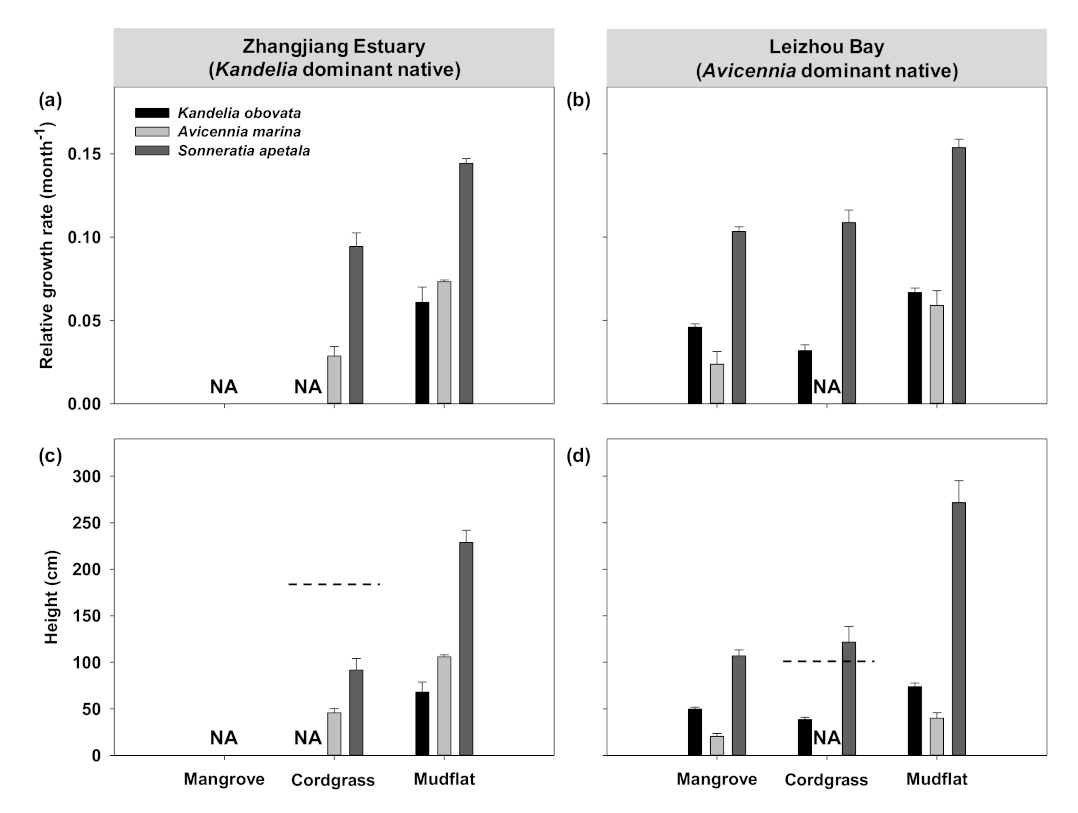
3.1. Transplant Experiment to Evaluate Early Establishment of Mangroves
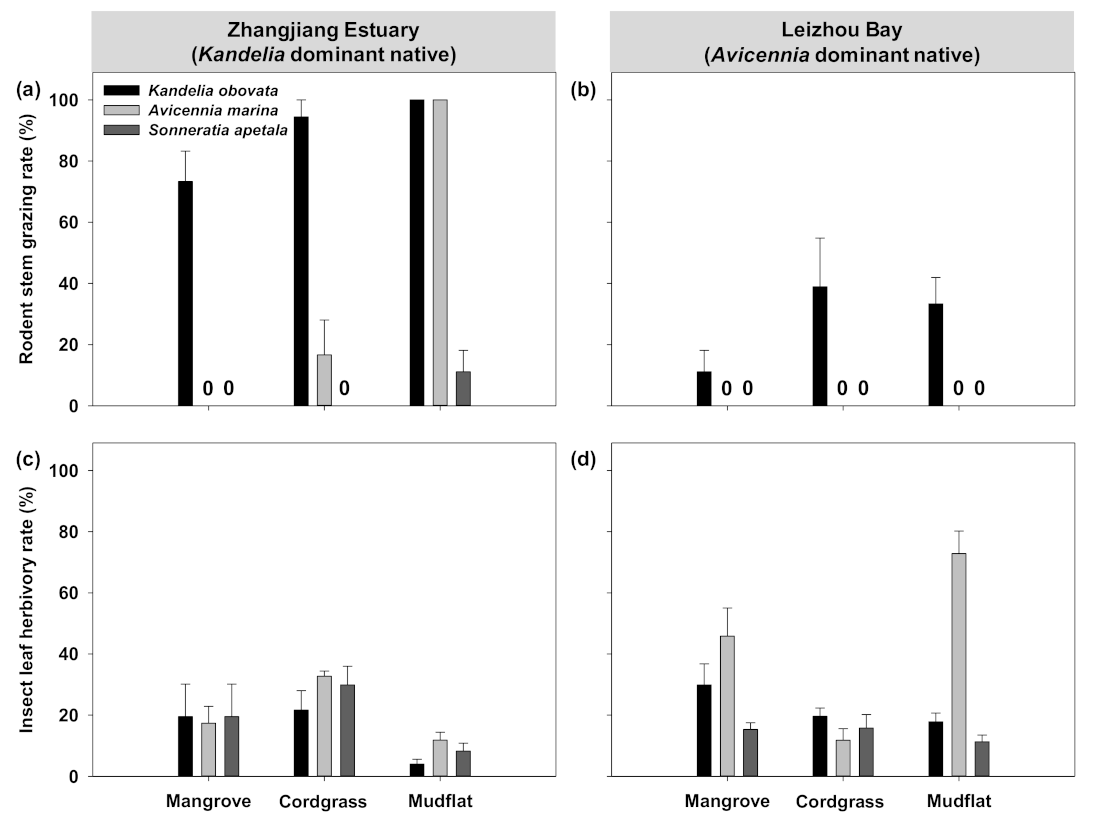
3.2. Effects of Herbivory
3.3. Effects of Abiotic Factors
3.4. Effects of Competition
3.5. Relative Importance of Biotic and Abiotic Factors
4. Discussion
4.1. Herbivory by Rodents and Insects Mediates Mangrove Establishment
4.2. Competition from Native Mangroves and Non-Native Cordgrass Mediates Mangrove Establishment
4.3. Novel Interaction between Non-Native Species in Southern China
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: New York, NY, USA, 2016. [Google Scholar]
- Ward, R.D.; Friess, D.A.; Day, R.H.; Mackenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Fu, H.; Lee, S.Y.; Fan, H.; Wang, M. Can strict protection stop the decline of mangrove ecosystems in China? From rapid destruction to rampant degradation. Forests 2020, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The state of the world’s mangrove forests: Past, present, and future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.-S.; Mukul, S.A. Publication performance and trends in mangrove forests: A bibliometric analysis. Sustainability 2021, 13, 12532. [Google Scholar] [CrossRef]
- McKee, K.L.; Rogers, K.; Saintilan, N. Response of salt marsh and mangrove wetlands to changes in atmospheric CO2, climate and sea level. In Global Change and the Function and Distribution of Wetlands, 1st ed.; Middleton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 63–96. [Google Scholar]
- Cavanaugh, K.C.; Kellner, J.R.; Forde, A.J.; Gruner, D.S.; Parker, J.D.; Rodriguez, W.; Feller, I.C. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc. Natl. Acad. Sci. USA 2014, 111, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, W.; Li, Q.Q.; Zhang, Y.; Yang, S.; Osland, M.J.; Huang, J.; Peng, C. Mangrove species’ responses to winter air temperature extremes in China. Ecosphere 2017, 8, e01865. [Google Scholar] [CrossRef]
- Biswas, S.R.; Biswas, P.L.; Limon, S.H.; Yan, E.; Xu, M.; Khan, M.S.I. Plant invasion in mangrove forests worldwide. For. Ecol. Manag. 2018, 429, 480–492. [Google Scholar] [CrossRef]
- Chen, L. Invasive plants in coastal wetlands: Patterns and mechanisms. In Wetlands: Ecosystem Services, Restoration and Wise Use, 1st ed.; An, S., Verhoeven, J., Eds.; Springer: Cham, Switzerland, 2019; Volume 238, pp. 97–128. [Google Scholar]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Guimarães Sampaio, J.A.; Gonçalves Reis, C.R.; Cunha-Lignon, M.; Nardoto, G.B.; Salemi, L.F. Plant invasion affects vegetation structure and sediment nitrogen stocks in subtropical mangroves. Mar. Environ. Res. 2021, 172, 105506. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Wang, W.; Chen, L.; Lin, G. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 2012, 93, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Guo, Q.; Liu, H.; Li, J.; Zhang, Q.; Xu, H.; Xu, F. Patterns of alien plant invasion across coastal bay areas in southern China. J. Coast. Res. 2014, 30, 448–455. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Smith, T.J.; Possley, J.; Collins, T.M.; Lee, D.; Namoff, S. Are mangroves in the tropical Atlantic ripe for invasion? Exotic mangrove trees in the forests of South Florida. Biol. Invasions 2010, 12, 2509–2522. [Google Scholar] [CrossRef]
- Saenger, P.; Bellan, M.F. The Mangrove Vegetation of the Atlantic Coast of Africa: A Review, 1st ed.; Université de Toulouse: Toulouse, France, 1995; pp. 1–62. [Google Scholar]
- Demopoulos, A.W.J.; Fry, B.; Smith, C.R. Food web structure in exotic and native mangroves: A Hawaii–Puerto Rico comparison. Oecologia 2007, 153, 675–686. [Google Scholar] [CrossRef]
- Devaney, J.L.; Lehmann, M.; Feller, I.C.; Parker, J.D. Mangrove microclimates alter seedling dynamics at the range edge. Ecology 2017, 98, 2513–2520. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Weaver, C.; Charles, S.P.; Whitt, A.; Dastidar, S.; D’Odorico, P.; Fuentes, J.D.; Kominoski, J.S.; Armitage, A.R.; Pennings, S.C. Coastal regime shifts: Rapid responses of coastal wetlands to changes in mangrove cover. Ecology 2017, 98, 762–772. [Google Scholar] [CrossRef]
- Saintilan, N.; Wilson, N.C.; Rogers, K.; Rajkaran, A.; Krauss, K.W. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Chang. Biol. 2014, 20, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.B.; de Freitas, D.M.; Rogers, K.; Woodroffe, C.D. Estuaries and coastal wetlands of the southern hemisphere—An Overview. Estuar. Coast. Shelf Sci. 2021, 250, 107125. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Eschtruth, A.K.; Battles, J.J. Assessing the relative importance of disturbance, herbivory, diversity, and propagule pressure in exotic plant invasion. Ecol. Monogr. 2009, 79, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Maron, J.L.; Vilà, M. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 2001, 95, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, S.; Spigler, R.B.; Horvitz, C.C. In a long-term experimental demography study, excluding ungulates reversed invader’s explosive population growth rate and restored natives. Proc. Natl. Acad. Sci. USA 2014, 111, 4501–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, D.E.; Potter, T.; Maron, J.L. Biotic resistance: Exclusion of native rodent consumers releases populations of a weak invader. J. Ecol. 2012, 100, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Meng, H.; Wang, Y.; He, Q. Herbivory enhances the resistance of mangrove forest to cordgrass invasion. Ecology 2018, 99, 1382–1390. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Schell, L.D.; Vanden Heuvel, B. How grazing and soil quality affect native and exotic plant diversity in rocky mountain grasslands. Ecol. Appl. 1999, 9, 45–64. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J.; Offenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Langston, A.K.; Kaplan, D.A.; Angelini, C. Predation restricts black mangrove (Avicennia germinans) colonization at its northern range limit along Florida’s gulf coast. Hydrobiologia 2017, 803, 317–331. [Google Scholar] [CrossRef]
- Bakker, J.; Wilson, S. Competitive abilities of introduced and native grasses. Plant Ecol. 2001, 157, 117–125. [Google Scholar] [CrossRef]
- Amsberry, L.; Baker, M.A.; Ewanchuk, P.J.; Bertness, M.D. Clonal integration and the expansion of Phragmites australis. Ecol. Appl. 2000, 10, 1110–1118. [Google Scholar] [CrossRef]
- Fine, P.V.A. The invasibility of tropical forests by exotic plants. J. Trop. Ecol. 2002, 18, 687–705. [Google Scholar] [CrossRef] [Green Version]
- Pranchai, A.; Jenke, M.; Vogt, J.; Grueters, U.; Yue, L.; Mehlig, U.; de Menezes, M.M.; Wagner, S.; Berger, U. Density-dependent shift from facilitation to competition in a dwarf Avicennia germinans forest. Wetl. Ecol. Manag. 2018, 26, 139–150. [Google Scholar] [CrossRef]
- Peng, D.; Chen, L.; Pennings, S.C.; Zhang, Y. Using a marsh organ to predict future plant communities in a Chinese estuary invaded by an exotic grass and mangrove. Limnol. Oceanogr. 2018, 63, 2595–2605. [Google Scholar] [CrossRef]
- An, S.; Gu, B.; Zhou, C.; Wang, Z.; Deng, Z.; Zhi, Y.; Li, H.; Chen, L.; Yu, D.; Liu, Y. Spartina invasion in China: Implications for invasive species management and future research. Weed Res. 2007, 47, 183–191. [Google Scholar] [CrossRef]
- Strong, D.R.; Ayres, D.R. Ecological and evolutionary misadventures of Spartina. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 389–410. [Google Scholar] [CrossRef]
- Milbrandt, E.C.; Tinsley, M.N. The role of saltwort (Batis maritima L.) in regeneration of degraded mangrove forests. Hydrobiologia 2006, 568, 369–377. [Google Scholar] [CrossRef]
- McKee, K.L.; Rooth, J.E.; Feller, I.C. Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol. Appl. 2007, 17, 1678–1693. [Google Scholar] [CrossRef]
- Liao, B.; Zheng, S.; Chen, Y.; Li, M.; Li, Y. Biological characteristics and ecological adaptability for non-indigenous mangrove species Sonneratia apetala. Chin. J. Ecol. 2004, 23, 10–15. [Google Scholar]
- Ren, H.; Lu, H.; Shen, W.; Huang, C.; Guo, Q.; Li, Z.; Jian, S. Sonneratia apetala Buch.Ham in the mangrove ecosystems of China: An invasive species or restoration species? Ecol. Eng. 2009, 35, 1243–1248. [Google Scholar] [CrossRef]
- Hong, P.; Wen, Y.; Xiong, Y.; Diao, L.; Gu, X.; Feng, H.; Yang, C.; Chen, L. Latitudinal gradients and climatic controls on reproduction and dispersal of the non-native mangrove Sonneratia apetala in China. Estuar. Coast. Shelf Sci. 2021, 248, 106749. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhang, Y.; Lin, G. Recent progresses in mangrove conservation, restoration and research in China. J. Plant Ecol. 2009, 2, 45–54. [Google Scholar] [CrossRef]
- Li, B.; Liao, C.; Zhang, X.; Chen, H.; Wang, Q.; Chen, Z.; Gan, X.; Wu, J.; Zhao, B.; Ma, Z.; et al. Spartina alterniflora invasions in the Yangtze River Estuary, China: An overview of current status and ecosystem effects. Ecol. Eng. 2009, 35, 511–520. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Zhang, Y. Recruitment and herbivory affect spread of invasive Spartina alterniflora in China. Ecology 2014, 95, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liao, B.; Liu, B.; Peng, C.; Zhang, Y.; Guan, W.; Zhu, Q.; Yang, G. Eradicating invasive Spartina alterniflora with alien Sonneratia apetala and its implications for invasion controls. Ecol. Eng. 2014, 73, 367–372. [Google Scholar] [CrossRef]
- Liu, W.; Maung-Douglass, K.; Strong, D.R.; Pennings, S.C.; Zhang, Y. Geographical variation in vegetative growth and sexual reproduction of the invasive Spartina alterniflora in China. J. Ecol. 2016, 104, 173–181. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M. The Mangrove of China, 1st ed.; Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Iftekhar, M.S.; Saenger, P. Vegetation dynamics in the Bangladesh Sundarbans mangroves: A review of forest inventories. Wetl. Ecol. Manag. 2008, 16, 291–312. [Google Scholar] [CrossRef]
- Zhao, S. On the prelimonary inestigation of turkestan rat, Rattus rattoides exiguus Howell, in sea beach of red-woods. Zool. Res. 1982, 3, 103. (In Chinese) [Google Scholar]
- Smith, A.T.; Xie, Y. A Guide to the Mammals of China, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2008; pp. 270–271. [Google Scholar]
- Li, Z.; Dai, J.; Ye, J.; Xu, H.; Han, S. Species, control status and outbreak causes of main pest insects in mangrove ecosystems in China. Acta Entomol. Sin. 2012, 55, 1109–1118. [Google Scholar]
- Lin, P. The Comprehensive Report of Science Investigation on the Natural Reserve of Mangrove Wetland of Zhangjiang Estuary in Fujian, 1st ed.; Xiamen University Press: Xiamen, China, 2001. (In Chinese) [Google Scholar]
- Zhu, X.; Meng, L.; Zhang, Y.; Weng, Q.; Morris, J. Tidal and meteorological influences on the growth of invasive Spartina alterniflora: Evidence from UAV remote sensing. Remote Sens. 2019, 11, 1208. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Pan, W.; Chen, Y.; Zhang, W.; He, T.; Liu, Y.; Liu, W.; Zhang, Y. Invasion of Spartina alterniflora and protection of mangroves in Guangdong Zhanjiang Mangrove National Nature Reserve and adjacent coastal area. For. Environ. Sci. 2018, 34, 58–63. [Google Scholar]
- Pennings, S.C.; Richards, C.L. Effects of wrack burial in salt-stressed habitats: Batis maritima in a southwest Atlantic salt marsh. Ecography 1998, 21, 630–638. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing, version 3.5.2; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Farnsworth, E.J.; Ellison, A.M. Global patterns of pre-dispersal propagule predation in mangrove forests. Biotropica 1997, 29, 318–330. [Google Scholar] [CrossRef]
- Minchinton, T.E.; Dalby-Ball, M. Frugivory by insects on mangrove propagules: Effects on the early life history of Avicennia marina. Oecologia 2001, 129, 243–252. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Silliman, B.R. Consumer control as a common driver of coastal vegetation worldwide. Ecol. Monogr. 2016, 86, 278–294. [Google Scholar] [CrossRef]
- Feller, I.C.; Ball, M.C.; Ellis, J.I.; Lovelock, C.E.; Reef, R.; Keith, S. Interactive effects of climate and nutrient enrichment on patterns of herbivory by different feeding guilds in mangrove forests. Glob. Ecol. Biogeogr. 2017, 26, 1326–1338. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B 2006, 273, 2575–2584. [Google Scholar] [CrossRef] [Green Version]
- Strauss, S.Y.; Zangrel, A.R. Plant–insect interactions in terrestrial ecosystems. In Plant–Animal Interactions. An Evolutionary Approach, 1st ed.; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Science: Oxford, UK, 2002; pp. 77–106. [Google Scholar]
- Pennings, S.C.; Bertness, M.D. Salt marsh communities. In Marine Community Ecology, 1st ed.; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 289–316. [Google Scholar]
- Pfeiffer, W.J.; Wiegert, R.G. Grazers on Spartina and their predators. In The Ecology of a Salt Marsh; Pomeroy, L.R., Wiegert, R.G., Eds.; Springer: New York, NY, USA, 1981; pp. 87–112. [Google Scholar]
- Greenberg, R.; Maldonado, J.E.; Droege, S.; McDonald, M.V. Tidal marshes: A global perspective on the evolution and conservation of their terrestrial vertebrates. BioScience 2006, 56, 675. [Google Scholar] [CrossRef] [Green Version]
- Crain, C.M. Interactions between marsh plant species vary in direction and strength depending on environmental and consumer context. J. Ecol. 2008, 96, 166–173. [Google Scholar] [CrossRef]
- Shaffer, G.P.; Day, J.W.; Hunter, R.G.; Lane, R.R.; Lundberg, C.J.; Wood, W.B.; Hillmann, E.R.; Day, J.N.; Strickland, E.; Kandalepas, D. System response, nutria herbivory, and vegetation recovery of a wetland receiving secondarily-treated effluent in coastal Louisiana. Ecol. Eng. 2015, 79, 120–131. [Google Scholar] [CrossRef]
- Pennings, S.C.; Silliman, B.R. Linking biogeography and community ecology: Latitudinal variation in plant-herbivore interaction strength. Ecology 2005, 86, 2310–2319. [Google Scholar] [CrossRef]
- Pennings, S.C.; Ho, C.-K.; Salgado, C.S.; Więski, K.; Davé, N.; Kunza, A.E.; Wason, E.L. Latitudinal cariation in herbivore pressure in Atlantic coast salt marshes. Ecology 2009, 90, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, X.; Li, M.; Lin, Y. Tannin contents in the different parts of alien mangrove Sonneratia apetala and leaf tannin structure. J. Xiamen Univ. 2008, 47, 186–191. [Google Scholar]
- Nasrin, S.; Hossain, M.; Alam, M.R. A Monograph on Sonneratia apetala Buch.-Ham, 1st ed.; Lap Lambert Academic Publishing: Saarbrucken, Germany, 2017; pp. 5–15. [Google Scholar]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Lan, Z.; Pennings, S.C. Biotic interactions mediate the expansion of black mangrove (Avicennia germinans) into salt marshes under climate change. Glob. Chang. Biol. 2013, 19, 2765–2774. [Google Scholar] [CrossRef]
- McKee, K.L.; Rooth, J.E. Where temperate meets tropical: Multi-factorial effects of elevated CO2, nitrogen enrichment, and competition on a mangrove-salt marsh community: Multi-factorial controls on vegetation shifts. Glob. Chang. Biol. 2008, 14, 971–984. [Google Scholar] [CrossRef]
- Chen, E.; Blaze, J.A.; Smith, R.S.; Peng, S.; Byers, J.E. Freeze tolerance of poleward-spreading mangrove species weakened by soil properties of resident salt marsh competitor. J. Ecol. 2020, 108, 1725–1737. [Google Scholar] [CrossRef]
- Gurevitch, J.; Morrison, J.A.; Hedges, L.V. The interaction between competition and predation: A meta-analysis of field experiments. Am. Nat. 2000, 155, 435–453. [Google Scholar] [CrossRef]
| Source of Variance | Light Intensity (×103 Lux) | Temperature of the Coldest Month (℃) | Sediment Water Content (%) | Sediment Porewater Salinity (PSU) |
|---|---|---|---|---|
| Zhangjiang Estuary | ||||
| Mangrove | 7.0 ± 1.1 e | 12.3 ± 0.1 b | 49.0 ± 0.2 a | 18.9 ± 1.0 ab |
| Cordgrass | 25.7 ± 4.3 d | 12.3 ± 0.1 b | 52.0 ± 0.5 a | 17.6 ± 0.8 b |
| Mudflat | 83.9 ± 1.3 b | 12.1 ± 0.2 b | 51.7 ± 0.4 a | 18.4 ± 0.9 ab |
| Leizhou Bay | ||||
| Mangrove | 37.7 ± 2.9 d | 16.3 ± 0.3 a | 34.1 ± 0.8 c | 21.6 ± 1.0 a |
| Cordgrass | 57.6 ± 4.8 c | 16.4 ± 0.1 a | 40.7 ± 1.1 b | 21.8 ± 0.8 a |
| Mudflat | 105.0 ± 4.8 a | 16.6 ± 0.0 a | 28.2 ± 0.5 d | 19.1 ± 0.8 ab |
| Species | Variable | Coef. | SE | t | p |
|---|---|---|---|---|---|
| Kandelia obovata | Rodent | −0.91 | 0.07 | −9.62 | <0.0001 |
| (Mallow’s Cp = 5.49, Adj R2 = 0.82) | Sediment porewater salinity | −0.08 | 0.09 | −0.89 | 0.38 |
| Light intensity | 0.12 | 0.08 | 1.51 | 0.14 | |
| Avicennia marina | Rodent | −0.18 | 0.12 | −1.49 | 0.14 |
| (Mallow’s Cp = 3.75, Adj R2 = 0.54) | Insect | −0.33 | 0.14 | −2.39 | 0.02 |
| Light intensity | 0.81 | 0.13 | 6.34 | <0.0001 | |
| Temperature | −0.36 | 0.14 | −2.48 | 0.02 | |
| Sonneratia apetala | Light intensity | 0.001 | 0.0001 | 5.09 | <0.0001 |
| (Mallow’s Cp = 5.36, Adj R2 = 0.72) | Temperature | 7.48 | 2.14 | 3.48 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, D.; Zhang, Y.; Wang, J.; Pennings, S.C. The Opposite of Biotic Resistance: Herbivory and Competition Suppress Regeneration of Native but Not Introduced Mangroves in Southern China. Forests 2022, 13, 192. https://doi.org/10.3390/f13020192
Peng D, Zhang Y, Wang J, Pennings SC. The Opposite of Biotic Resistance: Herbivory and Competition Suppress Regeneration of Native but Not Introduced Mangroves in Southern China. Forests. 2022; 13(2):192. https://doi.org/10.3390/f13020192
Chicago/Turabian StylePeng, Dan, Yihui Zhang, Jiayu Wang, and Steven Charles Pennings. 2022. "The Opposite of Biotic Resistance: Herbivory and Competition Suppress Regeneration of Native but Not Introduced Mangroves in Southern China" Forests 13, no. 2: 192. https://doi.org/10.3390/f13020192
APA StylePeng, D., Zhang, Y., Wang, J., & Pennings, S. C. (2022). The Opposite of Biotic Resistance: Herbivory and Competition Suppress Regeneration of Native but Not Introduced Mangroves in Southern China. Forests, 13(2), 192. https://doi.org/10.3390/f13020192






