The Impact of Forest Fertilization on the Ecological Quality of Two Hemiboreal Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design and Treatments
2.3. Surface Water Sample Collection and Analysis
2.4. Sampling and Analysis of Biological Quality Elements
2.5. Statistical Methods
3. Results
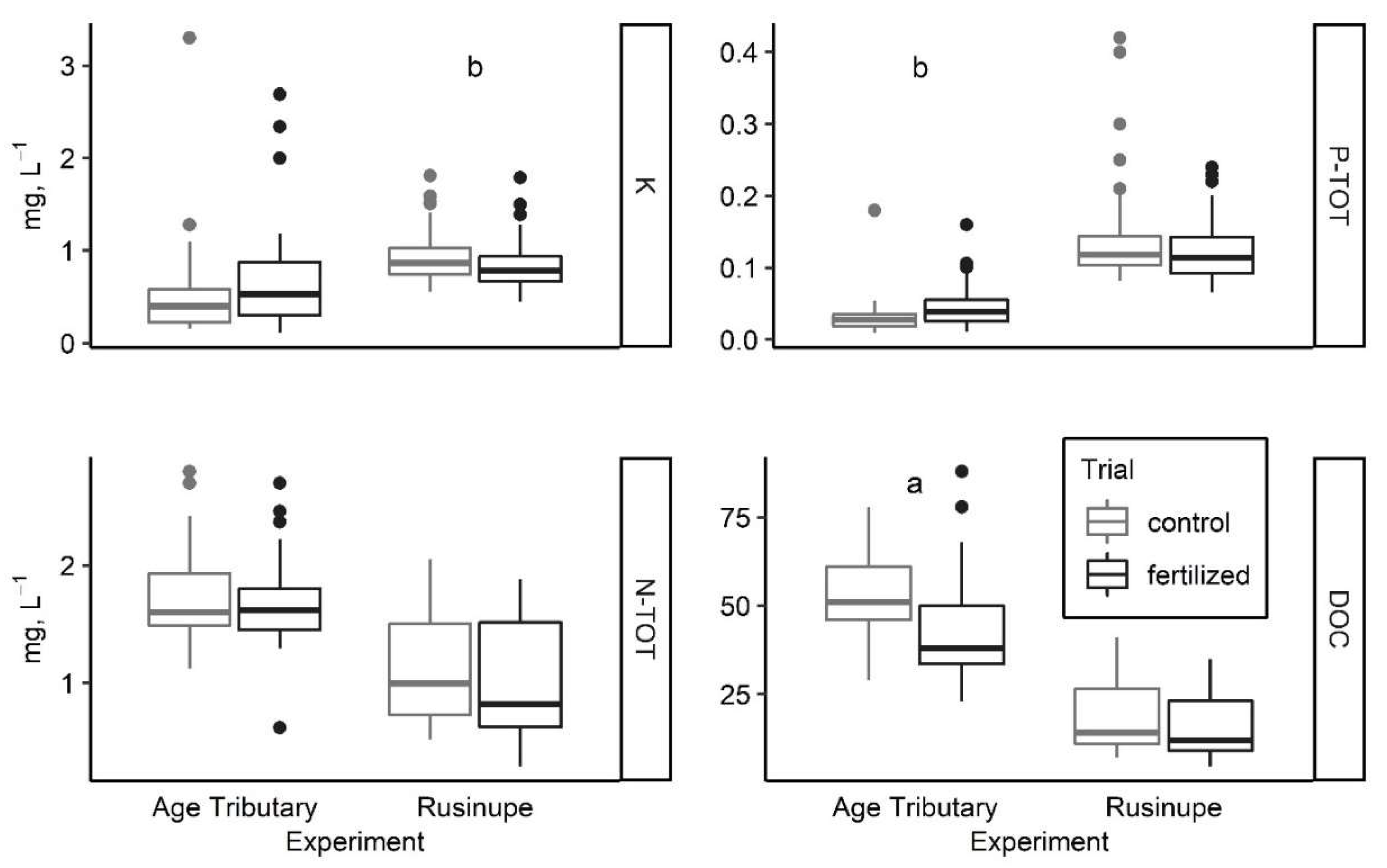
3.1. Chemistry of Surface Water
3.2. Macrophytes
3.3. Phytobenthos
3.4. Macroinvertebrates
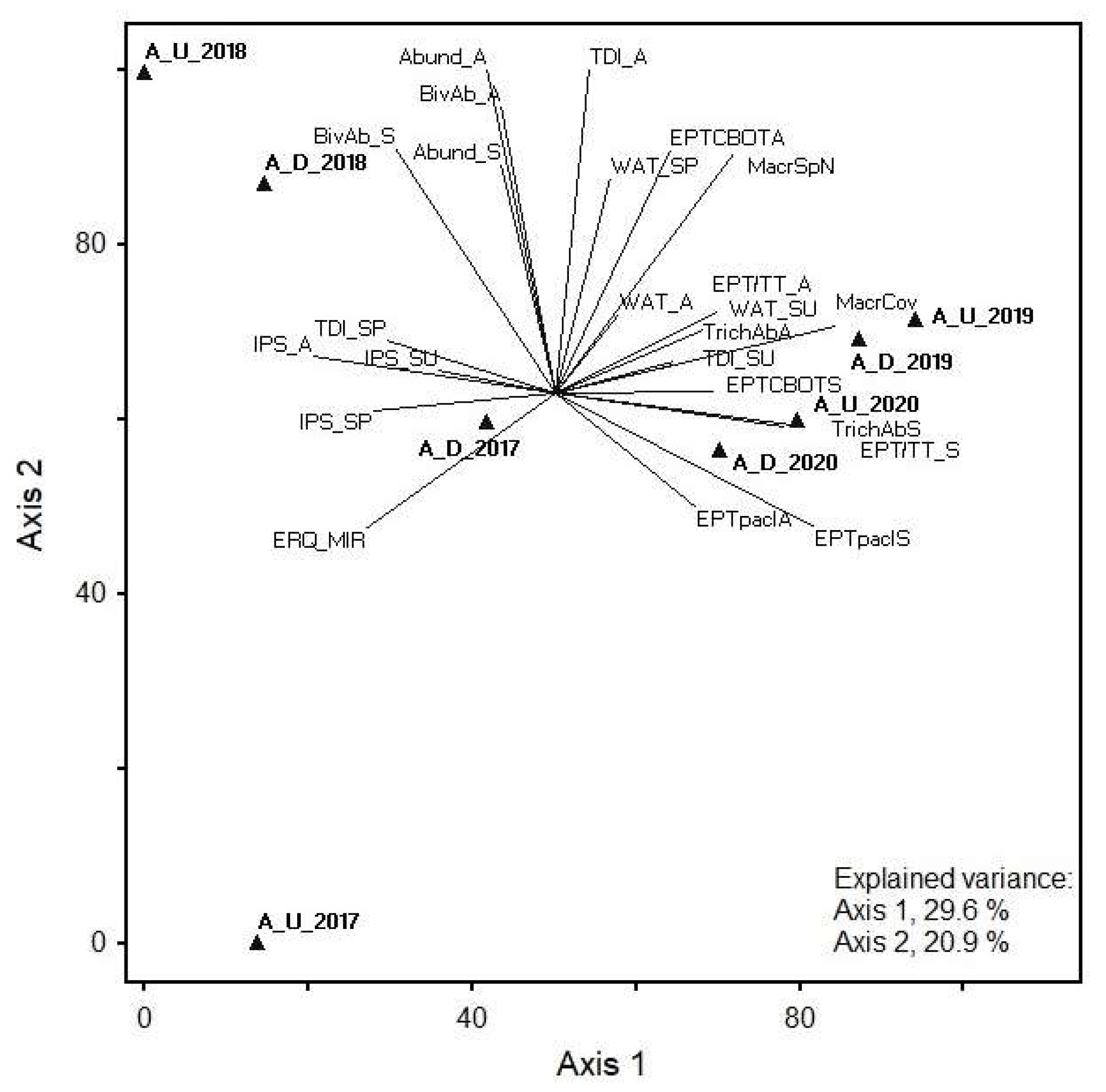
3.5. Indicator Species Analysis of the Age
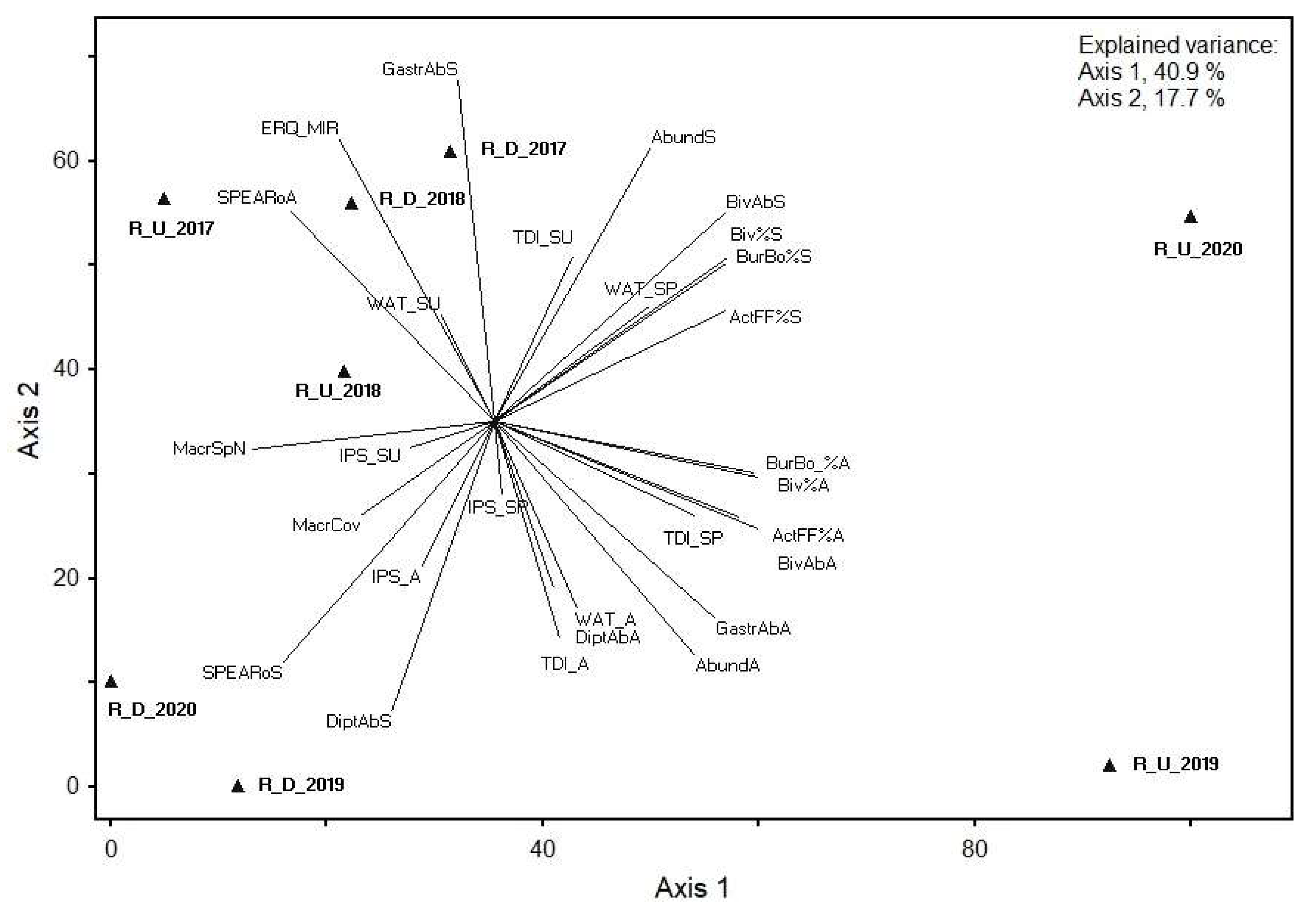
3.6. Indicator Species Analysis of the Rusinupe
4. Discussion
4.1. Water Chemistry
4.2. Biological Quality Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikström, U.; Almqvist, C.; Jansson, G. Growth of Pinus sylvestris after the application of wood ash or P and K fertilizer to a peatland in southern Sweden. Silva Fenn. 2010, 44, 411–425. [Google Scholar] [CrossRef] [Green Version]
- Pukkala, T. Optimal nitrogen fertilization of boreal conifer forest. For. Ecosyst. 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Lindkvist, A.; Kardell, Ö.; Nordlund, C. Intensive Forestry as Progress or Decay? An Analysis of the Debate about Forest Fertilization in Sweden, 1960–2010. Forests 2011, 2, 112–146. [Google Scholar] [CrossRef] [Green Version]
- Lundström, U.S.; Bain, D.C.; Taylor, A.F.S.; van Hees, P.A.W. Effects of Acidification and its Mitigation with Lime and Wood Ash on Forest Soil Processes: A Review. Water Air Soil Pollut. Focus 2003, 3, 5–28. [Google Scholar] [CrossRef]
- Aronsson, K.A.; Ekelund, N.G.A. Limnological effects on a first order stream after wood ash application to a boreal forest catchment in Bispgården, Sweden. For. Ecol. Manag. 2008, 255, 245–253. [Google Scholar] [CrossRef]
- Ring, E.; von Brömssen, C.; Losjö, K.; Sikström, U. Water chemistry following wood-ash application to a Scots pine stand on a drained peatland in Sweden. For. Stud. 2011, 54, 54–70. [Google Scholar] [CrossRef] [Green Version]
- Ring, E.; Jacobson, S.; Högbom, L. Long-term effects of nitrogen fertilization on soil chemistry in three Scots pine stands in Sweden. Can. J. For. Res. 2011, 41, 279–288. [Google Scholar] [CrossRef]
- Norström, S.H.; Bylund, D.; Vestin, J.L.K.; Lundström, U.S. Initial effects of wood ash application to soil and soil solution chemistry in a small, boreal catchment. Geoderma 2012, 187–188, 85–93. [Google Scholar] [CrossRef]
- Vejre, H.; Ingerslev, M.; Raulund-Rasmussen, K. Fertilization of Danish Forests: A Review of Experiments. Scand. J. For. Res. 2001, 16, 502–513. [Google Scholar] [CrossRef]
- Norwegian Ministry of Climate and Environment. Norway’s National Plan Related to the Decision of the EEA Joint committee No. 269/2019 of 25 October 2019. Available online: https://www.regjeringen.no/contentassets/31a96bc774284014b1e8e47886b3fa57/norways-national-plan-related-to-the-decision-of-the-eea-joint-committee-no.-269-2019-of-25-october-2019.pdf (accessed on 12 December 2021).
- Bardule, A.; Rancane, S.; Gutmane, I.; Berzins, P.; Stesele, V.; Lazdina, D.; Bardulis, A. The effect of fertiliser type on hybrid aspen increment and seed yield of perennial grass cultivated in the agroforestry system. Agron. Res. 2013, 11, 13–24. [Google Scholar]
- Okmanis, M.; Kalvis, T.; Lazdina, D. Initial evaluation of impact of evenness of spreading wood ash in forest on additional radial increment. In Proceedings of the 17th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 23–25 May 2018; Malinovska, L., Osadcuks, V., Eds.; Latvia University of Life Sciences and Technologies, Faculty of Engineering: Jelgava, Latvia, 2018. [Google Scholar]
- Bardule, A.; Bertins, M.; Busa, L.; Lazdina, D.; Viksna, A.; Tvrdonova, M.; Kanicky, V.; Vaculovic, T. Variation of major elements and heavy metals occurrence in hybrid aspen (Populus tremuloides Michx. × P. tremula L.) tree rings in marginal land. iForest 2020, 13, 24–32. [Google Scholar] [CrossRef]
- Bardule, A.; Grinfelde, I.; Lazdina, D.; Bardulis, A.; Sarkanabols, T. Macronutrient leaching in a fertilized juvenile hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantation cultivated in an agroforestry system in Latvia. Hydrol. Res. 2018, 49, 407–420. [Google Scholar] [CrossRef]
- Bardule, A.; Lazdins, A.; Sarkanabols, T.; Lazdina, D. Fertilized short rotation plantations of hybrid aspen (Populus tremuloides Michx. × Populus tremula L.) for energy wood or mitigation of GHG emissions. In Proceedings of the 15th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2016; Malinovska, L., Osadcuks, V., Eds.; Latvia University of Agriculture, Faculty of Engineering: Jelgava, Latvia, 2016. [Google Scholar]
- Ojanen, P.; Penttilä, T.; Tolvanen, A.; Hotanen, J.-P.; Saarimaa, M.; Nousiainen, H.; Minkkinen, K. Long-term effect of fertilization on the greenhouse gas exchange of low-productive peatland forests. For. Ecol. Manag. 2019, 432, 786–798. [Google Scholar] [CrossRef]
- Jurán, S.; Edwards-Jonášová, M.; Cudlín, P.; Zapletal, M.; Šigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. iForest 2018, 11, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Agyei, T.; Juráň, S.; Edwards-Jonášová, M.; Fischer, M.; Švik, M.; Komínková, K.; Ofori-Amanfo, K.K.; Marek, M.V.; Grace, J.; Urban, O. The Influence of Ozone on Net Ecosystem Production of a Ryegrass–Clover Mixture under Field Conditions. Atmosphere 2021, 12, 1629. [Google Scholar] [CrossRef]
- Tulonen, T.; Arvola, L.; Ollila, S. Limnological Effects of Wood Ash Application to the Subcatchments of Boreal, Humic Lakes. J. Environ. Qual. 2002, 31, 946. [Google Scholar] [CrossRef]
- Piirainen, S.; Domisch, T.; Moilanen, M.; Nieminen, M. Long-term effects of ash fertilization on runoff water quality from drained peatland forests. For. Ecol. Manag. 2013, 287, 53–66. [Google Scholar] [CrossRef]
- Renou-Wilson, F.; Farrell, E.P. Phosphorus in surface runoff and soil water following fertilization of afforested cutaway peatlands. Boreal Environ. Res. 2007, 12, 693–709. [Google Scholar]
- Xu, Z.; Xu, Y.J. Assessing effectiveness of long-term forestry best management practices on stream water quality at a basin scale—a case study in Southern USA. Environ. Monit. Assess. 2018, 190, 108. [Google Scholar] [CrossRef]
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban agriculture in the developing world: A review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef] [Green Version]
- Perrin, C.J.; Shortreed, K.S.; Stockner, J.G. An Integration of Forest and Lake Fertilization: Transport and Transformations of Fertilizer Elements. Can. J. Fish. Aquat. Sci. 1984, 41, 253–262. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Hensley, R.T.; Decker, P.H.; Flinders, C.; McLaughlin, D.; Schilling, E.; Cohen, M.J. Fertilization has negligible effects on nutrient export and stream biota in two North Florida forested watersheds. For. Ecol. Manag. 2020, 465, 118096. [Google Scholar] [CrossRef]
- Rimet, F. Diatoms: An Ecoregional Indicator of Nutrients, Organic Mater and Micropollutants Pollution. Ph.D. Thesis, Université de Grenoble, Grenoble, France, 2012. [Google Scholar]
- Gudmundsdottir, R.; Palsson, S.; Hannesdottir, E.R.; Olafsson, J.S.; Gislason, G.M.; Moss, B. Diatoms as indicators: The influences of experimental nitrogen enrichment on diatom assemblages in sub-Arctic streams. Ecol. Indic. 2013, 32, 74–81. [Google Scholar] [CrossRef]
- Kelly, M.G.; Whitton, B.A. The Trophic Diatom Index: A new index for monitoring eutrophication in rivers. J. Appl. Phycol. 1995, 7, 433–444. [Google Scholar] [CrossRef]
- Haury, J.; Peltre, M.C.; Trémolières, M.; Barbe, J.; Thiébaut, G.; Bernez, I.; Daniel, H.; Chatenet, P.; Haan-Archipof, G.; Muller, S.; et al. A new method to assess water trophy and organic pollution—The Macrophyte Biological Index for Rivers (IBMR): Its application to different types of river and pollution. Hydrobiologia 2006, 570, 153–158. [Google Scholar] [CrossRef]
- Willby, N.J.; Pitt, J.; Phillips, G.L. The Ecological Classification of UK Rivers Using Aquatic Macrophytes; Environment Agency: Bristol, UK, 2009; p. 222. [Google Scholar]
- Kuhar, U.; Germ, M.; Gaberščik, A.; Urbanič, G. Development of a River Macrophyte Index (RMI) for assessing river ecological status. Limnologica 2011, 41, 235–243. [Google Scholar] [CrossRef]
- Scott, W.A.; Adamson, J.K.; Rollinson, J.; Parr, T.W. Monitoring of aquatic macrophytes for detection of long-term change in river systems. Environ. Monit. Assess. 2002, 73, 131–153. [Google Scholar] [CrossRef]
- Birk, S.; Willby, N. Towards harmonization of ecological quality classification: Establishing common grounds in European macrophyte assessment for rivers. Hydrobiologia 2010, 652, 149–163. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Segurado, P.; Urbanič, G.; Cambra, J.; Chauvin, C.; Ciadamidaro, S.; Dörflinger, G.; Ferreira, J.; Germ, M.; Manolaki, P.; et al. Comparability of river quality assessment using macrophytes: A multi-step procedure to overcome biogeographical differences. Sci. Total Environ. 2014, 476–477, 757–767. [Google Scholar] [CrossRef]
- Hauer, F.R.; Lamberti, G.A. (Eds.) Methods in Stream Ecology, 1st ed.; Academic Press: New York, NY, USA, 1996; p. 674. [Google Scholar]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Gabriels, W.; Lock, K.; De Pauw, N.; Goethals, P.L.M. Multimetric Macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnol.-Ecol. Manag. Inland Waters 2010, 40, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Bušs, K. Meža Ekoloģija un Tipoloģija [Forest Ecology and Typology]; ‘Zinātne’: Riga, Latvia, 1981; p. 64. [Google Scholar]
- Avotniece, Z.; Aņiskeviča, S.; Maļinovskis, E. Climate Change Scenarios for Latvia; State Ltd. “Latvian Environment, Geology and Meteorology Centre”; 2017. p. 236. Available online: https://www4.meteo.lv/klimatariks/files/zinojums.pdf (accessed on 2 October 2021).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006; p. 145. [Google Scholar]
- Stoddard, J.L.; Larsen, D.P.; Hawkins, C.P.; Johnson, R.K.; Norris, R.H. Setting Expectations for the Ecological Condition of Streams: The Concept of Reference Condition. Ecol. Appl. 2006, 16, 1267–1276. [Google Scholar] [CrossRef]
- Lecointe, C.; Coste, M.; Prygel, J. ‘Omnidia’ software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia 1993, 269–270, 509–513. [Google Scholar] [CrossRef]
- Timm, H.; Vilbaste, S. Pinnavee Ökoloogilise Seisundi Hindamise Metoodika Bioloogiliste Kvaliteedielementide Alusel. Bentiliste Ränivetikate Kooslus jões. Suurselgrootute Põhjaloomade Kooslus jões. ja Järves. [Methodology for Assessing the Ecological Status of Surface Waters on the Basis of Biological Quality Elements. Composition of Benthic Siliceous Algae in the Rivers. A Community of Large Invertebrates in the Rivers and Lakes]. Tartu, Estonia, 2010; Volume 112.
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2019, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Uzule, L.; Jēkabsone, J. Fitting the Assessment System for Rivers in Latvia Using Macrophytes to the Results of the Central Baltic Geographical Intercalibration Group; Institute of Biology, University of Latvia: Salaspils, Latvia, 2016; Volume 16. [Google Scholar]
- Anonymous. AQEM European Stream Assessment Program; English Manual, Version 2.3; Published Online, April 2004. Available online: http://www.aqem.de/ (accessed on 12 December 2021).
- Ozolins, D.; Skuja, A. Fitting the New Latvian Macroinvertebrate Index (LMI) for Rivers to the Results of the Central-Baltic Geographical Intercalibration Group; Institute of Biology, University of Latvia: Salaspils, Latvia, 2016; Volume 20. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; MjM Solfware Desing: Lincoln, OR, USA, 2006. [Google Scholar]
- Bakker, J.D. Increasing the utility of Indicator Species Analysis. J. Appl. Ecol. 2008, 45, 1829–1835. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; 2018. Available online: https://www.R-project.org/ (accessed on 25 October 2021).
- Petaja, G.; Okmanis, M.; Makovskis, K.; Lazdiņa, D.; Lazdiņš, A. Forest Fertilization: Economic Effect and Impact on GHG Emissions in Latvia. Balt. For. 2018, 24, 9–16. [Google Scholar]
- Douglas, E.J.; Pilditch, C.A.; Hines, L.V.; Kraan, C.; Thrush, S.F. In situ soft sediment nutrient enrichment: A unified approach to eutrophication field experiments. Mar. Pollut. Bull. 2016, 111, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Klavins, M.; Rodinov, V.; Kokorite, I.; Klavina, I.; Apsite, E. Long-term and seasonal changes in chemical composition of surface waters in Latvia. Environ. Monit. Assess. 2001, 66, 233–251. [Google Scholar] [CrossRef]
- Karltun, E.; Saarsalmi, A.; Ingerslev, M.; Mandre, M.; Andersson, S.; Gaitnieks, T.; Ozolinčius, R.; Varnagiryte-Kabasinskiene, I. Wood Ash Recycling—Possibilities and Risks. In Sustainable Use of Forest Biomass for Energy; Röser, D., Asikainen, A., Raulund-Rasmussen, K., Stupak, I., Eds.; Sustainable Use of Forest Biomass for Energy; Springer: Dordrecht, The Netherlands, 2008; Volume 12, pp. 79–108. [Google Scholar] [CrossRef]
- Kahl, J.S.; Fernandez, I.J.; Rustad, L.E.; Peckenham, J. Threshold Application Rates of Wood Ash to an Acidic Forest Soil. J. Environ. Qual. 1996, 25, 220–227. [Google Scholar] [CrossRef]
- Teittinen, A.; Taka, M.; Ruth, O.; Soininen, J. Variation in stream diatom communities in relation to water quality and catchment variables in a boreal, urbanized region. Sci. Total Environ. 2015, 530–531, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Finlay, B. Hypothesis: The Rate and Scale of Dispersal of Freshwater Diatom Species is a Function of their Global Abundance. Protist 2002, 153, 261–273. [Google Scholar] [CrossRef]
- Franklin, P.; Dunbar, M.; Whitehead, P. Flow controls on lowland river macrophytes: A review. Sci. Total Environ. 2008, 400, 369–378. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Larsen, S.E.; Riis, T. Composition and Richness of Macrophyte Communitites in Small Danish Streams—Influence of Environmental Factors and Weed Cutting. Hydrobiologia 2003, 495, 171–179. [Google Scholar] [CrossRef]
- Bady, P.; Doledec, S.; Fesl, C.; Gayraud, S.; Bacchi, M.; Scholl, F. Use of invertebrate traits for the biomonitoring of European large rivers: The effects of sampling effort on genus richness and functional diversity. Freshw. Biol. 2005, 50, 159–173. [Google Scholar] [CrossRef]
- Schmera, D.; Erős, T.; Heino, J. Habitat filtering determines spatial variation of macroinvertebrate community traits in northern headwater streams. Community Ecol. 2013, 14, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E. Pattern of invertebrate community structure, abundance and standing crop in a Black Forest stream: Results of a 3-year study. SIL Proc. 1922–2010 1991, 24, 1840–1845. [Google Scholar] [CrossRef]
- Pliūraitė, V.; Kesminas, V. Ecological impact of Eurasian beaver (Castor fiber) activity on macroinvertebrate communities in Lithuanian trout streams. Cent. Eur. J. Biol. 2012, 7, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Washko, S.; Roper, B.; Atwood, T.B. Beavers alter stream macroinvertebrate communities in north-eastern Utah. Freshw. Biol. 2020, 65, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Mariani, F.; Ellwood, N.T.W.; Zuccarello, V.; Ceschin, S. Compatibility of the Invasive Alien Lemna minuta and Its Potential Biocontrol Agent Cataclysta lemnata. Water 2020, 12, 2719. [Google Scholar] [CrossRef]
- Johnson, R.C.; Carreiro, M.M.; Jin, H.S.; Jack, J.D. Within-year temporal variation and life-cycle seasonality affect stream macroinvertebrate community structure and biotic metrics. Ecol. Indic. 2012, 13, 206–214. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Garssen, A.; Göthe, E.; Hoffmann, C.C.; Oddershede, A.; Riis, T.; van Bodegom, P.; Larsen, S.E.; Soons, M. Structural and functional responses of plant communities to climate change-mediated alterations in the hydrology of riparian areas in temperate Europe. Ecol. Evol. 2018, 8, 4120–4135. [Google Scholar] [CrossRef] [Green Version]

| No | Forest Type | Tree Species | Stand Age 1 | Type of Fertilizer | Dose of Fertilizer, t ha−1 | Treated Area, ha | Date of Treatment | Coordinates |
|---|---|---|---|---|---|---|---|---|
| 1 | MTM 2 | Scots pine | 94 | WA 4 | 3 | 4.7 | 02.2018 | 57.3986, 24.5930 |
| 2 | M 3 | Scots pine | 62 | NH4NO3 5 | 0.44 | 5.5 | 07.2017 | 56.6666, 24.5170 |
| No | pHCaCl2 | Soil Bulk Density kg/m3 | Total C, g/kg | Total N, g/kg |
|---|---|---|---|---|
| 1 | 3.5 | 131.5 | 562.9 | 18.2 |
| 2 | 4.4 | 1265.7 | 7.6 | 0.4 |
| Taxon Name | Parameter for Group Defining | Observed Indicator Value | Indicator Values | p-Value | |
|---|---|---|---|---|---|
| Mean | Standart Deviation | ||||
| Year | |||||
| Pisidium sp. | 2018 | 31.8 | 28.2 | 1.4 | 0.01 |
| Acroloxus lacustris | 2018 | 44.6 | 32.4 | 7.53 | 0.04 |
| Ancylus fluviatilis | 2018 | 61.4 | 29.4 | 12.51 | 0.03 |
| Atherix ibis | 2018 | 51.3 | 32.1 | 8.45 | 0.02 |
| Limnius volckmari | 2018 | 51.2 | 33.8 | 8.41 | 0.03 |
| Hydrachnidia Gen. sp. | 2020 | 56.8 | 32.1 | 8.49 | 0.002 |
| Cataclysta lemnata | 2018 | 84.8 | 28 | 12.05 | 0.01 |
| Calopteryx virgo | 2019 | 51.8 | 32.7 | 8.84 | 0.02 |
| Baetis muticus | 2017 | 61.9 | 29.5 | 12.4 | 0.02 |
| Caenis luctuosa | 2020 | 41.5 | 32.2 | 3.39 | 0.01 |
| Site | |||||
| Sphaerium sp. | Downstream | 70.4 | 43.3 | 9.86 | 0.02 |
| Unio crassus | Downstream | 62.5 | 28.1 | 10.37 | 0.03 |
| Ceratopogonidae Gen. sp. | Upstream | 65.7 | 50 | 6.67 | 0.02 |
| Cyrnus trimaculatus | Upstream | 60.1 | 37.5 | 10.38 | 0.05 |
| Polycentropus irroratus | Upstream | 65.5 | 48.4 | 8.61 | 0.05 |
| Season | |||||
| Simuliidae Gen. sp. | Spring | 71.7 | 47.9 | 8.42 | 0.01 |
| Elmis aenea | Spring | 63.4 | 51.8 | 6.19 | 0.04 |
| Hydrachnidia Gen. sp. | Spring | 71.6 | 42.9 | 9.39 | 0.004 |
| Paraleptophlebia submarginata | Autumn | 66.3 | 45.8 | 9.37 | 0.03 |
| Nemoura flexuosa | Autumn | 82.1 | 48.2 | 8.63 | 0.0002 |
| Leuctra sp. | Spring | 95.9 | 40.5 | 9.84 | 0.0002 |
| Brachycentrus subnubilus | Autumn | 62.5 | 28.3 | 10.6 | 0.03 |
| Ithytrichia lamellaris | Spring | 61.6 | 40.7 | 10.16 | 0.04 |
| Halesus digitatus | Spring | 100 | 37.4 | 10.33 | 0.0002 |
| Limnephilus sp. | Autumn | 81.2 | 37.9 | 10.7 | 0.004 |
| Limnephilus lunatus | Spring | 62.5 | 28 | 10.24 | 0.03 |
| Lepidostoma hirtum | Spring | 69.9 | 47.9 | 8.36 | 0.02 |
| Taxon Name | Parameter for Group Defining | Observed Indicator Value | Indicator Values | p-Value | |
|---|---|---|---|---|---|
| Mean | Standart Deviation | ||||
| Year | |||||
| Dicranota sp. | 2017 | 81.9 | 27.9 | 12 | 0.01 |
| Nemoura cinerea | 2017 | 44.1 | 33 | 4.2 | 0.01 |
| Site | |||||
| Acroloxus lacustris | Downstream | 87.5 | 34.9 | 10.9 | 0.00 |
| Bithynia tentaculata | Downstream | 87.5 | 34.6 | 10.3 | 0.00 |
| Segmentina nitida | Upstream | 79 | 44 | 10.4 | 0.00 |
| Asellus aquaticus | Downstream | 60 | 54 | 3.1 | 0.04 |
| Calopteryx virgo | Downstream | 69 | 35.5 | 11.2 | 0.03 |
| Cloeon dipterum | Upstream | 62.5 | 28.5 | 11.3 | 0.02 |
| Hydropsyche angustipennis | Downstream | 65.8 | 37.4 | 10.4 | 0.02 |
| Athripsodes aterrimus | Downstream | 75 | 31 | 10.7 | 0.01 |
| Polycentropus irrratus | Downstream | 62.5 | 28.2 | 9.8 | 0.03 |
| Season | |||||
| Chironomidae Gen. sp. | Autumn | 59.4 | 52.7 | 2 | 0.00 |
| Limoniidae Gen. sp. | Autumn | 56 | 31.7 | 10.6 | 0.05 |
| Coenagrionidae Gen. sp. | Autumn | 62.5 | 28 | 10 | 0.02 |
| Baetis rhodani | Spring | 78.4 | 37.8 | 10.4 | 0.01 |
| Glyphotaelius pellucidus | Autumn | 62.5 | 28 | 10.6 | 0.02 |
| Limnephilus flavicornis | Spring | 70.9 | 34.6 | 10.3 | 0.01 |
| Lype reducta | Autumn | 73.3 | 40.2 | 9.8 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozoliņš, D.; Karklina, I.; Skuja, A.; Uzule, L.; Kokorite, I.; Medne-Peipere, M.; Lazdiņš, A. The Impact of Forest Fertilization on the Ecological Quality of Two Hemiboreal Streams. Forests 2022, 13, 196. https://doi.org/10.3390/f13020196
Ozoliņš D, Karklina I, Skuja A, Uzule L, Kokorite I, Medne-Peipere M, Lazdiņš A. The Impact of Forest Fertilization on the Ecological Quality of Two Hemiboreal Streams. Forests. 2022; 13(2):196. https://doi.org/10.3390/f13020196
Chicago/Turabian StyleOzoliņš, Dāvis, Ilze Karklina, Agnija Skuja, Linda Uzule, Ilga Kokorite, Madara Medne-Peipere, and Andis Lazdiņš. 2022. "The Impact of Forest Fertilization on the Ecological Quality of Two Hemiboreal Streams" Forests 13, no. 2: 196. https://doi.org/10.3390/f13020196





