Water Use Characteristics of Populus euphratica Oliv. and Tamarix chinensis Lour. at Different Growth Stages in a Desert Oasis
Abstract
:1. Introduction
2. Materials and Methods
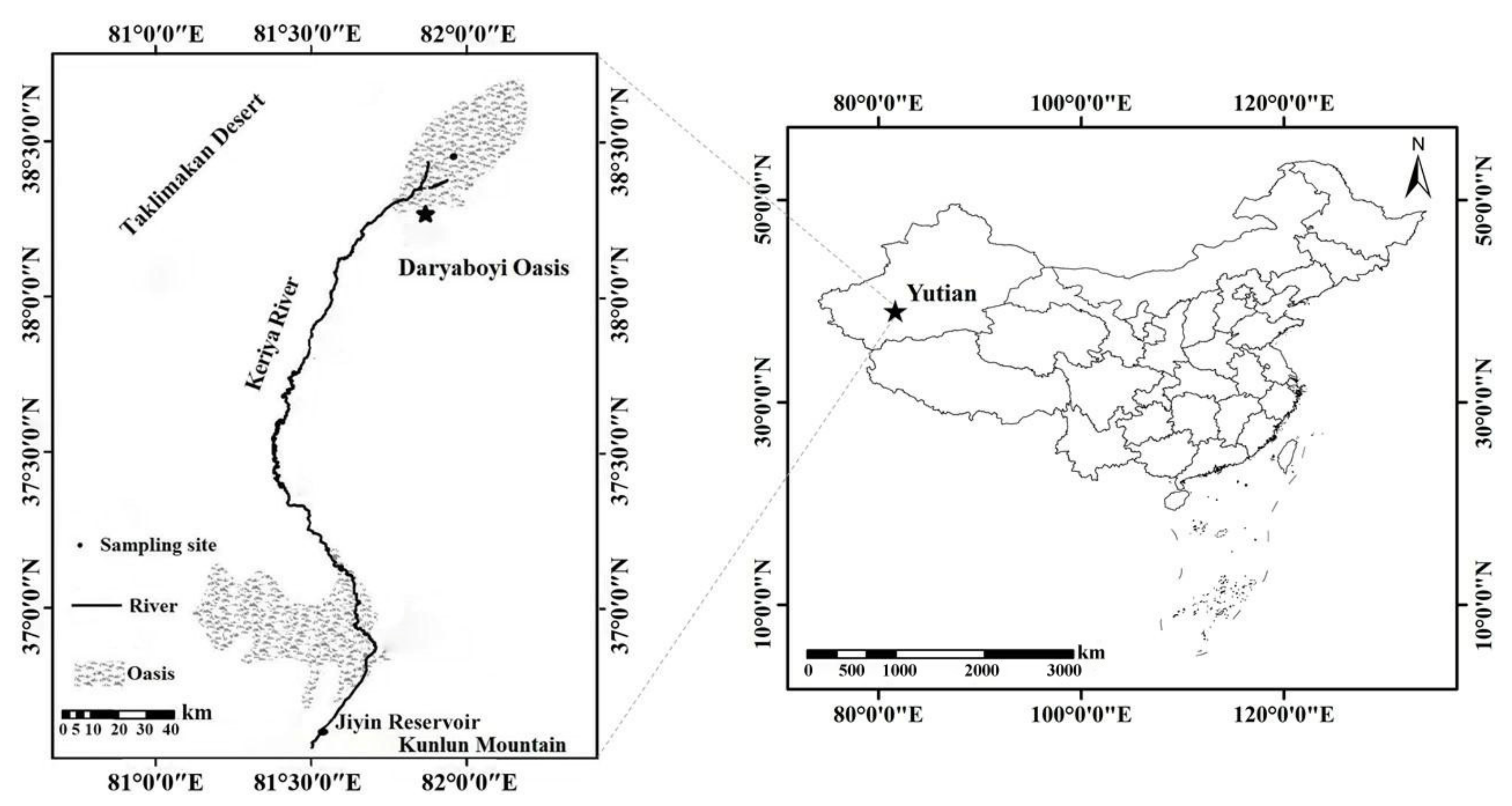
2.1. Study Area
2.2. Experimental Design
2.3. Sample Collection
2.4. Isotopic Analysis
2.5. The Division of Water Sources
2.6. Data Analysis
3. Results
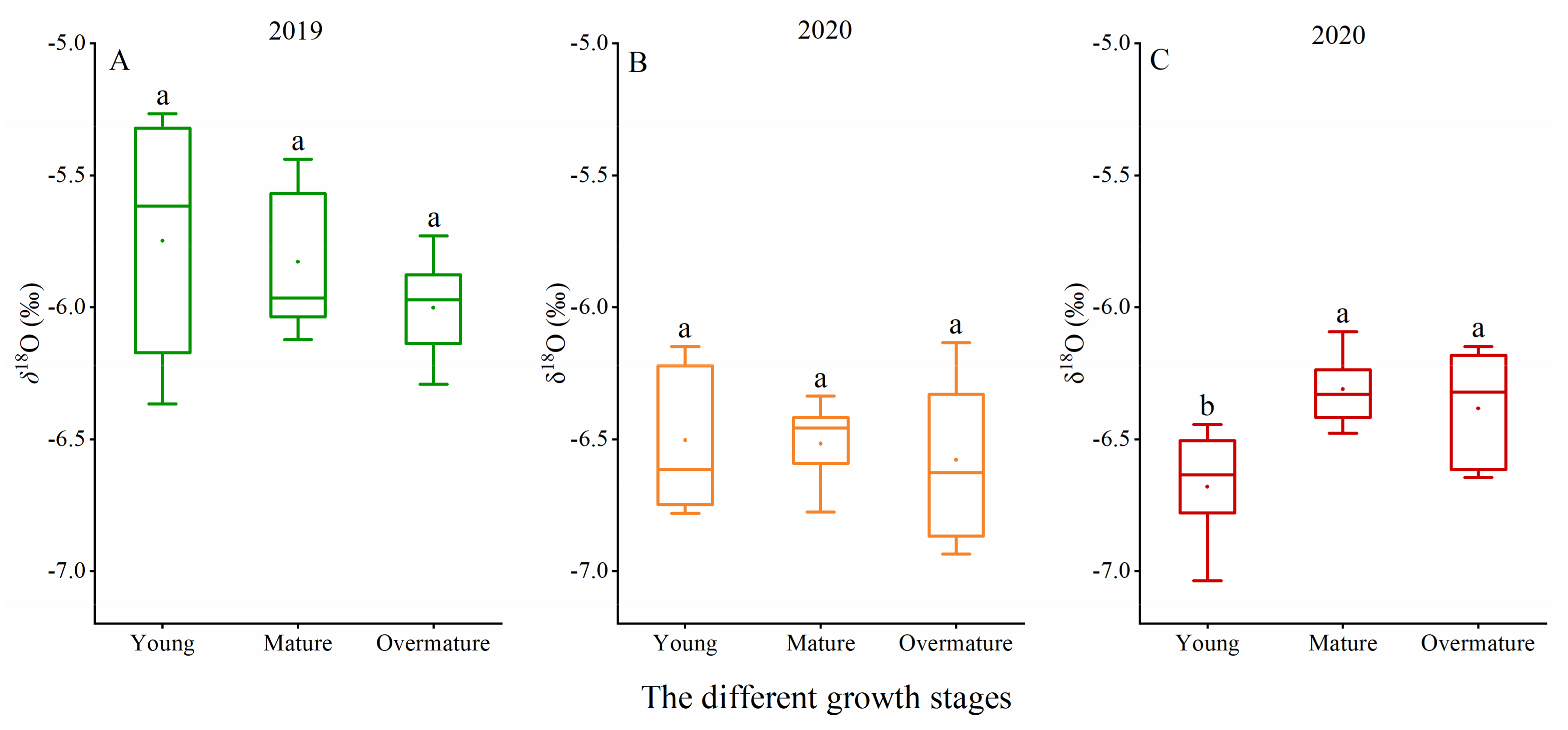
3.1. Variation of the Soil Water Content and δ18O Value
3.2. Isotopic Composition of Potential Water Sources and Xylem Water
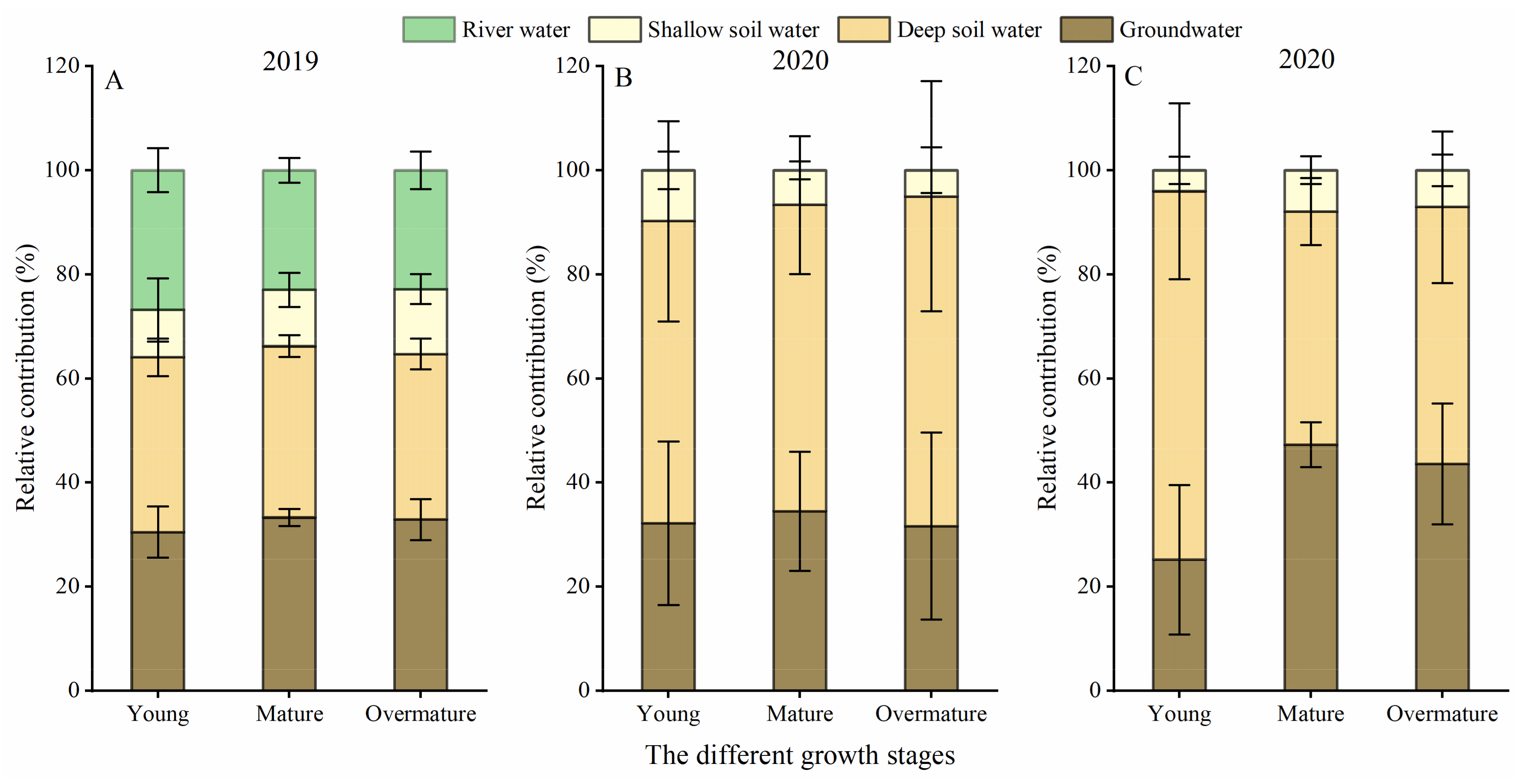
3.3. IsoSource Estimation of Feasible Potential Water Source Contributions
3.4. Variations in the δ13C Values of Plant Leaves
4. Discussion
4.1. Water Uptake Patterns of P. euphratica and T. chinensis at Different Growth Stages
4.2. Plant Leaf δ13C Values of P. euphratica and T. chinensis at Different Growth Stages
4.3. Implications for Desert Oasis Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lang, P.; Jeschke, M.; Wommelsdorf, T.; Backes, T.; Lv, C.; Zhang, X.; Thomas, F.M. Wood Harvest by Pollarding Exerts Long-Term Effects on Populus Euphratica Stands in Riparian Forests at the Tarim River, NW China. For. Ecol. Manag. 2015, 353, 87–96. [Google Scholar] [CrossRef]
- Aishan, T.; Halik, Ü.; Betz, F.; Gärtner, P.; Cyffka, B. Modeling Height–Diameter Relationship for Populus Euphratica in the Tarim Riparian Forest Ecosystem, Northwest China. J. For. Res. 2016, 27, 889–900. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cooper, T.A. Correlations between Carbon Isotope Ratio and Microhabitat in Desert Plants. Oecologia 1988, 76, 562–566. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Virginia, R.A.; Kemp, P.R.; Soyza, A.D.; Tremmel, D.C. Impact of Drought on Desert Shrubs: Effects of Seasonality and Degree of Resource Island Development. Ecol. Monogr. 1999, 69, 69–106. [Google Scholar] [CrossRef]
- Si, J.; Feng, Q.; Cao, S. Water Use Sources of Desert Riparian Populus Euphratica Forests. Environ. Monit. Assess. 2014, 186, 5469–5477. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Dawson, T.E.; Adams, H.D.; Collins, A.D.; Dickman, L.T.; Newman, B.D.; Stockton, E.A.; McDowell, N.G. Warming Combined with More Extreme Precipitation Regimes Modifies the Water Sources Used by Trees. New Phytol. 2017, 213, 584–596. [Google Scholar] [CrossRef]
- Harrison, J.L.; Blagden, M.; Green, M.B.; Salvucci, G.D.; Templer, P.H. Water Sources for Red Maple Trees in a Northern HardWood Forest under a Changing Climate. Ecohydrology 2020, 13, e2248. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Jiao, L.; Lu, N.; Wang, L. Age-Related Water Use Characteristics of Robinia Pseudoacacia on the Loess Plateau. Agric. For. Meteorol. 2021, 5, 301–302. [Google Scholar] [CrossRef]
- Dawson, T.E.; Ehleringer, J.R. Streamside Trees that Do Not use Stream Water. Nature 1991, 350, 335–337. [Google Scholar] [CrossRef]
- Lin, G.; Sternberg, S.; Ehleringer, J.R.; Hall, A.E.; Farquhar, G.D. Hydrogen Isotopic Fractionation by Plant Roots during Water Uptake in Coastal Wetland Plants. In Stable Isotopes and Plant Carbon-Water Relations; Academic Press: Cambridge, MA, USA, 1993; pp. 497–510. [Google Scholar] [CrossRef]
- Ellsworth, P.Z.; Williams, D.G. Hydrogen Isotope Fractionation during Water Uptake by Woody Xerophytes. Plant Soil 2007, 291, 93–107. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Zhang, Y.; Meng, F. Plant Water Use Strategies in the Shapotou Artificial Sand-Fixed Vegetation of the Southeastern Margin of the Tengger Desert, Northwestern China. J. Mt. Sci. 2019, 16, 898–908. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Chen, C. How Do Plants Share Water Sources in a Rubber-Tea Agroforestry System during the Pronounced Dry Season? Agric. Ecosyst. Environ. 2017, 236, 69–77. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, X.; Tang, L.; Li, Y. Stable Oxygen Isotopes Reveal Distinct Water Use Patterns of Two Haloxylon Species in the Gurbantonggut Desert. Plant Soil 2015, 389, 73–87. [Google Scholar] [CrossRef]
- Gow, L.J.; Barrett, D.J.; O’grady, A.P.; Renzullo, L.J.; Phinn, S.R. Subsurface Water-Use Strategies and Physiological Responses of Subtropical Eucalypt Woodland Vegetation under Changing Water-Availability Conditions. Agric. For. Meteorol. 2018, 248, 348–360. [Google Scholar] [CrossRef]
- Song, L.; Zhu, J.; Li, M.; Zhang, J. Water Use Patterns of Pinus Sylvestris Var. Mongolica Trees of Different Ages in a Semiarid Sandy Lands of Northeast China. Environ. Exp. Bot. 2016, 129, 94–107. [Google Scholar] [CrossRef]
- Volkmann, T.H.; Haberer, K.; Gessler, A.; Weiler, M. High-Resolution Isotope Measurements Resolve Rapid Ecohydrological Dynamics at the Soil-Plant Interface. New Phytol. 2016, 210, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Feng, Q.; Si, J.; Chang, Z.; Zhuo, M.; Xi, H.; Su, Y. Summary on the Plant Water Use Efficiency at Leaf Level. Acta Ecol. Sin. 2009, 29, 3882–3892. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, X.; Huang, Y.; Su, N.; Feng, J. Research Progress on Plant Water Use Efficiency and its Determination Methods. J. Desert Res. 2009, 29, 648–655. [Google Scholar]
- Martin, B.; Thorstenson, Y.R. Stable Carbon Isotope Composition (13C), Water Use Efficiency, and Biomass Productivity of Lycopersicon Esculentum, Lycopersicon Pennellii, and the F1 Hybrid. Plant Physiol. 1988, 88, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richard, R.A. Carbon Isotope Fractionation and Plant Water-Use Efficiency; Springer: New York, NY, USA, 1989; pp. 21–40. [Google Scholar]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus Sargentii and Larix Kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Stokes, V.J.; Morecroft, M.D.; Morison, J. Comparison of Leaf Water Use Efficiency of Oak and Sycamore in the Cnopy over Two Growing Seasons. Trees 2010, 24, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Baruch, Z. Leaf Trait Variation of a Dominant Neotropical Savanna Tree across Rainfall and Fertility Gradients. Oecologica 2011, 37, 455–461. [Google Scholar] [CrossRef]
- Robinson, D.; Handley, L.L.; Scrimgeour, M.C.; Gordon, C.D.; Forster, P.B. Using Stable Isotope Natural Abundances (δ15N and δ13C) to Integrate the Stress Responses of Wild Barley (Hordeum Spontaneum C. Koch.) Genotypes. J. Exp. Bot. 2000, 51, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igamberdiev, A.U.; Mikkelsen, T.N.; Mbus, P.; Bauwe, H.; Lea, P.J.; Gardestrm, P. Photorespiration Contributes to Stomatal Regulation and Carbon Isotope Fractionation: A Study with Barley, Potato and Arabidopsis Plants Deficient in Glycine DecarBoxylase. Photosynth. Res. 2004, 81, 139–152. [Google Scholar] [CrossRef]
- Li, H.; Shi, Q.; Wan, Y.; Shi, H.; Imin, B. Using Sentinel-2 Images to Map the Populus Euphratica Distribution Based on the Spectral Difference Acquired at the Key Phenological Stage. Forests 2021, 12, 147. [Google Scholar] [CrossRef]
- Shi, H.; Shi, Q.; Zhou, X.; Imin, B.; Li, H.; Zhang, W.; Kahaer, Y. Effect of the Competition Mechanism of between Co-Dominant Species on the Ecological Characteristics of Populus Euphratica under a Water Gradient in a Desert Oasis. Glob. Ecol. Conserv. 2021, 27, e01611. [Google Scholar] [CrossRef]
- Thomas, F.M.; Bruelheide, H.; Foetzki, A.; Gries, D.; Runge, M. Ecological Basis for a Sustainable Management of the Indigenous Vegetation in a Central-Asian Desert: Presentation and First Results. J. Appl. Bot. 2000, 74, 212–219. [Google Scholar]
- Li, T.; Luo, G.; Dong, K.; Peng, L.; Dai, Y. Water Usage of Populus Euphratica in Different Development Stages Grown near the Riverbank at the Tail of the Keriya River. Chin. J. Ecol. 2021, 40, 989–997. [Google Scholar] [CrossRef]
- Marhaba, N.; Bilal, E.; Shi, Q.; Yue, D.; Shi, H. Water Sources of Tamarix sp. Seedlings at the Daryaboyi Oasis in the Hinterland of Desert. J. Arid. Land Resour. Environ. 2021, 35, 159–166. [Google Scholar] [CrossRef]
- Cyffka, B.; Wang, J.; Halik, U.; Osman, K.; Peng, S. Study on DBH-Structure of Populus Euphratica and their Spatial Distribution in the Lower Reaches of the Tarim River. Chin. Bull. Bot. 2008, 25, 728–733. [Google Scholar]
- Li, C.; Guo, J.; Zeng, F.; Guo, Z. Shoot and Root Architectural Variance and Adaptability of in Different Ages. J. Desert Res. 2015, 35, 365–372. [Google Scholar] [CrossRef]
- Schultz, N.M.; Griffis, T.J.; Lee, X.H.; Baker, J.M. Identification and Correction of Spectral Contamination in H2/H1 and O18/O16 Measured in Leaf, Stem, and Soil Water. Rapid Commun. Mass Spectrom. 2011, 25, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Gregg, J.W. Source Partitioning Using Stable Isotopes: Coping with too Many Sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Li, W.; Wang, R.; Zhou, Y.; Zhang, J. Water Sources of Typical Desert Riparian Plants in the Lower Reaches of Tarim River. J. Desert Res. 2017, 37, 1150–1157. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Chen, Y.; Friedman, J.M.; Hati, J.H.A.; Fang, G. Use of H2 and O18 Stable Isotopes to Investigate Water Sources for Different Ages of Populus Euphratica along the lower Heihe River. Ecol. Res. 2015, 30, 581–587. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, X.; Wang, Q.; Li, P.; Jiapaer, G. Retrieval of Leaf Biochemical Properties by Inversed Prospect Model and HyperSpectral Indices: An Application to Populus Euphratica Polymorphic Leaves. J. Arid Land 2012, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Overdieck, D.; Ziche, D.; Yu, R.D. Gas Exchange of Populus Euphratica Leaves in a Riparian Zone. J. Arid Land 2013, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Li, J.; Huang, Z.; Baskin, C.C.; Baskin, J.M.; Hao, P. Reproductive Characteristics of a Populus Euphratica Population and Prospects for its Restoration in China. PLoS ONE 2012, 7, e39121. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Li, J.; Li, J.; Cheng, C.; Wang, X. The Characteristics of Root Suckers of Populus Euphratica Oliv. in Three Habitats of Ejina Oasis. Acta Ecol. Sin. 2008, 28, 4703–4709. [Google Scholar] [CrossRef]
- Tsheboeng, G.; Murray-Hudson, M.; Kashe, K. Response of Riparian Plant Communities to Distance from Surface Water in the Okavango Delta, Botswana. Afr. J. Ecol. 2017, 55, 402–410. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, C.; Wang, D.; Fu, S.; Zhou, T. Water Use Strategies of Populus Euphratica and Tamarix Ramosissima at Different Ages in Tarim River Basin. J. Soil Water Conserv. 2017, 31, 157–163. [Google Scholar] [CrossRef]
- Pierret, A.; Maeght, J.L.; Clément, C.; Montoroi, J.P.; Hartmann, C.; Gonkhamdee, S. Understanding Deep Roots and their Functions in Ecosystems: An Advocacy for more Unconventional Research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhou, H.; Zheng, X.; Li, Y.; Tang, L. Seasonal Changes in the Water Use Strategies of Three Co-Occurring Desert Shrubs. Hydrol. Process. 2014, 28, 6265–6275. [Google Scholar] [CrossRef]
- Nardini, A.; Casolo, V.; Dal, B.A.; Savi, T.; Stenni, B.; Bertoncin, P.; Zini, L. Rooting Depth, Water Relations and Non-Structural Carbohydrate Dynamics in Three Woody Angiosperms Differentially Affected by an Extreme Summer Drought. Plant Cell Environ. 2016, 39, 618–627. [Google Scholar] [CrossRef]
- Farquhar, G.; Richards, R. Isotopic Composition of Plant Carbon Correlates with Water-Use Efficiency of Wheat Genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Ma, W.; Wu, S. Responses of Net Photosynthetic Rate of Populus Euphratica at Different Ages Toecological and Physiological Factors. J. Yunnan Univ. 2020, 42, 1004–1013. [Google Scholar]
- Zheng, Y.; Zhao, Z.; Zhou, J.; Zhou, H.; Liang, Z.; Luo, Z. The Importance of Slope Aspect and Stand Age on the Photosynthetic Carbon Fixation Capacity of Forest: A Case Study with Black Locust (Robinia Pseudoacacia) Plantations on the Loess Plateau. Acta Physiol. Plant 2011, 33, 419–429. [Google Scholar] [CrossRef]
- Brown, R.H.; Byrd, G.T. Relationships between Specific Leaf Weight and Mineral Concentration among Genotypes. Field Crop. Res. 1997, 54, 19–28. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Bares, J.C.; Craine, J.M.; Oieksyn, J.; Walter, M.B.; Reich, P.B.; Westoby, M. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Carl, C.; Biber, P.; Veste, M.; Landgraf, D.; Pretzsch, H. Key Drivers of Competition and Growth Partitioning among Robinia Pseudoacacia L. Trees. For. Ecol. Manag. 2018, 430, 86–93. [Google Scholar] [CrossRef]
- Ale, R.; Zhang, L.; Li, X.; Raskoti, B.B.; Pugnaire, F.I.; Luo, T. Leaf δ13C as an Indicator of Water Availability along Elevation Gradients in the Dry Himalayas. Ecol. Indic. 2018, 94, 266–273. [Google Scholar] [CrossRef]
- Liu, M.; Shi, J.; Wang, X.; Aijier, A. Photosynthetic and Transpiration Characteristics of Natural Populus Euphratica Forests in Different Habitats in the Middle Reaches of Tahe River. J. Northwest For. Coll. 2021, 36, 9–15. [Google Scholar] [CrossRef]
- Deng, W.; Li, X.; Zhang, X.; Ye, W.; Andrea, F.; Michael, R. The Studies about the Photosynthetic Response of the Four Desert Plants. Acta Ecol. Sin. 2003, 23, 598–605. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Kim, H.S. Evaluation of Morphological, Physiological, and Biochemical Traits for Assessing Drought Resistance in Eleven Tree Species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Feng, Q.; Su, Y.; Chang, Z.; Xi, H. Research on the Water Use Efficiency and Foliar Nutrient Status of Populus Euphratica and Tamarix Ramosissima in the Extreme Arid Region of China. Environ. Earth Sci. 2011, 62, 1597–1607. [Google Scholar] [CrossRef]


| Species | Growth Stages | Average Tree Height (m) | Average Diameter at Breast Height (cm) | Average Canopy Width (m) |
|---|---|---|---|---|
| Populus euphratica Oliv. | Young trees | 3.84 ± 1.40 | 8.75 ± 1.83 | 2.52 ± 0.35 |
| Mature trees | 8.64 ± 0.89 | 32.41 ± 3.66 | 5.78 ± 1.47 | |
| Overmature trees | 9.62 ± 1.26 | 58.76 ± 12.35 | 9.57 ± 3.52 | |
| Tamarix chinensis Lour. | Young shrubs | 0.79 ± 0.25 | – | 0.56 ± 0.28 |
| Mature shrubs | 2.01 ± 0.40 | – | 2.99 ± 2.12 | |
| Overmature shrubs | 2.85 ± 0.41 | – | 5.80 ± 3.15 |
| δ18O | 2019 | 2020 |
|---|---|---|
| River water | −7.43 ± 0.65‰ | – |
| Shallow soil water (0–140 cm) | −1.29 ± 3.22‰ | −2.39 ± 4.65‰ |
| Deep soil water (140–300 cm) | −5.92 ± 0.50‰ | −7.06 ± 0.86‰ |
| Groundwater | −5.72 ± 0.07‰ | −6.26 ± 0.18‰ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Shi, Q.; Dai, Y.; Marhaba, N.; Peng, L.; Peng, L.; Shi, H. Water Use Characteristics of Populus euphratica Oliv. and Tamarix chinensis Lour. at Different Growth Stages in a Desert Oasis. Forests 2022, 13, 236. https://doi.org/10.3390/f13020236
Wan Y, Shi Q, Dai Y, Marhaba N, Peng L, Peng L, Shi H. Water Use Characteristics of Populus euphratica Oliv. and Tamarix chinensis Lour. at Different Growth Stages in a Desert Oasis. Forests. 2022; 13(2):236. https://doi.org/10.3390/f13020236
Chicago/Turabian StyleWan, Yanbo, Qingdong Shi, Yue Dai, Nijat Marhaba, Liping Peng, Lei Peng, and Haobo Shi. 2022. "Water Use Characteristics of Populus euphratica Oliv. and Tamarix chinensis Lour. at Different Growth Stages in a Desert Oasis" Forests 13, no. 2: 236. https://doi.org/10.3390/f13020236






