Tree-to-Shrub Shift Benefits the Survival of Quercus mongolica Fisch. ex Ledeb. at the Xeric Timberline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Design
2.2. Soil Available Water Calculation and Physical Property Measurement
2.3. Tree Age and Chromosome Ploidy Measurements
2.4. Tree Architecture Measurement
2.5. Hydraulic Architecture Measurement
2.6. Leaf Carbon Isotope Measurement
2.7. Nonstructural Carbohydrate Measurement
2.8. Statistical Analysis
3. Results
3.1. Chromosomeploidy of Q. mongolica Trees and Shrubs
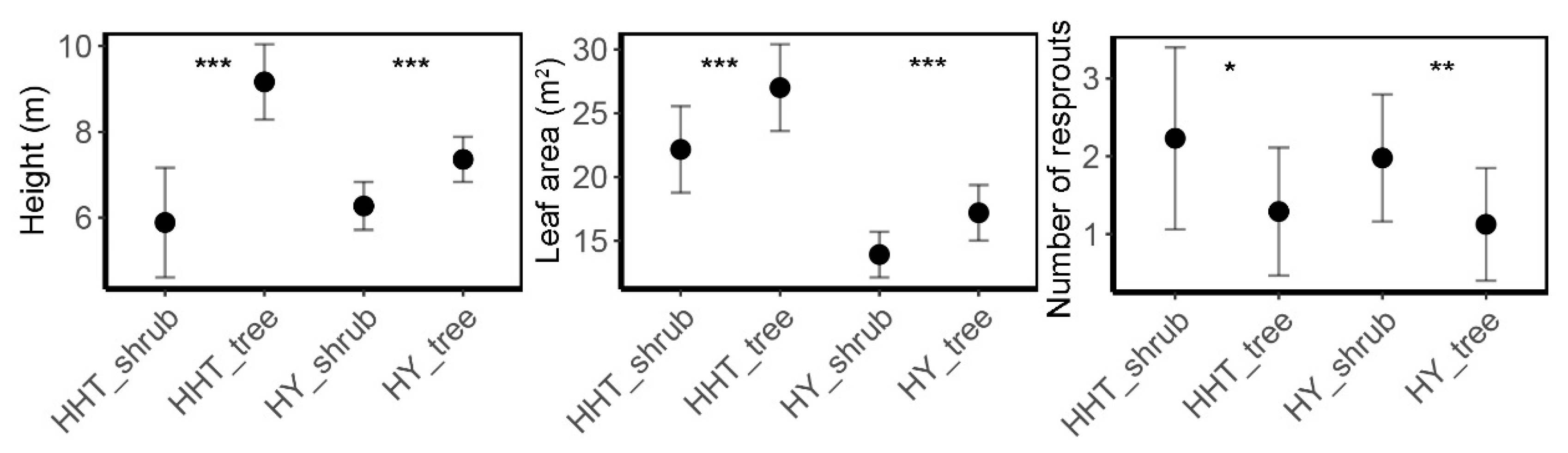
3.2. Architecture of Q. mongolica Trees and Shrubs
3.3. Soil Available Water of Q. mongolica Trees and Shrubs
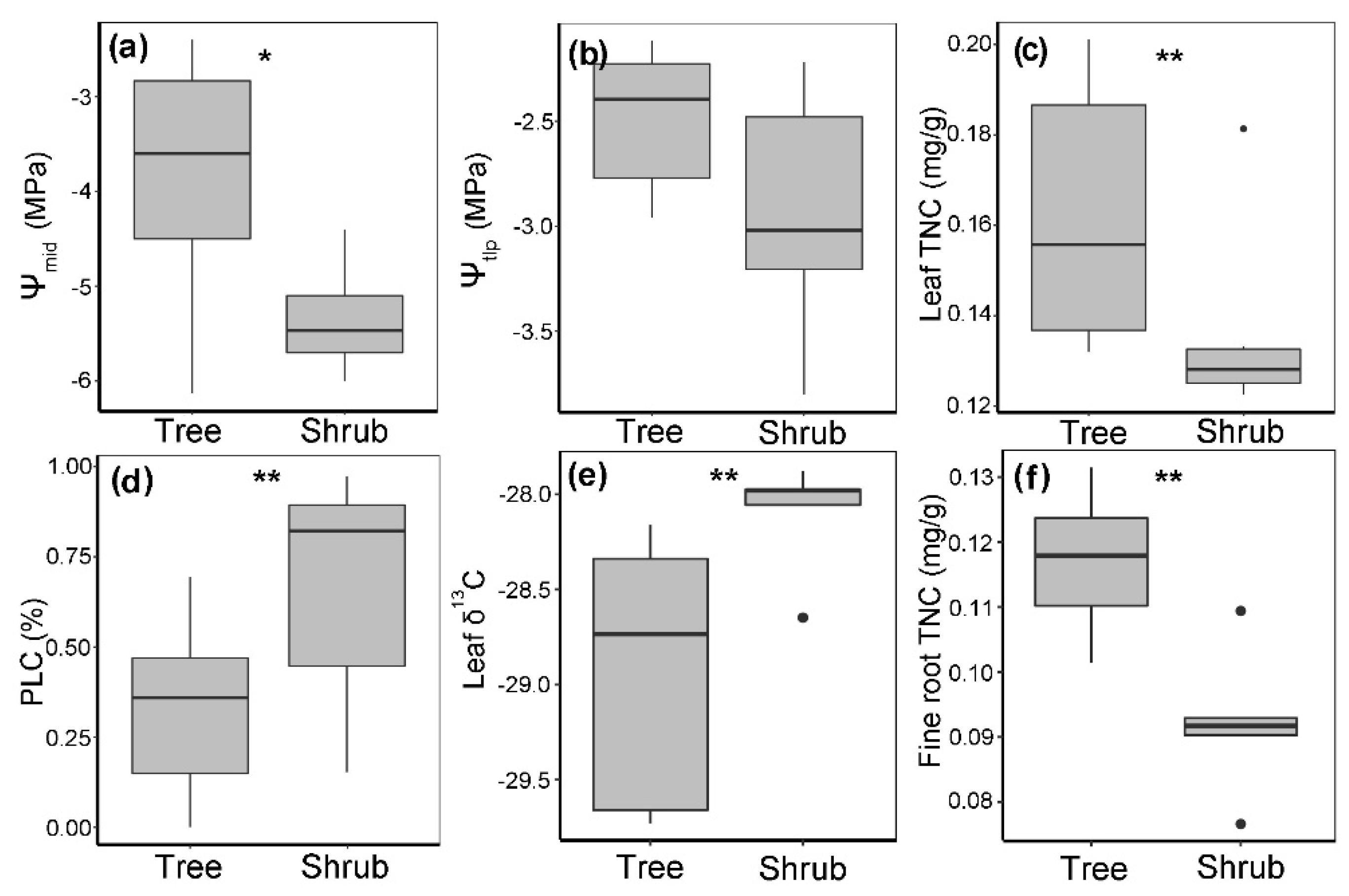
3.4. Quantification of Drought Stress in Q. mongolica Trees and Shrubs
3.5. Drought Tolerance of Q. mongolica Trees and Shrubs
3.6. Nonstructural Carbohydrates of Q. mongolica Trees and Shrubs
4. Discussion and Concluding Remarks
4.1. The Tree-to-Shrub Shift Physiologically Improves Plant Drought Tolerance
4.2. Implications for Fire Ecology
4.3. Further Considerations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene Name | Primer | Product Length (bp) | Primer Amplification Efficiency |
|---|---|---|---|
| NDH8 | qPCR ndh8-F: TCATCACTGTCGGAATTGGGT | 92 | 2.08 |
| qPCR ndh8-R: TTATCGAACGAACCGCACTC | |||
| RPL23 | qPCR rpl23-F: ATCGGGATCAACTAGGACAGA | 174 | 2.1 |
| qPCR rpl23-R: CCCATTCTTCTACCCTTTCCCG |
| Gene Name | NDH8 | RPL23 |
|---|---|---|
| Slope | −3.15 | −3.21 |
| Efficiency | 2.08 | 2.10 |
| Error | 0.18 | 0.12 |
| R2 | 0.99 | 1.00 |
| Y intercept | 16.19 | 15.75 |
References
- Scheffer, M.; Vergnon, R.; Cornelissen, J.H.C.; Hantson, S.; Holmgren, M.; van Nes Egbert, H.; Xu, C. Why trees and shrubs but rarely trubs? Trends Ecol. Evol. 2014, 29, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.; Harja, D. Exploring ecological significance of tree crown plasticity through three-dimensional modelling. Ann. Bot. 2007, 101, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archibald, S.; Bond, W.J. Growing tall vs growing wide: Tree architecture and allometry of Acacia karroo in forest, savanna, and arid environments. Oikos 2003, 102, 3–14. [Google Scholar] [CrossRef]
- Wieser, G.; Tausz, M. Current concepts for treelife limitation at the upper timberline. In Trees at their Upper Limit: Treelife Limitation at the Alpine Timberline; Wieser, G., Tausz, M., Eds.; Springer: Amsterdam, The Netherlands, 2007; pp. 1–18. [Google Scholar]
- Malanson, G.P.; Trabaud, L. Vigour of post-fire resprouting by Quercus Coccifera L. J. Ecol. 1988, 76, 351–365. [Google Scholar] [CrossRef]
- Krivtsov, V.; Legg, C. Modelling soil moisture deficit and moisture content of ground vegetation: Progress towards development of a fire weather index system appropriate to the UK. Fire Technol. 2011, 47, 539–548. [Google Scholar] [CrossRef]
- Matyas, C.; Vendramin, G.G.; Fady, B. Forests at the limit: Evolutionary-genetic consequences of environmental changes at the receding (xeric) edge of distribution. Report from a research workshop. Ann. For. Sci. 2009, 66, 800. [Google Scholar] [CrossRef]
- Liu, H.Y.; He, S.Y.; Anenkhonov, O.A.; Hu, G.Z.; Sandanov, D.V.; Badmaeva, N.K. Topography-controlled soil water content and the coexistence of forest and steppe in northern China. Physcal Geogr. 2012, 33, 561–573. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J.; Asensio, D.; Llusià, J. Chlorophyll fluorescence responses to temperature and water availability in two co-dominant Mediterranean shrub and tree species in a long-term field experiment simulating climate change. Environ. Exp. Bot. 2011, 71, 123–127. [Google Scholar] [CrossRef]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Dai, J.Y.; Liu, H.Y.; Wang, Y.C.; Guo, Q.H.; Hu, T.Y.; Quine, T.; Green, S.; Hartmann, H.; Xu, C.Y.; Liu, X.; et al. Drought-modulated allometric patterns of trees in semi-arid forests. Commun. Biol. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Bader, M.; Osokhjargal, D.; Oyungerel, S.; Nyambayar, S.; Runge, M.; Leuschner, C. Water relations and photosynthetic performance in Larix sibirica growing in the forest-steppe ecotone of northern Mongolia. Tree Physiol. 2009, 29, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Krestov, P.V. Forest vegetation of easternmost Russia (Russian Far East). In Forest Vegetation of Northeast Asia; Kolbek, J., Šrůtek, M., Box, E.O., Eds.; Springer: Amsterdam, The Netherlands, 2003; pp. 93–180. [Google Scholar]
- Xu, X.T.; Wang, Z.H.; Rahbek, C.; Sanders, N.J.; Fang, J.Y. Geographical variation in the importance of water and energy for oak diversity. J. Biogeogr. 2016, 43, 279–288. [Google Scholar] [CrossRef]
- Gao, W.B.; Ding, W.; Li, S.; Wang, J.Z.; Chen, X.B. Study on site suitable to Quercus mongolica in natural hag in Changbai Mountain forest region. J. Beihua Univ. (Nat. Sci.) 2000, 1, 77–81. (In Chinese) [Google Scholar]
- Yang, D.Y.; Guo, L.R.; Yang, X.K.; Yang, Y.C.; Chen, H.M.; Deng, P.J. A method freed from endogenous reference gene for estimating copy number of transgenes in genetically mod ified plants by TaqMan quantitative PCR. Chin. J. Health Lab. 2008, 6, 992–996. (In Chinese) [Google Scholar]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Li, X.M.; Blackman, C.J.; Choat, B.; Duursma, R.A.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant Cell Environ. 2018, 41, 646–660. [Google Scholar] [CrossRef]
- Maréchaux, I.; Bartlett, M.K.; Sack, L.; Baraloto, C.; Engel, J.; Joetzjer, E.; Chave, J. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 2015, 29, 1268–1277. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.W.; Cao, K.F.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Holbrook, N.M. Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L.) and red spruce (Picea rubens Sarg.). Plant Cell Environ. 1998, 21, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Liu, H.Y.; Zhao, F.J.; Liu, X. Inconsistent changes of biomass and species richness along a precipitation gradient in temperate steppe. J. Arid Environ. 2016, 132, 42–48. [Google Scholar] [CrossRef]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef]
- Guo, Q.; Li, J.Y.; Zhang, Y.X.; Zhang, J.X.; Lu, D.L.; Korpelainen, H.; Li, C.Y. Species-specific competition and N fertilization regulate non-structural carbohydrate contents in two Larix species. For. Ecol. Manag. 2016, 364, 60–69. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.Y.; Shangguan, H.L.; Zhou, M.; Airebule, P.; Zhao, P.W.; He, W.Q.; Xiang, C.L.; Wu, X.C. Differentiated responses of nonstructural carbohydrate allocation to climatic dryness and drought events in the Inner Asian arid timberline. Agric. For. Meteorol. 2019, 271, 355–361. [Google Scholar] [CrossRef]
- Midgley, J.J. Is bigger better in plants? The hydraulic costs of increasing size in trees. Trends Ecol. Evol. 2003, 18, 5–6. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Cruiziat, P.; Cochard, H.; Ameglio, T. Hydraulic architecture of trees: Main concepts and results. Ann. For. Sci. 2002, 59, 723–752. [Google Scholar] [CrossRef] [Green Version]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Perea, R.; López-Sánchez, A.; Pallarés, J.; Gordaliza, G.G.; González-Doncel, I.; Gil, L.; Rodríguez-Calcerrada, J. Tree recruitment in a drought- and herbivory-stressed oak-beech forest: Implications for future species coexistence. For. Ecol. Manag. 2020, 477, 118489. [Google Scholar] [CrossRef]
- Valbuena-Carabaña, M.; Gil, L. Genetic resilience in a historically profited root sprouting oak (Quercus pyrenaica Willd.) at its southern boundary. Tree Genet. Genomes 2013, 9, 1129–1142. [Google Scholar] [CrossRef]
- Peña-Rojas, K.; Aranda, X.; Joffre, R.; Fleck, I. Leaf morphology, photochemistry and water status changes in resprouting Quercus ilex during drought. Funct. Plant Biol. 2005, 32, 117–130. [Google Scholar] [CrossRef]
- Krivtsov, V.; Vigy, O.; Legg, C.; Curt, T.; Rigolot, E.; Lecomte, I.; Jappiot, M.; Lampin-Maillet, C.; Fernandes, P.; Pezzatti, B. Fuel modelling in terrestrial ecosystems: An overview in the context of the development of an object-orientated database for wild fire analysis. Ecol. Model. 2009, 220, 2915–2926. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E.; Schwilk, D.W. Flammability as an ecological and evolutionary driver. J. Ecol. 2017, 105, 289–297. [Google Scholar] [CrossRef]
- Legg, C.; Pezzatti, G.; Rigolot, E.; Vigy, O.; Lecomte, I.; Mårell, A.; Krivtsov, V. Development of an object-orientated database for wildfire modelling. Model. Monit. Manag. For. Fires 2008, 119, 39–47. [Google Scholar]
- Sun, Y.; Abbott, R.J.; Li, L.; Li, L.; Zou, J.; Liu, J. Evolutionary history of Purple cone spruce (Picea purpurea) in the Qinghai–Tibet Plateau: Homoploid hybrid origin and Pleistocene expansion. Mol. Ecol. 2014, 23, 343–359. [Google Scholar] [CrossRef]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Seddon, A.W.R.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [Green Version]
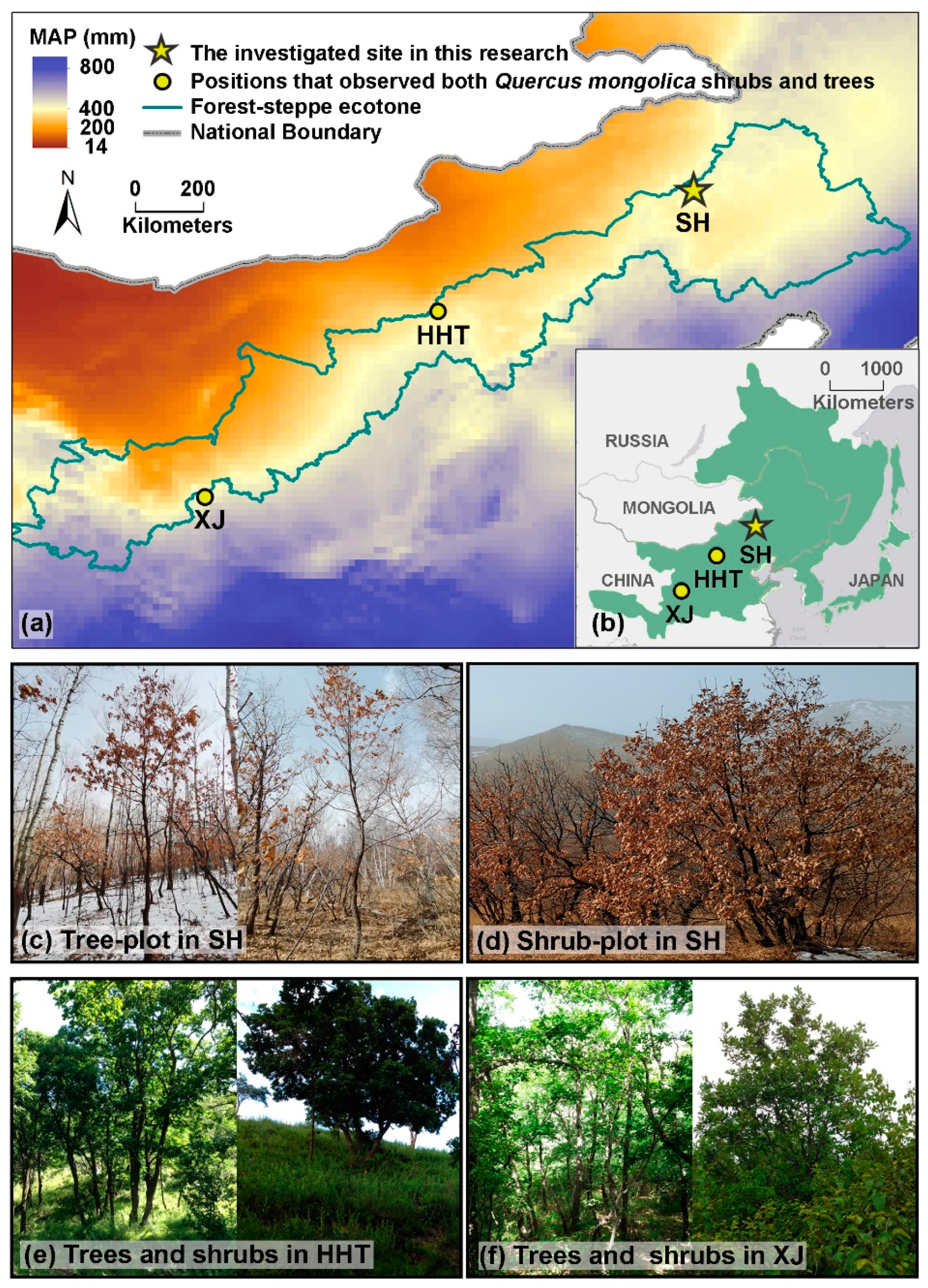

| Plot name | Latitude (°N) | Longitude (°E) | Altitude (m a.s.l.) | Slope (°) | Aspect | Average tree age (years) |
| Tree | 44.22 | 118.74 | 1195 | 12 | North | 60 |
| Shrub | 44.22 | 118.75 | 1207 | 6 | South | 58 |
| Plot name | Bulk density (g/cm3) | Field capacity (%) | Soil depth (m) | |||
| Tree | 1.04 | 41.56 | 0.49 | |||
| Shrub | 1.19 | 32.69 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Lu, S.; Qi, Y.; Liu, H. Tree-to-Shrub Shift Benefits the Survival of Quercus mongolica Fisch. ex Ledeb. at the Xeric Timberline. Forests 2022, 13, 244. https://doi.org/10.3390/f13020244
Dai J, Lu S, Qi Y, Liu H. Tree-to-Shrub Shift Benefits the Survival of Quercus mongolica Fisch. ex Ledeb. at the Xeric Timberline. Forests. 2022; 13(2):244. https://doi.org/10.3390/f13020244
Chicago/Turabian StyleDai, Jingyu, Surui Lu, Yang Qi, and Hongyan Liu. 2022. "Tree-to-Shrub Shift Benefits the Survival of Quercus mongolica Fisch. ex Ledeb. at the Xeric Timberline" Forests 13, no. 2: 244. https://doi.org/10.3390/f13020244
APA StyleDai, J., Lu, S., Qi, Y., & Liu, H. (2022). Tree-to-Shrub Shift Benefits the Survival of Quercus mongolica Fisch. ex Ledeb. at the Xeric Timberline. Forests, 13(2), 244. https://doi.org/10.3390/f13020244








