Direct and Indirect Effects of Habitat Disturbances on Caribou Terrestrial Forage Lichens in Montane Forests of British Columbia
Abstract
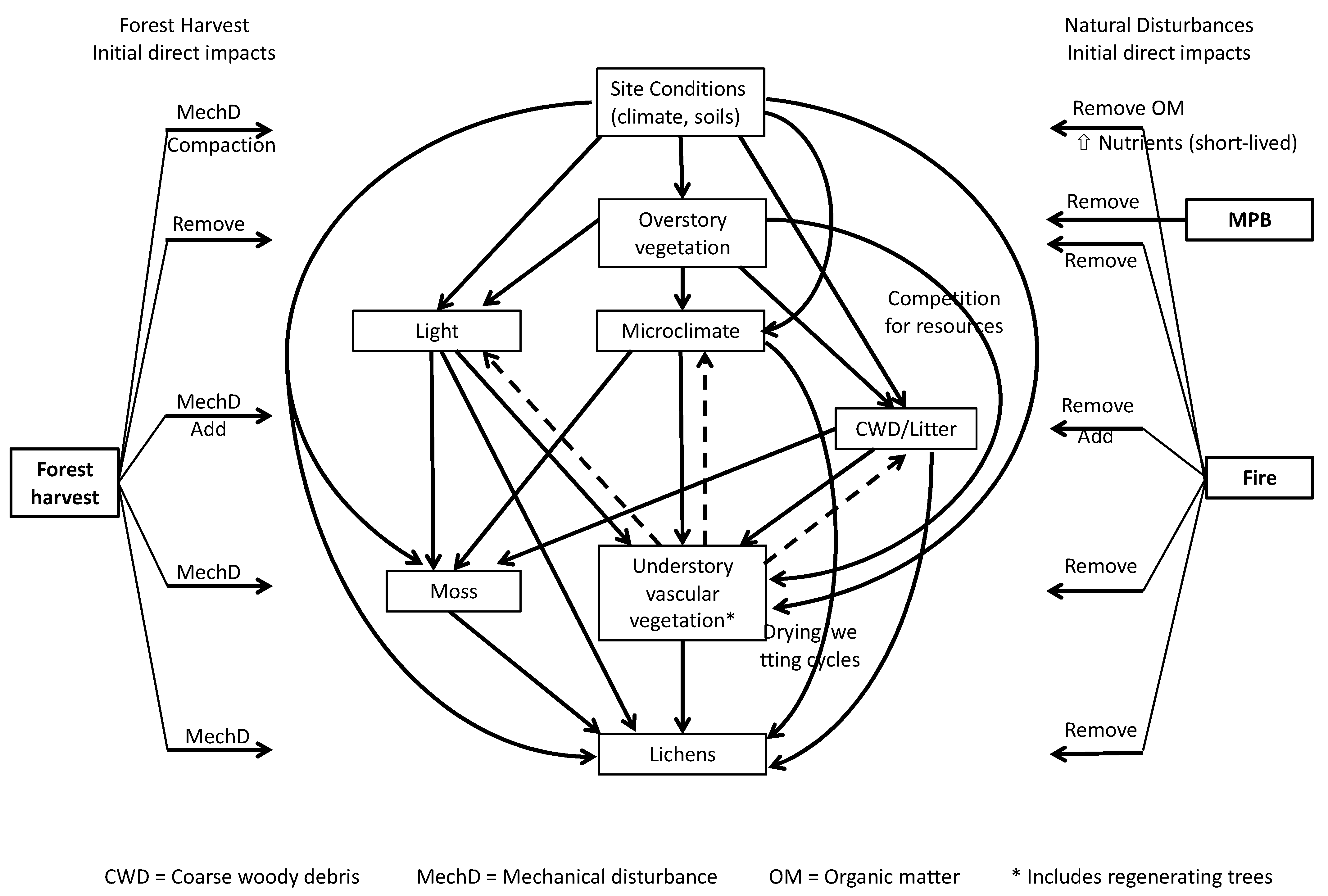
:1. Introduction
2. Materials and Methods
2.1. Study Area
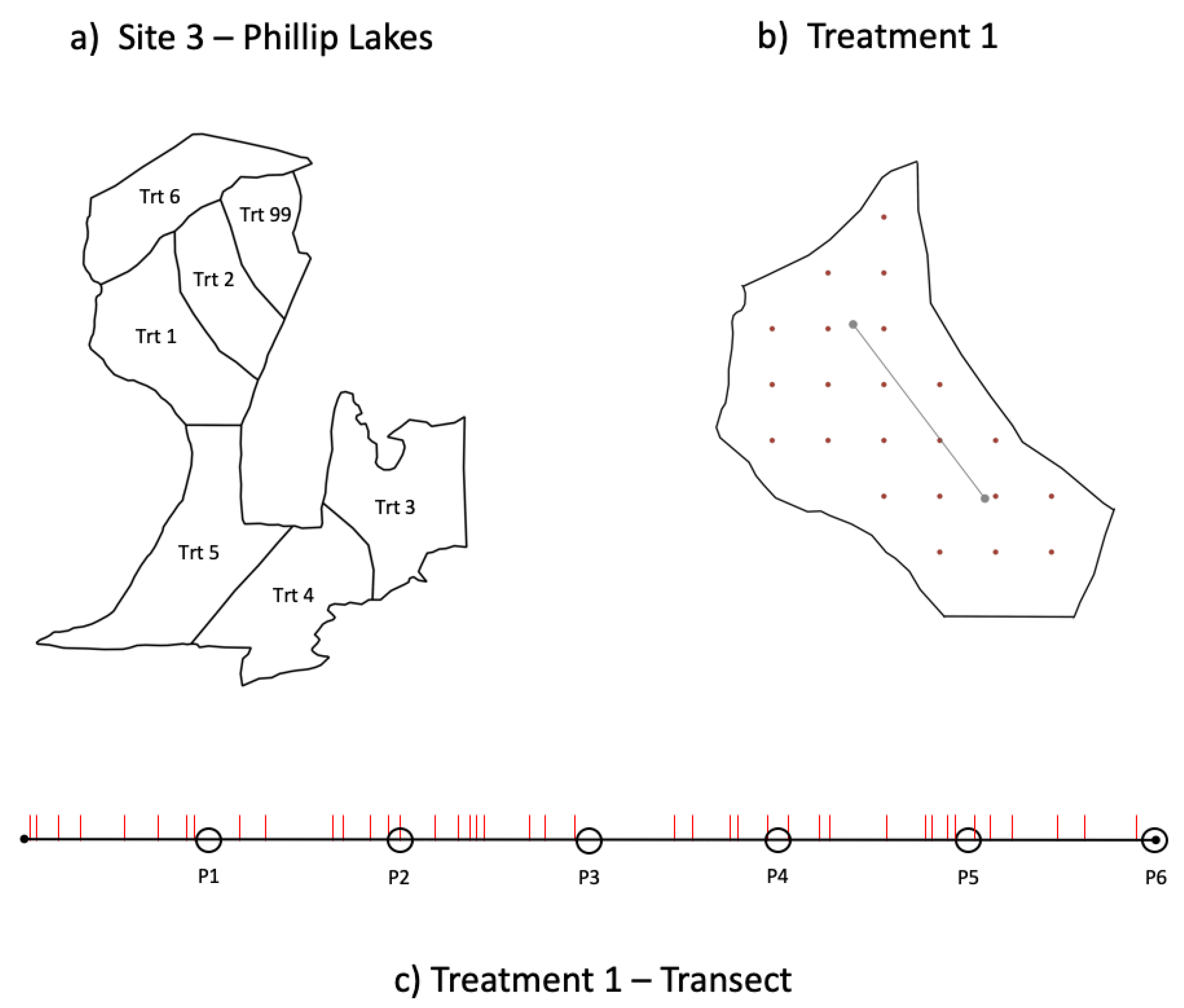
2.2. Treatment Descriptions
2.2.1. Managed Disturbances
2.2.2. Natural Disturbances
2.3. Data Collection Methods
2.4. Data Analyses
3. Results
3.1. Effects of Forest Harvesting, Prescribed Burning and MPB on Total Forage Lichen Abundance
3.2. Overall Site and Disturbance Influences on Abundance of Total Forage Lichens
3.3. Factors Influencing Recovery Rates of Forage Lichen Abundances after Disturbance
3.3.1. MPB Disturbance
3.3.2. Effects of Forest Harvesting
3.3.3. Effects of Prescribed Burning
4. Discussion
4.1. Effects of Disturbances on Terrestrial Lichen Dynamics
4.1.1. Forest Harvesting
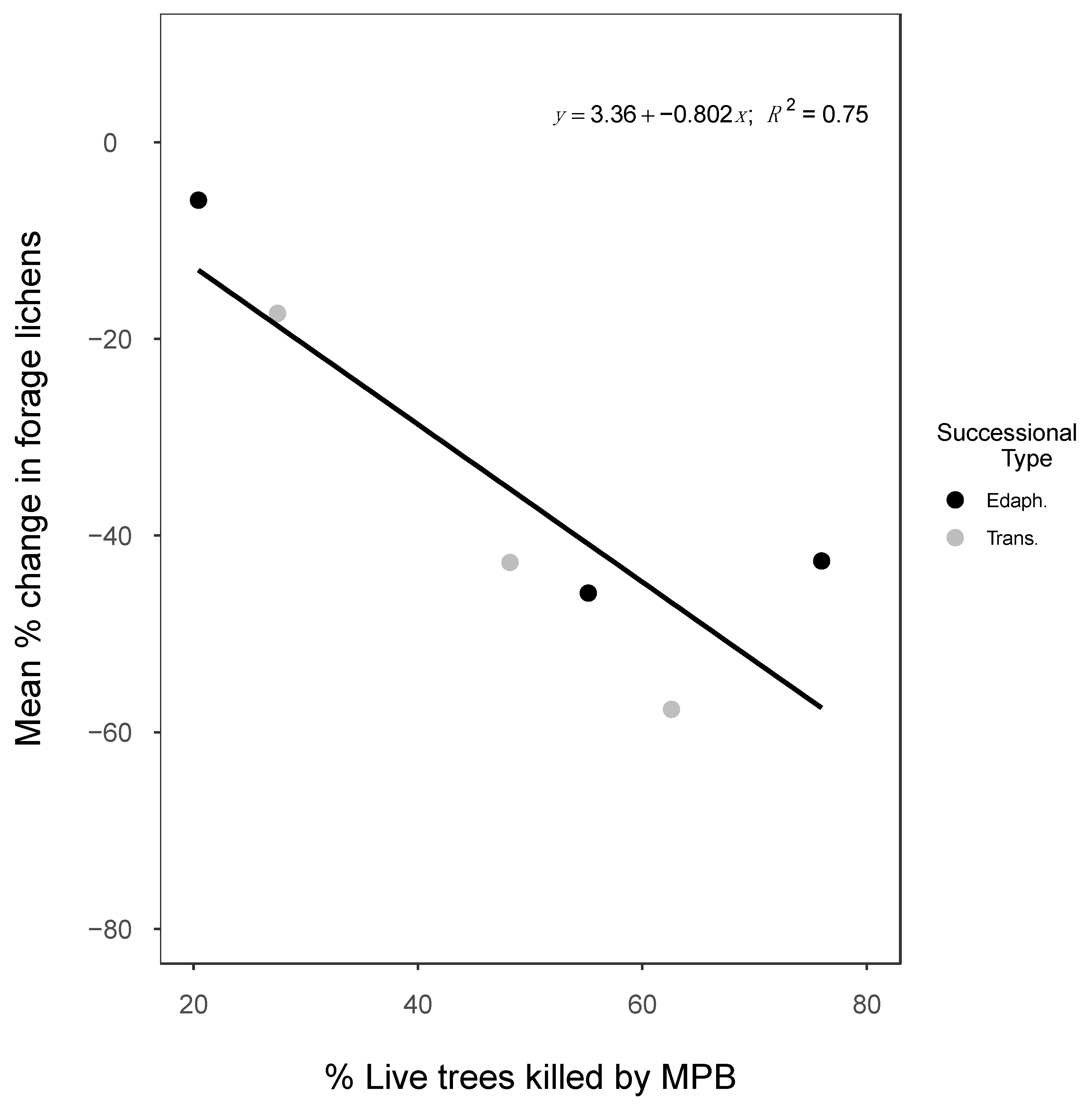
4.1.2. Mountain Pine Beetles
4.1.3. Fire
4.2. Considerations for Management
Suggested Guidelines and Recommendations for Sustaining Forage Lichens in Forests
- Target edaphic sites with low levels of MPB attack as a priority for retention. Edaphic sites with low levels of MPB attack will provide the best conditions for retaining terrestrial lichen abundance in the short term.
- Target all edaphic sites for retention. Despite decreases in forage lichen abundance following MPB on edaphic sites, cover of forage lichens in MPB stands continued to exceed cover of forage lichens in harvested areas, 12–14 years post-harvest. Retaining all edaphic sites for retention will provide more lichen for caribou in the short term. Currently, most caribou populations in southern and central BC are declining while habitat alteration rates are high; therefore, management strategies need to support short-term objectives for halting these declines.
- Retention areas, especially those with low levels of MPB attack, should be designed to reduce the potential for windthrow. Small pockets of retention within large clearcuts (e.g., the 98-Mile control) that will result in a high degree of blowdown in retention areas will not provide conditions favourable to forage lichens.
- Where forest harvesting does occur on edaphic sites, conduct harvesting during winter months to retain as much of the lichen mat as possible. Although we were unable to detect a difference between effects of winter and summer harvesting on terrestrial lichens, other studies suggest that winter harvesting can benefit terrestrial lichens. Forest harvesting on edaphic sites should focus on maximizing retention of the existing lichen mat.
- Target transitional sites, especially those in later stages of succession and with higher levels of MPB attack for re-establishing conditions favourable for forage lichens. Forest harvesting on transitional sites where moss has outcompeted forage lichens should focus on re-establishing conditions that promote lichen growth, such as making the site less hospitable for mosses and shade-tolerant competing plant species by opening up the canopy.
- Finally, we also recommend that researchers distinguish between edaphic and transitional sites when conducting studies on forage lichens, and effects of disturbance on forage lichens. Few studies on the effects of disturbance on forage lichens differentiate between edaphic and transitional ecological conditions in their analyses and interpretations. Because forest floor vegetation dynamics following disturbance vary with ecological conditions, it is important to distinguish between site types when analyzing data, interpreting results and developing recommendations. In addition, research on forest harvesting should also consider harvest method (whole tree and cut to length), season (winter and summer), regeneration strategy (planting and natural) and site preparation technique.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Environment Canada. Recovery Strategy for the Woodland Caribou, Southern Mountain Population (Rangifer tarandus caribou) in Canada; Species at Risk Act Recovery Strategy Series; Environment Canada: Ottawa, ON, Canada, 2014; pp. viii + 103. [Google Scholar]
- Helle, T.; Aspi, J.; Tarvainen, L. The growth rate of Cladonia rangiferina and C. mitis in relation to forest characteristics in northeastern Finland. Rangifer 1983, 3, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Cichowski, D.; Williston, P.; Haeussler, S. The Response of Caribou Terrestrial Forage Lichens to Mountain Pine Beetles and Forest Harvesting in the East Ootsa and Entiako Areas: Annual Report—2007/08—Year 7; Morice-Lakes Innovative Forest Practices Agreement: Prince George, BC, Canada; Bulkley Valley Centre for Natural Resources Research and Management: Smithers, BC, Canada; Ministry of Environment: Prince George, BC, Canada, 2008; 46p. [Google Scholar]
- Ahti, T. Lichens of the boreal coniferous zone. In Lichen Ecology; Seaward, M.R.D., Ed.; Academic Press: London, UK, 1977; pp. 145–181. [Google Scholar]
- Rowe, J. Lichen woodland in northern Canada. In Northern Ecology and Resource Management; Univ. Alta. Press: Edmonton, Alta, 1984; pp. 225–237. [Google Scholar]
- Thompson, I.D.; Wiebe, P.A.; Mallon, E.; Rodgers, A.R.; Fryxell, J.M.; Baker, J.A.; Reid, D. Factors influencing the seasonal diet selection of woodland caribou (Rangifer tarandus caribou) in boreal forests in Ontario. Can. J. Zool. 2015, 93, 87–98. [Google Scholar] [CrossRef]
- Silva, J.; Nielsen, S.; Lamb, C.; Hague, C.; Boutin, S. Modelling lichen abundance for woodland caribou in a fire-driven boreal landscape. Forests 2019, 10, 962. [Google Scholar] [CrossRef] [Green Version]
- Morneau, C.; Payette, S. Postfire lichen—spruce woodland recovery at the limit of the boreal forest in northern Quebec. Can. J. Bot. 1989, 67, 2770–2782. [Google Scholar] [CrossRef]
- Brulisauer, A.; Bradfield, G.; Maze, J. Quantifying organizational change after fire in lodgepole pine forest understorey. Can. J. Bot. 1996, 74, 1773–1782. [Google Scholar] [CrossRef]
- Maikawa, E.; Kershaw, K.A. Studies on lichen-dominated systems. XIX. The postfire recovery sequence of black spruce—lichen woodland in the Abitau Lake Region, N.W.T. Can. J. Bot. 1976, 54, 2679–2687. [Google Scholar] [CrossRef]
- Sulyma, R.; Coxson, D. Microsite displacement of terrestrial lichens by feather moss mats in late seral pine-lichen woodlands of north-central British Columbia. Bryologist 2001, 104, 505–516. [Google Scholar] [CrossRef]
- Coxson, D.; Marsh, J. Lichen chronosequences (postfire and postharvest) in lodgepole pine (Pinus contorta) forests of northern interior British Columbia. Can. J. Bot. 2001, 79, 1449–1464. [Google Scholar] [CrossRef]
- Cichowski, D.; Haeussler, S. The Response of Caribou Terrestrial Forage Lichens to Mountain Pine Beetles, Forest Harvesting and Fire in the East Ootsa and Entiako Areas: 2016/17 (Year 16) Field Season Report; Bulkley Valley Centre for Natural Resources Research and Management: Smithers, BC, Canada; Habitat Conservation Trust Foundation: Victoria, BC, Canada; Canadian Forest Products Ltd.; Ministry of Forests, Lands and Natural Resources Operations and Rural Development: Smithers, BC, Canada, 2020. [Google Scholar]
- McNay, S.; Heard, D.; Sulyma, R.; Ellis, R. A Recovery Action Plan for Northern Caribou Herds in North-Central British Columbia; FORREX Series 22; FORREX Forest Research Extension Partnership: Kamloops, BC, Canada, 2008. [Google Scholar]
- Meidinger, D.; Pojar, J. (Eds.) Ecosystems of British Columbia. Special Report Series 6; British Columbia Ministry of Forests, Research Branch: Victoria, BC, Canada, 1991. [Google Scholar]
- DeLong, C. A Field Guide to Site Identification and Interpretation for the North Central Portion of the Northern Interior Forest Region; Land Management Handbook No. 54; BC Ministry of Forests: Victoria, BC, Canada, 2004. [Google Scholar]
- DeLong, C.; Tanner, D.; Jull, M. A Field Guide for Site Identification and Interpretation for the Southwest Portion of the Prince George Forest Region; Land Management Handbook No. 24; BC Ministry of Forests: Victoria, BC, Canada, 1993. [Google Scholar]
- Cichowski, D.; Sutherland, G.; McNay, R.S. Effects of Habitat Alteration on Caribou Terrestrial Forage Lichens in the Omineca Area—Annual Report 2017/18; Wildlife Infometrics Inc. Report No. 618; Wildlife Infometrics Inc.: Mackenzie, BC, Canada, 2018. [Google Scholar]
- Sulyma, R.; Sulyma, S. Adaptive Management of Forestry Practices in Pine-Lichen Woodlands in North-Central British Columbia: Post Treatment Data Summary; Resource Interface Ltd.: Fort St. James, BC, Canada, 2006; p. 38. [Google Scholar]
- Sulyma, R. Rehabilitation of Caribou Winter Range Following Attack by Mountain Pine Beetle: Pre-Treatment Site Monitoring UWR U-7-012; Wildlife Infometrics Inc. Report No. 312; Wildlife Infometrics Inc.: Mackenzie, BC, Canada, 2009. [Google Scholar]
- Sulyma, R.; McNay, R.S. Identifying Factors affecting the Succession and Availability of Terrestrial Lichen Communities in the Omineca Region of North-Central British Columbia; Wildlife Infometrics Inc. Report No. 322; Wildlife Infometrics Inc.: Mackenzie, BC, Canada, 2009. [Google Scholar]
- Sulyma, R.; McNay, R.S.; Brumovsky, V. Identifying factors affecting the succession and availability of terrestrial lichen communities in the Omineca Region of north-central British Columbia: Year 2; Wildlife Infometrics Inc. Report No. 337; Wildlife Infometrics Inc.: Mackenzie, BC, Canada, 2010. [Google Scholar]
- Westfall, J.; Ebata, T. Summary of Forest Health Conditions in British Columbia; Ministry of Forests and Range: Victoria, BC, Canada, 2008. Available online: https://www2.gov.bc.ca/assets/gov/environment/research-monitoring-and-reporting/monitoring/aerial-overview-survey-documents/2008-fh-bc-overview.pdf (accessed on 3 November 2021).
- Westfall, J.; Ebata, T. Summary of Forest Health Conditions in British Columbia; Lands and Natural Resource Operations; Ministry of Forests: Victoria, BC, Canada, 2011. Available online: https://www2.gov.bc.ca/assets/gov/environment/research-monitoring-and-reporting/monitoring/aerial-overview-survey-documents/2011-fh-bc-overview.pdf (accessed on 3 November 2021).
- BC Ministry of Environment, Lands and Parks (MOELP); Ministry of Forests (MOF). Field Manual for Describing Terrestrial Ecosystems; Land Manage. Handbook No. 25; BC Ministry of Forests: Victoria, BC, Canada, 1998. [Google Scholar]
- Trowbridge, R.; Hawkes, B.; Macadam, A.; Parminter, J. Field Handbook for Prescribed Fire Assessments in British Columbia: Logging Slash Fuels; FRDA Handbook 001; BC Ministry of Forests, Research Branch and Forestry Canada, Pacific Forestry Centre: Victoria, BC, Canada, 1989. [Google Scholar]
- De’ath, G. Boosted trees for ecological modeling and prediction. Ecology 2007, 88, 243–251. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Naujokaitis-Lewis, I.; Curtis, J.M.R. Advances in global sensitivity analysis of demographic-based species distribution models to address uncertainties in dynamic landscapes. PeerJ 2016, 4, e2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürkner, P. Advanced Bayesian multilevel modeling with the R package brms. R J. 2019, 10, 395–411. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Greenwell, B.; Boehmke, B.; Cunningham, J.; GBM Developers. gbm: Generalized Boosted Regression Models, R Package Version 2.1.8; 2020. Available online: https://CRAN.R-project.org/package=gbm (accessed on 3 November 2021).
- Bürkner, P. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Kranrod, K. Effects of Timber Harvesting Methods on Terrestrial Lichens and Understory Plants in West-Central Alberta. Master’s Thesis, University of Alberta, Edmonton, Alberta, 1996. [Google Scholar]
- Bråkenhielm, S.; Liu, Q. Long-term effects of clear-felling on vegetation dynamics and species diversity in a boreal pine forest. Biod. Cons. 1998, 7, 207–220. [Google Scholar] [CrossRef]
- Miège, D.; Armleder, H.; Waterhouse, M.; Goward, T. A Pilot Study of Silvicultural Systems for Northern Caribou Winter Range: Lichen Response; Working Paper 56; British Columbia Ministry of Forests Research Branch: Victoria, BC, Canada, 2001; 56p. [Google Scholar]
- Gough, A. Effects of Historical Timber Harvesting Practices on Caribou Forage Lichen Abundance Near Marsh Lake, Yukon; Forest Management Branch, Government of Yukon: Whitehorse, YK, Canada, 2010. [Google Scholar]
- Waterhouse, M.; Armleder, H.; Nemec, A. Terrestrial lichen response to partial cutting in lodgepole pine forests on caribou winter range in west-central British Columbia. Rangifer 2011, 19, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Kembel, S.; Waters, I.; Shay, J. Short-term effects of cut-to-length versus full-tree harvesting on understorey plant communities and understorey-regeneration associations in Manitoba boreal forests. For. Ecol. Manag. 2008, 255, 1848–1858. [Google Scholar] [CrossRef]
- Snyder, J.; Woodard, P. Lichen Regeneration Rates in Alberta Following Various Types of Logging and Wildfire Disturbances; Final Report; University of Alberta: Edmonton, Alta, 1992; 117p. [Google Scholar]
- Harris, A. Post-logging regeneration of reindeer lichens (Cladina spp.) as related to woodland caribou winter habitat. NWST Technical Report TR-69; Ontario Ministry of Natural Resources: Thunder Bay, ON, Canada, 1996. [Google Scholar]
- Webb, E. Survival, persistence, and regeneration of the reindeer lichens, Cladina stellaris, C. rangiferina, and C. mitis following clearcut logging and forest fire in northwestern Ontario. Rangifer 1998, 10, 41–47. [Google Scholar] [CrossRef]
- Kuzyk, R. Terrestrial Lichen Abundance in Relation to Stand Structure and Silvicultural History. Master’s Thesis, Lakehead University, Thunder Bay, On, Canada, 2013. [Google Scholar]
- Lafleur, B.; Zouaoui, S.; Fenton, N.; Drapeau, P.; Bergeron, Y. Short-term response of Cladonia lichen communities to logging and fire in boreal forests. For. Ecol. Manag. 2016, 372, 44–52. [Google Scholar] [CrossRef]
- Heinken, T. Dispersal patterns of terricolous lichens by thallus fragments. Lichenologist 1999, 31, 603–612. [Google Scholar] [CrossRef]
- Vitt, D.H.; Finnegan, L.; House, M. Terrestrial bryophyte and lichen responses to canopy opening in pine-moss-lichen forests. Forests 2019, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Kranabetter, J.M.; Haeussler, S.; Wood, C. Vulnerability of boreal indicators (ground-dwelling beetles, understory plants and ectomycorrhizal fungi) to severe forest soil disturbance. For. Ecol. Manag. 2017, 402, 213–222. [Google Scholar] [CrossRef]
- Cichowski, D.; Macadam, A.; Haeussler, S. Mountain Pine Beetle/Lichen Project—Quesnel TSA—Year 4—2008/09; Ministry of Environment: Williams Lake, BC, Canada, 2009; 79p. [Google Scholar]
- Cichowski, D.; Haeussler, S. The Response of Caribou Terrestrial Forage Lichens to Mountain Pine Beetles and Forest Harvesting in the East Ootsa and Entiako Areas: Annual Report—2012/13—Year 11; Bulkley Valley Centre for Natural Resources Research and Management, Ministry of Forests, Lands and Natural Resources Operations: Smithers, BC, Canada; Habitat Conservation Trust Foundation: Victoria, BC, Canada, 2013; 49p. [Google Scholar]
- Seip, D.; Jones, E. Response of Woodland Caribou to Partial Retention Logging of Winter Ranges Attacked by Mountain Pine Beetle; Annual Report; BC Ministry of Forests: Prince George, BC, Canada, 2010; 27p. [Google Scholar]
- McNay, R.S.; Giguere, L.; Brumovsky, V. A Validation Assessment of UWR U-7-007: Terrestrial Lichen Component; Wildlife Infometrics Inc. Report No. 458; Wildlife Infometrics Inc.: Mackenzie, BC, Canada, 2014. [Google Scholar]
- Kovacic, D.; Dyer, M.; Cringan, A. Understory biomass in ponderosa pine following mountain pine beetle infestation. For. Ecol. Manag. 1985, 13, 53–67. [Google Scholar] [CrossRef]
- Stone, W.; Wolfe, M. Response of understory vegetation to variable tree mortality following a mountain pine beetle epidemic in lodgepole pine stands in northern Utah. Vegetatio 1996, 122, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pec, G.; Karst, J.; Sywenky, A.; Cigan, P.; Erbilgin, N.; Simard, S.; Cahill, J., Jr. Rapid increases in forest understory diversity and productivity following a mountain pine beetle (Dendroctonus ponderosae) outbreak in pine forests. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, E.; Hollingsworth, T.; Chapin, F.S., III. Fire severity mediates climate-driven shifts in understorey community composition of black spruce stands of interior Alaska. J. Veg. Sci. 2011, 22, 32–44. [Google Scholar] [CrossRef]
- Pinno, B.; Errington, R. Burn severity dominates understory plant community response to fire in xeric jack pine forests. Forests 2016, 7, 83. [Google Scholar] [CrossRef]
- Johansson, P.; Reich, P. Population size and fire intensity determine post-fire abundance in grassland lichens. Appl. Veg. Sci. 2005, 8, 193–198. [Google Scholar] [CrossRef]
- Cichowski, D.; Haeussler., S. Lichen Dynamics on Terrestrial Lichen Sites 11–13 years Following Fire—Annual Report—2017/18; Habitat Conservation Trust Foundation: Victoria, BC, Canada; BC Parks and BC Ministry of Forests, Lands, Natural Resource Operations and Rural Development: Smithers, BC, Canada, 2018. [Google Scholar]
- Ruokolainen, L.; Salo, K. The effect of fire intensity on vegetation succession on a sub-xeric heath during years after wildfire. Ann. Bot. Fenn. 2009, 46, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Girard, F.; Payette, S.; Delwaide, A. Patterns of early postfire succession of alpine, subalpine and lichen-woodland vegetation: 21 years of monitoring from permanent plots. Forests 2017, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Loehman, R.; Saperstein, L.; Macander, M.; Nelson, P.; Paradis, D.; Natali, S. Multi-decadal patterns of vegetation succession after tundra fire on the Yukon-Kuskokwim Delta, Alaska. Environ. Res. Lett. 2020, 15, 025003. [Google Scholar] [CrossRef]
- Greuel, R. Abundance of forage lichens for boreal caribou in the Boreal Shield Ecozone of Saskatchewan. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Russell, K.; Johnson, C. Post-fire dynamics of terrestrial lichens: Implications for the recovery of woodland caribou winter range. For. Ecol. Manag. 2019, 434, 1–17. [Google Scholar] [CrossRef]
- Carroll, S.; Bliss, L. Jack Pine—Lichen Woodland on Sandy Soils in Northern Saskatchewan and Northeastern Alberta. Can. J. Bot. 1982, 60, 2270–2282. [Google Scholar] [CrossRef]
- Lesmerises, R.; Ouellet, J.M.R.; St-Laurent, M.-H. Assessing terrestrial lichen biomass using ecoforest maps: A suitable approach to plan conservation areas for forest-dwelling caribou. Can. J. For. Res. 2011, 41, 632–642. [Google Scholar] [CrossRef]
- Boudreault, C.; Drapeau, P.; Bouchard, M.; St-Laurent, M.-H.; Imbeau, L.; Bergeron, Y. Contrasting responses of epiphytic and terricolous lichens to variations in forest characteristics in northern boreal ecosystems. Can. J. For. Res. 2015, 45, 595–606. [Google Scholar] [CrossRef]
- Pharo, E.; Vitt, D. Local variation in bryophyte and macro-lichen cover and diversity in montane forests of western Canada. Bryologist 2000, 103, 455–466. [Google Scholar] [CrossRef]
- Haughian, S.; Burton, P. Microhabitat associations of lichens, feathermosses, and vascular plants in a caribou winter range, and their implications for understory development. Botany 2015, 92, 1–11. [Google Scholar] [CrossRef]
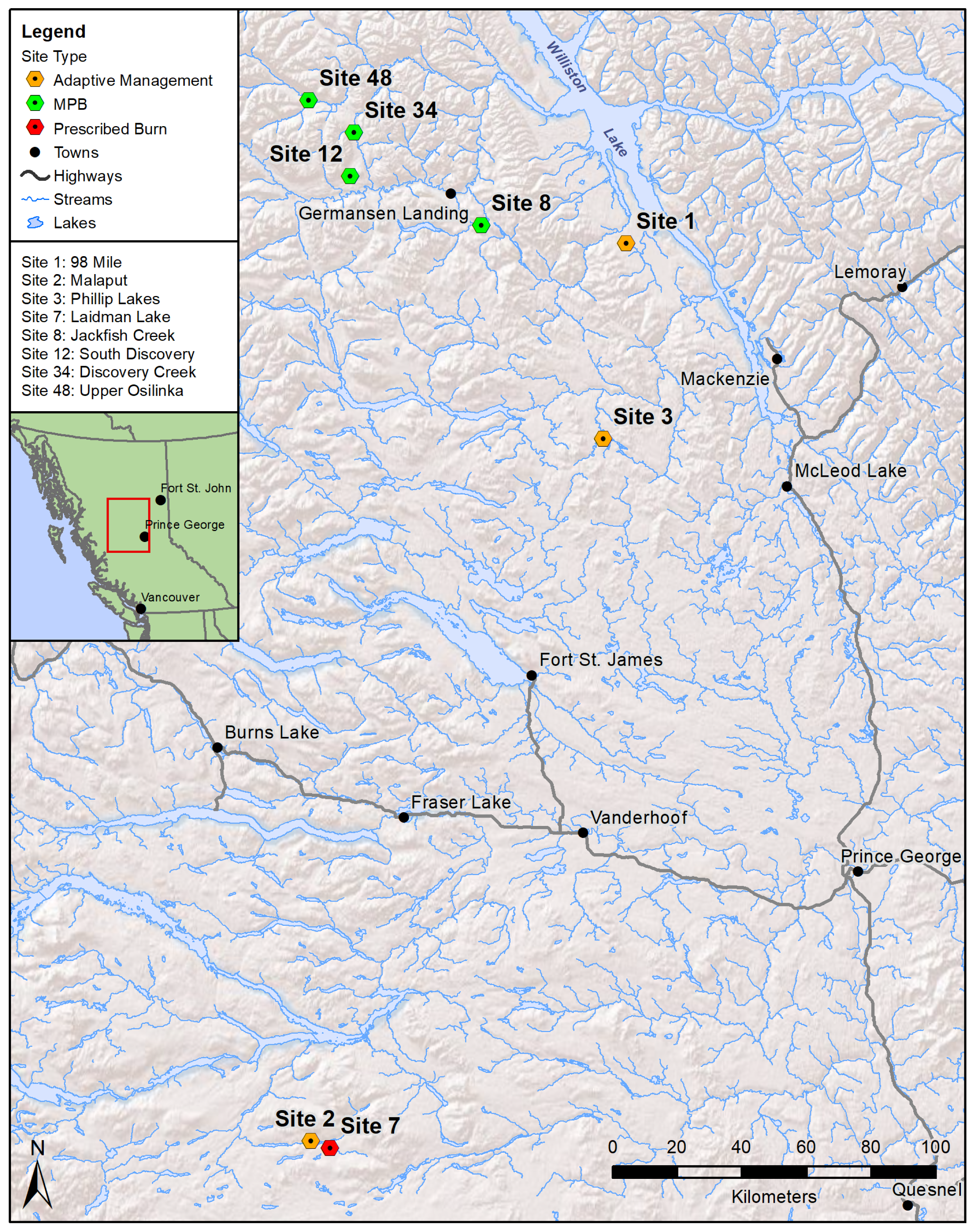

| Site | Name | BEC 1 | Successional Type 2 | Treatment | Trees (Average) | Sampling Session | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Disturbance | 2nd Disturbance | ||||||||||||
| Type | Year 3 | Type | Year 3 | Age (years) | dbh (cm) | Height (m) | 1 | 2 | 3 | ||||
| 1 | 98-Mile | SBSmk2 | transitional | harvest | 2002 | MPB 5 | 2009 | 148 | 16.7 | 14.8 | 2001 | 2003 | 2016 |
| 2 | Malaput 4 | SBSmc | transitional | MPB | 2001 | harvest | 2004 | 112 | 19.4 | 17.0 | 2001 | 2005 | 2017 |
| 3 | Phillip Lakes | SBSmk1 | edaphic | harvest | 2004 | MPB 5 | 2009 | 195 | 14.7 | 12.6 | 2002 | 2005 | 2016 |
| 7 | Laidman Lake | SBPSmc | transitional | MPB | 2002 | prescribed burn | 2009 | 103 | 17.6 | - | 2008 | 2010 | 2017 |
| 8 | Jackfish Creek 6 | BWBSdk | transitional | MPB | 2009 | - | 106 | 17.9 | 19.8 | - | 2009 | 2017 | |
| 12 | S. Discovery Creek | BWBSdk | transitional | MPB | 2011 | - | 114 | 15.0 | 17.8 | - | 2008 | 2016 | |
| 34 | Discovery Creek | BWBSdk | edaphic | MPB | 2011 | - | 126 | 11.5 | 11.5 | - | 2008 | 2016 | |
| 48 | Upper Osilinka | BWBSdk | edaphic | MPB | 2011 | - | 114 | 14.1 | 14.1 | - | 2008 | 2016 | |
| Trt 1 | Treatment Regime Code 2 | Harvesting Method | Harvesting Season | Site Preparation | Regeneration Method | Predicted Conditions for Lichens 3 | Sites Treated 4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Amount of Debris | Disturbance to Lichens | Regenerating Canopy | T | E | ||||||
| 1 | W-W-N-N | Whole tree | Winter | None | Natural | Best | Small | Low | More open | 1, 2 | 3 |
| 2 | C-W-N-N | Cut to length | Winter | None | Natural | Good | Large | Low | More open | 1 | 3 |
| 3 | C-S-N-N | Cut to length | Summer | None | Natural | Moderate | Large | Moderate | More open | 1 | 3 |
| 4 | C-S-N-P | Cut to length | Summer | None | Plant | Moderate | Large | Moderate | More closed | 1 | 3 |
| 5 | C-S-S-N | Cut to length | Summer | Drag scarify | Natural | Worst | Large | Very high | More open | 1 | 3 |
| 6 | W-S-N-N | Whole tree | Summer | None | Natural | Good | Small | High | More open | 1, 2 | 3 |
| 7 | W-S-N-P | Whole tree | Summer | None | Plant | Moderate | Small | High | More closed | 2 | |
| 8 | W-S-S-N | Whole tree | Summer | Drag scarify | Natural | Worst | Small | Very high | More open | 2 | |
| 9 | W-S-S-P | Whole tree | Summer | Drag scarify | Plant | Worst | Small | Very high | More closed | 2 | |
| 99 | No Harvest | NA5 | NA | NA | Natural | NA | NA | NA | NA | 1 | 3 |
| Question | Period 1 | Metric | ||
|---|---|---|---|---|
| I | R1 | R2 | ||
| 1. How well did lichens survive disturbance? | Change in lichen abundance | |||
| 2. By 12–14 years post-harvest, has lichen abundance recovered to pre-harvest levels? | Change in lichen abundance | |||
| 3. By 12–14 years post-harvest, how well are lichens recovering after harvest? | Annual rate of change in lichen abundance | |||
| Treatment 1 | 99 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Method 2 | MPB | W | C | C | C | C | W | W | W | W | B |
| Season 3 | - | W | W | S | S | S | S | S | S | S | - |
| Site prep 4 | - | N | N | N | N | S | N | N | S | S | - |
| Regen 5 | N | N | N | N | P | N | N | P | N | P | N |
| Site 1—98-Mile—Transitional (2016 = 14 years post-harvest) | |||||||||||
| R2 (2016) | 0.2 | 1.6 | 1.0 | 0.7 | 1.2 | 1.0 | 2.2 | ||||
| R1 (2003) | 1.2 | 0.5 | 0.2 | <0.1 | 0.2 | 0.1 | 0.8 | ||||
| I (2001) | 1.8 | 1.2 | 0.6 | 0.3 | 0.4 | 2.9 | 1.7 | ||||
| Change/year (R1 to R2) | −0.08 | 0.08 | 0.06 | 0.05 | 0.08 | 0.07 | 0.11 | ||||
| Change/year SE | 0.04 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | ||||
| Site 2—Malaput—Transitional (2017 = 13 years post-harvest) | |||||||||||
| R2 (2017) | 2.8 | 2.9 | 3.0 | 1.6 | 2.1 | ||||||
| R1 (2005) | 0.9 | 1.4 | 1.1 | 0.6 | 1.2 | ||||||
| I (2001) | 3.6 | 4.0 | 3.0 | 3.0 | 6.5 | ||||||
| Change/year (R1 to R2) | 0.16 | 0.12 | 0.16 | 0.08 | 0.08 | ||||||
| Change/year SE | 0.04 | 0.03 | 0.04 | 0.01 | 0.01 | ||||||
| Site 3—Phillip Lakes—Edaphic (2016 = 12 years post-harvest) | |||||||||||
| R2 (2016) | 19.5 | 13.6 | 14.5 | 9.3 | 7.9 | 2.8 | 5.0 | ||||
| R1 (2005) | 28.6 | 14.5 | 14.6 | 10.7 | 9.0 | 2.6 | 4.5 | ||||
| I (2002) | 35.9 | 34.1 | 45.9 | 28.7 | 37.4 | 31.0 | 29.7 | ||||
| Change/year (R1 to R2) | −0.83 | −0.08 | −0.01 | −0.14 | −0.10 | 0.03 | 0.05 | ||||
| Change/year SE | 0.12 | 0.09 | 0.11 | 0.12 | 0.08 | 0.05 | 0.09 | ||||
| Site 7—Laidman Lake—Transitional (2017 = 8 years post-burn) | |||||||||||
| R2 (2017) | 2.4 | 0 | |||||||||
| R1 (2010) | NA 6 | <0.1 | |||||||||
| I (2008) | 4.3 | 7.2 | |||||||||
| Site | Plot Establishment (I) | 2nd Re-Measurement (R2) | Years Since Attack | Change/Yr 1 (I to R2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name (No.) | BEC | Type | % MPB 2 | Year | Mean | SE | Year | Mean | SE | Mean | SE | |
| Phillip Lakes (3) | SBSmk1 | Edaphic | 55 | 2002 | 35.9 | 2.9 | 2016 | 19.5 | 1.8 | 14 | −1.18 | 0.06 |
| Discovery Creek (34) | BWBSdk1 | Edaphic | 20 | 2008 | 28.0 | 2.5 | 2016 | 26.4 | 2.1 | 8 | −0.21 | 0.15 |
| Upper Osilinka (48) | BWBSdk1 | Edaphic | 76 | 2008 | 43.0 | 3.8 | 2016 | 24.7 | 2.3 | 8 | −2.29 | 0.26 |
| South Discovery Creek (12) | BWBSdk1 | Trans. | 27 | 2008 | 7.4 | 1.8 | 2016 | 6.1 | 1.7 | 8 | −0.16 | 0.06 |
| 98-Mile (1) | SBSmk2 | Trans. | 54 | 2001 | 1.8 | 0.6 | 2016 | 0.2 | 0.2 | 16 | −0.10 | 0.04 |
| Laidman Lake (7) | SBPSmc | Trans. | 48 | 2008 | 4.3 | 1.1 | 2017 | 2.4 | 0.7 | 10 | −0.20 | 0.05 |
| Jackfish Creek (8) | BWBSdk1 | Trans. | 63 | 2009 | 8.7 | 0.8 | 2017 | 3.3 | 0.8 | 10 | −0.56 | 0.10 |
| Predictor 1 | Sampling Scale | Relative Influence 2 | Number of Interactions 3 |
|---|---|---|---|
| Years since 1st disturbance | treatment | 27.9 | 2 |
| Moss cover | quadrat | 13.3 | 3 |
| Total debris cover | treatment | 12.3 | 4 |
| Successional type | treatment | 10.2 | 5 |
| Density of regenerating trees (stems/ha) | treatment | 8.4 | N/A |
| Live tree density (stems/ha) | treatment | 7.3 | 2 |
| Years since 2nd disturbance | quadrat | 7.1 | N/A |
| Vascular plant cover | treatment | 6.1 | 2 |
| Exposed soil cover | treatment | 3.5 | 2 |
| Organic matter disturbance | quadrat | 2.8 | 0 |
| Type of 2nd disturbance | treatment | 0.5 | N/A |
| Disturbance regime | treatment | 0.4 | N/A |
| % overstory trees killed by MPB (stems/ha) | treatment | 0.1 | N/A |
| Type of 1st disturbance | treatment | <0.1 | N/A |
| Predictors | Successional Type | ||||||
|---|---|---|---|---|---|---|---|
| Harvesting Treatment | Edaphic (n = 247) 1 | Transitional (n = 472) | |||||
| No. 2 | Regime | Coefficient 3 | LCI 4 | UCI 4 | Coefficient 3 | LCI 4 | UCI 4 |
| Question 1: How well did forage lichens survive disturbance (i.e., absolute change in lichen abundance from pre-harvest to first re-measurement post-harvest)? | |||||||
| 5 5 | C-S-S-N | −8.25 | −16.35 | 0.53 | −1.15 | −2.35 | 0.25 |
| 1 | W-W-N-N | 0.80 | −1.42 | 3.22 | 0.32 | −0.01 | 0.67 |
| 2 | C-W-N-N | −1.52 | −3.67 | 0.67 | 0.27 | −0.09 | 0.65 |
| 3 | C-S-N-N | 2.34 | 0.13 | 4.66 | 0.29 | −0.06 | 0.67 |
| 4 | C-S-N-P | −0.09 | −2.26 | 2.10 | 0.31 | −0.05 | 0.70 |
| 6 | W-S-N-N | 0.29 | −0.06 | 0.67 | 0.34 | −0.01 | 0.68 |
| 7 | W-S-N-P | - | - | - | 0.59 | 0.24 | 1.08 |
| 8 | W-S-S-N | - | - | - | 0.22 | −0.19 | 0.67 |
| 9 | W-S-S-P | - | - | - | 0.04 | −0.37 | 0.46 |
| Question 2: By 12–14 years post-harvest, has forage lichen abundance recovered to pre-harvest levels (i.e., absolute change in lichen abundance from pre-harvest to second re-measurement post-harvest)? | |||||||
| 5 | C-S-S-N | −1.93 | −4.99 | 1.21 | −0.12 | −0.86 | 1.48 |
| 1 | W-W-N-N | 0.40 | −0.18 | 0.98 | −0.08 | −0.02 | 0.18 |
| 2 | C-W-N-N | −0.24 | −0.81 | 0.33 | 0.07 | −0.04 | 0.18 |
| 3 | C-S-N-N | 0.53 | −0.05 | 1.12 | 0.06 | −0.05 | 0.17 |
| 4 | C-S-N-P | −0.10 | −0.66 | 0.47 | 0.12 | 0.01 | 0.25 |
| 6 | W-S-N-N | 0.17 | −0.41 | 0.74 | 0.03 | −0.10 | 0.16 |
| 7 | W-S-N-P | - | - | - | 0.23 | 0.09 | 0.37 |
| 8 | W-S-S-N | - | - | - | 0.08 | −0.02 | 0.18 |
| 9 | W-S-S-P | - | - | - | −0.05 | −0.18 | 0.08 |
| Question 3: By 12–14 years post-harvest, how well are forage lichens recovering after harvest (i.e., annual rate of change in lichen abundance from the first re-measurement post-harvest to the second re-measurement post-harvest)? | |||||||
| 5 | C-S-S-N | −0.04 | −2.94 | 3.06 | 0.22 | −0.49 | 0.80 |
| 1 | W-W-N-N | 0.02 | −0.22 | 0.24 | 0.01 | −0.03 | 0.06 |
| 2 | C-W-N-N | 0.10 | −0.14 | 0.34 | −0.01 | −0.05 | 0.05 |
| 3 | C-S-N-N | −0.08 | −0.31 | 0.15 | −0.02 | −0.07 | 0.04 |
| 4 | C-S-N-P | −0.03 | −0.27 | 0.19 | −0.00 | −0.05 | 0.05 |
| 6 | W-S-N-N | −0.02 | −0.22 | 0.24 | −0.00 | −0.05 | 0.05 |
| 7 | W-S-N-P | - | - | - | −0.01 | −0.07 | 0.05 |
| 8 | W-S-S-N | - | - | - | 0.02 | −0.03 | 0.08 |
| 9 | W-S-S-P | - | - | - | −0.01 | −0.07 | 0.05 |
| Predictors | Successional Type | ||||||
|---|---|---|---|---|---|---|---|
| Harvest Method Components | Edaphic (n = 247) | Transitional (n = 472) | |||||
| Coefficient 1 | LCI 2 | UCI 2 | Coefficient 1 | LCI 2 | UCI 2 | ||
| Question 1: How well did forage lichens survive disturbance, i.e., absolute change in lichen abundance from pre-harvest to first re-measurement post-harvest)? | |||||||
| (intercept) 3 | −8.35 | −16.62 | −0.24 | −1.13 | −2.33 | 0.39 | |
| Method | Whole tree (W) | −0.04 | −1.58 | 1.55 | 0.07 | −0.17 | 0.34 |
| Season | Winter (W) | −1.46 | −3.03 | 0.11 | −0.05 | −0.27 | 0.17 |
| Site Prep | None (N) | 1.15 | −0.83 | 3.10 | 0.29 | 0.10 | 0.48 |
| Regeneration | Plant (P) | −1.20 | −3.23 | 0.75 | 0.02 | −0.17 | 0.23 |
| Question 2: By 12–14 years post-harvest, has forage lichen abundance recovered to pre-harvest levels (i.e., absolute change in lichen abundance from pre-harvest to second re-measurement post-harvest)? | |||||||
| (intercept) 3 | −1.90 | −5.00 | 1.22 | −0.19 | −0.81 | 0.41 | |
| Method | Whole tree (W) | 0.15 | −0.26 | 0.55 | 0.02 | −0.05 | 0.09 |
| Season | Winter (W) | −0.26 | −0.66 | 0.13 | −0.05 | −0.12 | 0.01 |
| Site Prep | None (N) | −0.25 | −0.78 | 0.28 | 0.14 | 0.08 | 0.20 |
| Regeneration | Plant (P) | −0.35 | −0.87 | 0.18 | 0.01 | −0.05 | 0.07 |
| Question 3: By 12–14 years post-harvest, how well are forage lichens recovering after harvest (i.e., annual rate of change in lichen abundance from the first re-measurement post-harvest to the second re-measurement post-harvest)? | |||||||
| (intercept) 3 | −0.07 | −3.07 | 2.98 | 0.15 | −0.39 | 1.04 | |
| Method | Whole tree (W) | 0.04 | −0.12 | 0.20 | 0.01 | −0.02 | 0.04 |
| Season | Winter (W) | 0.05 | −0.12 | 0.21 | 0.01 | −0.02 | 0.04 |
| Site Prep | None (N) | −0.01 | −0.23 | 0.20 | −0.01 | −0.04 | 0.02 |
| Regeneration | Plant (P) | −0.04 | −0.26 | 0.19 | −0.01 | −0.04 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichowski, D.; Sutherland, G.D.; McNay, R.S.; Sulyma, R. Direct and Indirect Effects of Habitat Disturbances on Caribou Terrestrial Forage Lichens in Montane Forests of British Columbia. Forests 2022, 13, 251. https://doi.org/10.3390/f13020251
Cichowski D, Sutherland GD, McNay RS, Sulyma R. Direct and Indirect Effects of Habitat Disturbances on Caribou Terrestrial Forage Lichens in Montane Forests of British Columbia. Forests. 2022; 13(2):251. https://doi.org/10.3390/f13020251
Chicago/Turabian StyleCichowski, Deborah, Glenn D. Sutherland, R. Scott McNay, and Randy Sulyma. 2022. "Direct and Indirect Effects of Habitat Disturbances on Caribou Terrestrial Forage Lichens in Montane Forests of British Columbia" Forests 13, no. 2: 251. https://doi.org/10.3390/f13020251





