Phenotypic Comparison of Three Populations of Juniperus turbinata Guss. in North-Eastern Morocco
Abstract
1. Introduction
2. Materials and Methods
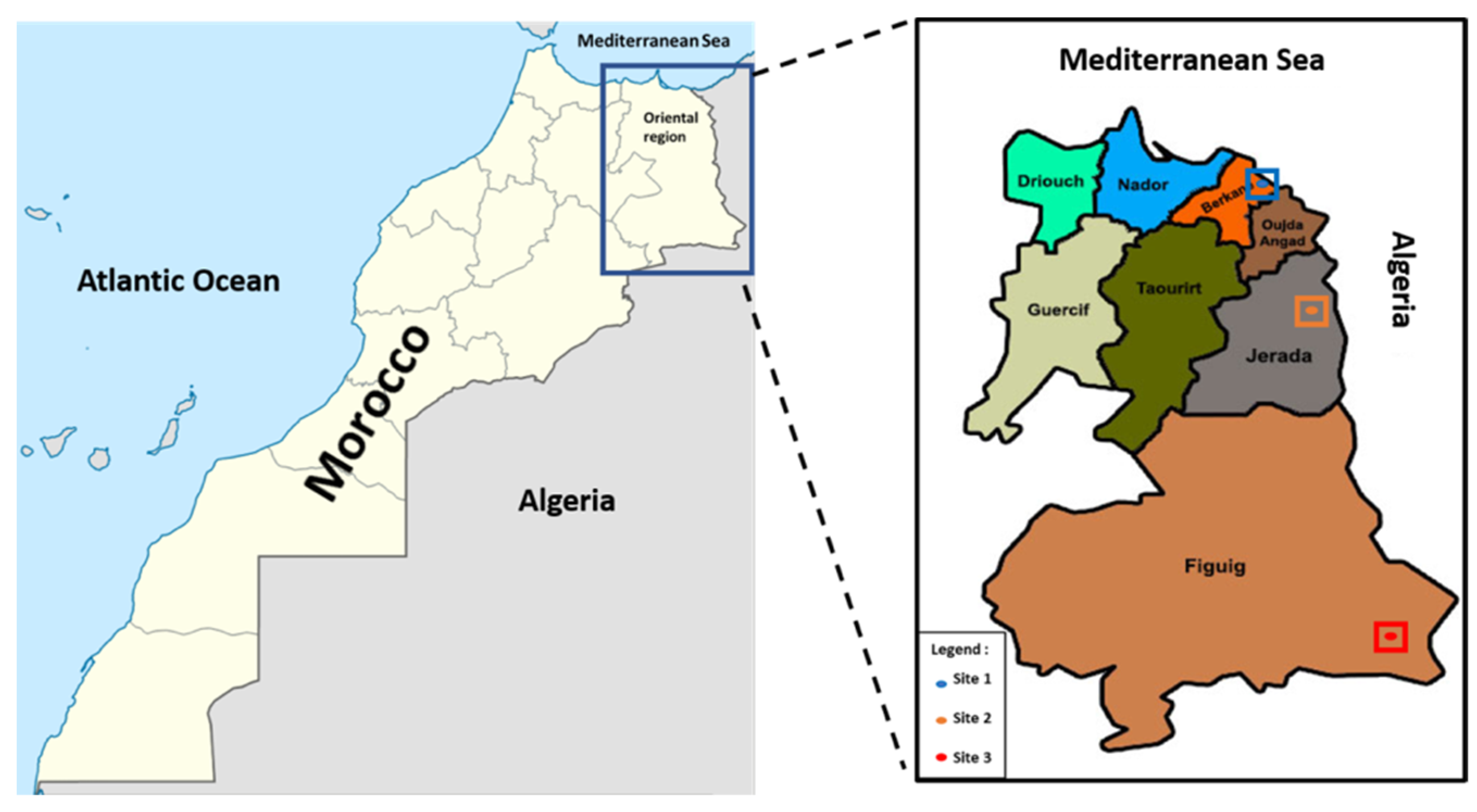
2.1. Plant Material
2.2. Climate Assessment
2.3. Data Analysis
3. Results
3.1. Assessment of Characters
3.2. Differences between Populations
3.2.1. Descriptive Statistics and ANOVA
3.2.2. Discriminant Canonical Analysis
4. Discussion
4.1. Taxonomic Classification and Differences between the Three Populations
4.2. Influence of Abiotic Factors Related to Biogeographical Pattern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farjon, A. A Handbook of the World’s Conifers; EJ Brill: Leiden, The Netherlands; Boston, MA, USA, 2010; 1073p. [Google Scholar]
- Romo, A.; Mazur, M.; Salva-Catarineu, M.; Boraty’nski, A. A re-evaluated taxon: Genetic values and phenotypical characters support the recognition of the Canary Island juniper of the phoenicea group at a specific level. Phytotaxa 2019, 40, 64–70. [Google Scholar] [CrossRef]
- Quézel, P.; Gast, M. Genévriers. In Encyclopédie Berbère; online; Peeters Publishers: Louvain, Belgium, 1998; p. 20. [Google Scholar] [CrossRef]
- Quézel, P.; Médail, F. Écologie et Biogéographie des Forêts du Bassin Méditerranéen; Éditions scientifiques; Elsevier: Amsterdam, The Netherlands, 2003; 574p. [Google Scholar]
- Benabid, A.; Fennane, M. Connaissance sur la Végétation du Maroc. Phytogéographie, Phytosociologie et séries de végétation. Lazaroa 1994, 14, 21–97. [Google Scholar]
- Benabid, A. Grands écosystèmes naturels marocains, équilibre de fonctionnement, Perturbation, préservation et restauration. In Grande Encyclopédie du Maroc; GEM: Rabat, Morocco, 1986; pp. 117–190. [Google Scholar]
- Medail, F.; Quezel, P. The phytogeographical significance of S.W. Morocco compared to the Canary Islands. Plant Ecol. 1999, 140, 221–244. [Google Scholar] [CrossRef]
- Adams, R.P. Junipers of the World: The genus Juniperus, 4th ed.; Trafford Publishing: Trafford, UK, 2014. [Google Scholar]
- Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore Pratique du Maroc; Institut scientifique, Université Mohammed V: Rabat, Morocco, 1999; Volume 1, 558p. [Google Scholar]
- Valdés, B.; Rejdali, M.; EI Kadmiri, A.A.; Jury, J.L.; Montserrat, J.M. Catalogue des Plantes Vasculaires du Nord du Maroc, Incluant des Clés D’identification; Consejo Superior de Investigaciones: Madrid, Spain, 2002; Volume 1, 497p. [Google Scholar]
- Benabid, A. Flore et Ecosystèmes du Maroc. Évaluation et Préservation de la Biodiversité; Ibis Press: Paris, France; Librairies et éditions Kalila Wa Dimna: Rabat, Morocco, 2000; 359p. [Google Scholar]
- Adams, R.P.; Nguyen, S.; Achak, N. Geographic variation in Juniperus phoenicea (Cupressaceae) from the Canary Islands, Morocco and Spain based on RAPDs analysis. Phytologia 2006, 88, 270–278. [Google Scholar]
- Mazur, M.; Klajbor, K.; Kielich, M.; Sowińska, M.; Romo, A.; Montserrat, J.M.; Boratyński, A. Intra-specific differentiation of Juniperus phoenicea in the western Mediterranean region revealed in morphological multivariate analysis. Dendrobiology 2010, 63, 21–31. [Google Scholar]
- Dzialuk, A.; Mazur, M.; Boratyńska, K.; Montserrat, J.M.; Romo, A.; Boratyński, A. Population genetic structure of Juniperus phoenicea (Cupressaceae) in the western Mediterranean Basin: Gradient of diversity on a broad geographical scale. Ann. Sci. 2011, 68, 1341–1350. [Google Scholar] [CrossRef]
- Mazur, M.; Boratynska, K.; Marcysiak, K.; Gomez, D.; Tomaszewski, D.; Didukh, Y.; Boratynski, A. Phenotypical variability of Juniperus phoenicea (Cupressaceae) from three distant localities on Iberian Peninsula. Acta Soc. Bot. Pol. 2003, 72, 71–78. [Google Scholar] [CrossRef]
- Pavon, D.; Véla, E.; Médail, F. Are Meditterranean trees well known? Juniperus turbinata (Cupressaceae) a common but misunderstood taxons. Ecol. Med. 2021, 46, 77–104. [Google Scholar]
- Sánchez-Gómez, P.; Jiménez, J.F.; Cánovas, J.L. Genetic structure and phylogeography of Juniperus phoenicea complex throughout Mediterranean and Macaronesian regions: Different stories in one. Ann. For. Sci. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Montserrat, S.C.; Angel, R.; Małgorzata, M.; Zielińska, M.; Minissale, P.; Dönmez, A.A.; Boratyńska, K.; Boratyński, A. Past, present, and future geographic range of the relict Mediterranean and Macaronesian Juniperus phoenicea complex. Ecol. Evol. 2021, 11, 5075–5095. [Google Scholar] [CrossRef]
- Maire, R. Flore de l’Afrique du Nord; Paul Lechevalier Editore: Paris, France, 1952; Volume 1, pp. 114–115. [Google Scholar]
- Lebreton, P.; Thivend, S. Sur une sous-espèce du Genévrier de Phénicie Juniperus phoenicea L., définie à partir de critères biochimiques Naturalia monspeliensia Sér: Université de Montpelier, France. Bot 1981, 45, 1–12. [Google Scholar]
- Fennane, M.; Ibn Tattou, M. Flore Vasculaire du Maroc. Inventaire et Chorologie; Institut scientifique, Université Mohammed V: Rabat, Morocco, 2005; Volume 1, 483p. [Google Scholar]
- Lebreton, P.; Pérez de Paz, P.L. Définition du genévrier de Phénicie (Juniperus phoenicea), reconsidéré à ses limites biogéographiques: Méditerranée orientale (Crète et Chypre) et Atlantiques (Îles Canaries). Bull. Mens. Soc. linn. Lyon. 2001, 70, 73–92. [Google Scholar] [CrossRef]
- Camarero, J.J.; Valeriano, C.; Gazol, A.; Colangelo, M.; Sánchez-Salguero, R. Climate Differently Impacts the Growth of Coexisting Trees and Shrubs under Semi-Arid Mediterranean Conditions. Forests 2021, 12, 381. [Google Scholar] [CrossRef]
- García Morote, F.A.; Andrés Abellán, M.; Rubio, E.; Pérez Anta, I.; García Saucedo, F.; López Serrano, F.R. Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain. Forests 2021, 12, 1340. [Google Scholar] [CrossRef]
- Goldstein, G.M.; Simonetti, M. Watschinger, Guida al Riconoscimento Delgi Alberi d’Europa; Arnold Mondadori Editore: Milano, Italy, 1985. [Google Scholar]
- Farjon, A. World Checklist and Bibliography of Conifers, 2nd ed.; Kew: The Royal Botanic Gardens; The University of Chicago Press: Chicago, IL, USA, 2001; 316p. [Google Scholar]
- Kavetsou, E.; Pitterou, I.; Katopodi, A.; Petridou, G.; Adjali, A.; Grigorakis, S.; Detsi, A. Preparation, Characterization, and Acetylcholinesterase Inhibitory Ability of the Inclusion Complex of β-Cyclodextrin–Cedar (Juniperus phoenicea) Essential Oil. Micro 2021, 1, 250–266. [Google Scholar] [CrossRef]
- Acuña-Míguez, B.; Valladares, F.; Martín-Forés, I. Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons. Forests 2020, 11, 960. [Google Scholar] [CrossRef]
- Marcysiak, K.; Mazur, M.; Romo, A.; Montserrat, J.M.; Didukh, Y.; Boratyńska, K.; Jasińska, A.; Kosiński, P.; Boratyński, A. Numerical taxonomy of Juniperus thurifera, J. excelsa and J. foetidissima (Cupressaceae) basedon phenotypical characters. Bot. J. Linn. Soc. 2007, 155, 483–495. [Google Scholar] [CrossRef]
- Stewart, P. Quotient pluviothermique et dégradation biosphérique. Bull. Soc. Hist. Nat. Afr. Nord. 1969, 59, 23–36. [Google Scholar]
- Daget, P. Le bioclimat méditerranéen, caractères généraux, méthodes de classification. Vegetatio 1977, 34, 1–20. [Google Scholar] [CrossRef]
- Rivas-Martinez, S.; Mapa de Series, Geoseries y Geopermaseries de Vegetación de España. Memoria del Mapa de Vegetación Potencial de España. Partie I. 2005. Available online: http://www.globalbioclimatics.org (accessed on 18 July 2021).
- Le Houérou, H.N. An agro-bioclimatic classification of arid and semi-arid lands in the isoclimatic Mediterranean zones. Arid Land Res. Manag. 2004, 18, 301–346. [Google Scholar] [CrossRef]
- Mokhtari, N.; Mrabet, R.; Lebailly, P.; Bock, L. Spatialisation des bioclimats, de l’aridité et des étages de végétation du Maroc. Rev. Mar. Sci. Agron. Vét. 2013, 2, 50–66. [Google Scholar]
- Mazur, M.; Minissale, P.; Sciandrello, S.; Boratyński, A. Morphological and ecological comparison of populations of Juniperus turbinata Guss. and J. phoenicea L. from the Mediterranean region. Plant Biosyst. 2016, 150, 313–322. [Google Scholar] [CrossRef]
- Adams, R.P.; Boratynski, A.; Arista, M.; Schwarzbach, A.E.; Leschner, H.; Liber, Z.; Manolis, A. Analysis of Juniperus phoenicea from throughout its range in the Mediterranean using DNA sequence data from nrDNA and petN-psbM: The case for the recognition of J. turbinate Guss. Biol. Veg. Ecol. 2013, 95, 202–209. [Google Scholar]
- Boulli, A.; Baaziz, M.; M’Hirit, O. Polymorphism of natural populations of Pinus halepensis Mill. in Morocco as revealed by morphological characters. Euphytica 2001, 119, 309–316. [Google Scholar] [CrossRef]
- Panetsos, K.P. Natural hybridization between Pinus halpensis and Pinus brutia in Greece. Silvae Genet. 1975, 24, 163–168. [Google Scholar]
- Matziris, D. Genetic variation in cone and seed characteristics in a clonal seed orchard of Aleppo pine grown in Greece. Silvae Genet. 1998, 47, 37–41. [Google Scholar]
- Debazac, E.-F.; Tomassone, R. Contribution à une étude comparée des Pins Mediterranéens de la Section Halepensis. Ann. Sci Nancy 1965, 21, 213–256. [Google Scholar] [CrossRef]
- Mazur, M.; Zielińska, M.; Boratyńska, K.; Romo, A.; Salva-Catarineu, M.; Marcysiak, K.; BoratyŃski, A. Taxonomic and geographic differentiation of Juniperus phoenicea agg. based on cone, seed, and needle characteristics. Syst. Biodivers. 2018, 16, 469–482. [Google Scholar] [CrossRef]
- Meloni, M.; Perini, D.; Filigheddu, R.; Binelli, G. Genetic Variation in Five Mediterranean Populations of Juniperus phoenicea as Revealed by Inter-Simple Sequence Repeat (ISSR) Markers. Ann. Bot. 2006, 97, 299–304. [Google Scholar] [CrossRef]
- Boratynski, A.; Lewandowski, A.; Boratyn´ska, K.; Montserrat, J.M.; Romo, A. High level of genetic differentiation of Juniperus phoenicea (Cupressaceae) in the Mediterranean region: Geographic implications. Plant Syst. Evol. 2009, 277, 163–172. [Google Scholar] [CrossRef]
- Mao, K.; Hao, G.; Liu, J.Q.; Adams, R.P.; Milne, R.I. Diversification and biogeography of Juniperus (Cupressaceae): Variable diversification rates and multiple intercontinental dispersals. New Phytol. 2010, 188, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Eliades, N.G.H.; Aravanopoulos, F.A.; Christou, A.K. Mediterranean Islands Hosting Marginal and Peripheral Forest Tree Populations: The Case of Pinus brutia Ten. in Cyprus. Forests 2018, 9, 514. [Google Scholar] [CrossRef]
- Fady, B.; Conord, C. Macroecological patterns of species and genetic diversity in vascular plants of the Mediterranean basin. Divers. Distrib. 2010, 16, 53–64. [Google Scholar] [CrossRef]
- Aussenac, G. Effets de conditions microclimatiques différentes sur la morphologie et la structure anatomique des aiguilles de quelques résineux. Ann. Sci. For. 1973, 30, 375–392. [Google Scholar] [CrossRef][Green Version]
- Quézel, P.; Barbero, M. Contribution à l’étude des formations présteppiques à genévriers au Maroc. Bol. Soc. Ser. 1981, 2, 1137–1160. [Google Scholar]
- Sahib, N. How species colonise gaps after soil in temporary ponds? Implication of species traits. J. Water Land Dev. 2018, 38, 137–145. [Google Scholar] [CrossRef][Green Version]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbo, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Klimko, M.; Boratynska, K.; Montserrat, J.M.; Didukh, Y.; Romo, A.; Gomez, D.; Boratynski, A. Morphological variation of Juniperus oxycedrus subsp. oxycedrus (Cupressaceae) in the Mediterranean region. Flora-Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 133–147. [Google Scholar] [CrossRef]

| Aridity | Continentality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Altitude (m.a.s.l) | Latitude | Longitude | M (°C) | m (°C) | P (mm) | Q2 | Bioclimates | Ic | Type | Subtypes |
| Site 1 Coastline | 4 | 35°11′ | 2°28′ | 31.83 | 7.03 | 315.04 | 44.4 | Semi-arid hot winter | 24.80 | Continental | Subcontinental |
| Site 2 Semi-continental | 951 | 34°41′ | 1°88′ | 33.20 | 0.14 | 359.16 | 37.9 | Semi-arid cool winter | 33.06 | Continental | Eucontinental attenuate |
| Site 3 Continental | 1500 | 32°22′ | 1°66′ | 41.31 | 2.02 | 12.15 | 12.6 | Saharan cool winter | 39.29 | Continental | Eucontineantal accentuated |
| Characters | Total | Coastline | Semi-Continental | Continental | Signification |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| (1) Recta number of cones (4 or 6) | 4 ± 0.021 | 4.02 ± 0.80 a | 4.05 ± 0.50 b | 4.02 ± 0.24 b | *** |
| (2) Length of cone (mm) | 7.82 ± 0.040 | 8.78 ± 0.91 b | 7.94 ± 0.86 b | 6.73 ± 0.58 a | *** |
| (3) Diameter of cone (mm) “average of 2 independent measures of angle of 90°” | 7.05 ± 0.036 | 8.02 ± 0.77 c | 7.08 ± 0.6 b | 6.02 ± 0.56 a | *** |
| (4) Number of scales of cone | 6.76 ± 0.038 | 6.77 ± 1.31 b | 7.22 ± 1.04 c | 6.27 ± 0.73 a | *** |
| (5) Number of leaves per 5mm section of ultimate lateral branchlet | 22.45 ± 0.106 | 19.85 ± 1.81 a | 24.72 ± 2.32 c | 22.78 ± 2.85 b | *** |
| (6) Thickness of the ultimate lateral branchlet and leaves (mm) | 1.12 ± 0.004 | 1.19 ± 0.14 c | 1.09 ± 0.11 b | 1.06 ± 0.11 a | *** |
| (7) Number of seeds | 4.84 ± 0.048 | 3.86 ± 1.33 a | 5.94 ± 1.06 c | 4.71 ± 0.92 b | *** |
| (8) Length of seeds (mm) | 4.01 ± 0.025 | 4.76 ± 0.55 c | 3.82 ± 0.49 b | 3.42 ± 0.42 a | *** |
| (9) Width of seeds(mm) | 1.85 ± 0.014 | 2.05 ± 0.44 | 1.77 ± 0.29 | 1.73 ± 0.41 | ns |
| (10) Length/diameter (of cone) | 1.12 ± 0.004 | 1.09 ± 0.11 | 1.12 ± 0.1 | 1.12 ± 0.14 | ns |
| (11) Length/width (of seed) | 2.21 ± 0.013 | 2.39 ± 0.43 c | 2.19 ± 0.36 b | 2.03 ± 0.33 a | *** |
| (12) Cone diameter/number-of-seeds | 1.63 ± 0.024 | 2.33 ± 0.82 b | 1.22 ± 0.25 a | 1.32 ± 0.27 a | *** |
| (13) Cone diameter/width-of-seeds | 3.92 ± 0.026 | 4.05 ± 0.8 b | 4.08 ± 0.74 b | 3.61 ± 0.71 a | *** |
| (14) Thickness-of-branchlet/number-of-leaves | 0.05 ± 0.0003 | 0.60 ± 0.009 c | 0.04 ± 0.006 a | 0.05 ± 0.009 b | *** |
| (15) Cone diameter/recta-number-of-cone | 1.89 ± 0.021 | 2.39 ± 0.8 c | 1.77 ± 0.32 b | 1.50 ± 0.15 a | *** |
| (16) Cone length/number-of-leaves | 0.36 ± 0.0028 | 0.44 ± 0.05 c | 0.32 ± 0.04 b | 0.30 ± 0.5 a | *** |
| (17) Cone number-of-scales/cone length | 0.88 ± 0.0057 | 0.78 ± 0.16 a | 0.91 ± 0.16 b | 0.93 ± 0.13 b | *** |
| Characters | Total | Coas | Smc | Con | p Value of Tukey t-Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | CV (%) | Min | Max | CV (%) | Min | Max | CV (%) | Min | Max | CV (%) | Coas/ Smc | Coas/ Con | Smc/ Con | |
| Recta number of cones (4 or 6) | 4 | 6 | 15.82 | 4 | 6 | 22.99 | 4 | 6 | 12.35 | 4 | 6 | 5.97 | 0.000 | 0.000 | 0.79 |
| Length of cone (mm) | 5 | 15 | 14.83 | 5.70 | 11.50 | 10.36 | 5 | 15 | 10.83 | 5.50 | 9.5 | 8.62 | 0.000 | 0.000 | 0.000 |
| Diameter of cone (mm) “average of 2 independent measures of angle of 90°” | 2.2 | 10 | 14.96 | 6 | 10 | 9.60 | 5.6 | 8.80 | 9.04 | 2.2 | 7.8 | 9.30 | 0.000 | 0.000 | 0.000 |
| Number of scales of cone | 4 | 13 | 16.67 | 4 | 13 | 19.35 | 4 | 9 | 14.40 | 5 | 9 | 1.64 | 0.000 | 0.000 | 0.000 |
| Number of leaves per 5 mm section of ultimate lateral branchlet | 10 | 29 | 13.80 | 15 | 24 | 9.12 | 19 | 29 | 9.39 | 10 | 28 | 12.52 | 0.000 | 0.000 | 0.000 |
| Thickness of the ultimate lateral branchlet and leaves (mm) | 0.13 | 1.66 | 12.06 | 0.85 | 1.66 | 11.76 | 0.13 | 1.43 | 10.09 | 0.74 | 1.41 | 10.38 | 0.000 | 0.000 | 0.015 |
| Number of seeds | 2 | 9 | 29.09 | 2 | 9 | 34.46 | 3 | 9 | 17.85 | 2 | 7 | 19.53 | 0.000 | 0.000 | 0.000 |
| Length of seeds (mm) | 2.18 | 6 | 18.70 | 2.4 | 6 | 11.55 | 2.47 | 5.52 | 12.83 | 2.18 | 5.12 | 12.28 | 0.000 | 0.000 | 0.000 |
| Width of seeds(mm) | 0.88 | 6.3 | 22.29 | 1.13 | 5.16 | 21.46 | 0.88 | 2.58 | 16.38 | 0.88 | 6.30 | 23.70 | 0.000 | 0.000 | 0.39 |
| Length/diameter (of cone) | 0.77 | 3.18 | 10.68 | 0.78 | 1.57 | 9.17 | 0.77 | 1.76 | 8.93 | 0.92 | 3.18 | 12.50 | 0.025 | 0.027 | 1 |
| Length/width (of seed) | 0.56 | 4 | 17.92 | 0.91 | 3.83 | 17.15 | 1.42 | 4 | 15.53 | 0.56 | 3.09 | 16.26 | 0.000 | 0.000 | 0.000 |
| Cone diameter/number-of-seeds | 0.37 | 4.5 | 44.19 | 0.94 | 4.50 | 35.19 | 0.76 | 2.33 | 19.67 | 0.37 | 2.90 | 20.45 | 0.000 | 0.000 | 0.071 |
| Cone diameter/width-of-seeds | 0.83 | 7.02 | 19.76 | 1.55 | 6.67 | 19.75 | 2.6 | 7.02 | 17.40 | 0.83 | 5.71 | 19.67 | 0.97 | 0.000 | 0.000 |
| Thickness-of-branchlet/number-of-leaves | 0.01 | 0.09 | 21.29 | 0.04 | 0.09 | 15.00 | 0.01 | 0.06 | 15.00 | 0.03 | 0.09 | 22.50 | 0.000 | 0.000 | 0.000 |
| Cone diameter/recta-number-of-cone | 0.55 | 4.5 | 33.38 | 1.33 | 4.50 | 33,47 | 1.08 | 4 | 18.08 | 0.55 | 2.37 | 10.00 | 0.000 | 0.000 | 0.000 |
| Cone length/number-of-leaves | 0.19 | 0.68 | 23.27 | 0.32 | 0.63 | 11,36 | 0.19 | 0.68 | 12.50 | 0.21 | 0.65 | 16.67 | 0.000 | 0.000 | 0.000 |
| Cone number-of-scales/cone length | 0.44 | 1.6 | 19.09 | 0.44 | 1.57 | 20,51 | 0.53 | 1.6 | 16.48 | 0.67 | 1.53 | 13.98 | 0.000 | 0.000 | 0.26 |
| Characters | Discrimination Power | ||
|---|---|---|---|
| Wilks’ Lambda | F | p | |
| Recta number of cones (4 or 6) | 0.73 | 14.83 | 0.000 |
| Number of scales of cone | 0.76 | 16.57 | 0.000 |
| Number of leaves per 5mm section of ultimate lateral shoot | 0.2 | 160.8 | 0.000 |
| Thickness of the ultimate lateral shoot and leaves (mm) | 0.51 | 37.61 | 0.000 |
| Number of seeds | 0.12 | 283.26 | 0.000 |
| Width of seeds(mm) | 0.63 | 23.19 | 0.000 |
| Length/diameter (of cone) | 0.91 | 3.37 | 0.028 |
| Length/width (of seed) | 0.57 | 30.00 | 0.000 |
| Cone diameter/number-of-seeds | 0.06 | 547.6 | 0.000 |
| Cone diameter/width-of-seeds | 0.73 | 15.01 | 0.000 |
| Thickness-of-shoot/number-of-leaves | 0.19 | 172.6 | 0.000 |
| Cone diameter/recta number of cone | 0.37 | 67.74 | 0.000 |
| Cone length/number-of leaves | 0.09 | 371.43 | 0.000 |
| Cone number-of-scales/cone length | 0.55 | 32.09 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahib, N.; Boumediene, M.; Abid, M.; Mihamou, A.; Serghini-Caid, H.; Elamrani, A.; Hano, C.; Addi, M. Phenotypic Comparison of Three Populations of Juniperus turbinata Guss. in North-Eastern Morocco. Forests 2022, 13, 287. https://doi.org/10.3390/f13020287
Sahib N, Boumediene M, Abid M, Mihamou A, Serghini-Caid H, Elamrani A, Hano C, Addi M. Phenotypic Comparison of Three Populations of Juniperus turbinata Guss. in North-Eastern Morocco. Forests. 2022; 13(2):287. https://doi.org/10.3390/f13020287
Chicago/Turabian StyleSahib, Nargis, Mehdi Boumediene, Malika Abid, Aatika Mihamou, Hana Serghini-Caid, Ahmed Elamrani, Christophe Hano, and Mohamed Addi. 2022. "Phenotypic Comparison of Three Populations of Juniperus turbinata Guss. in North-Eastern Morocco" Forests 13, no. 2: 287. https://doi.org/10.3390/f13020287
APA StyleSahib, N., Boumediene, M., Abid, M., Mihamou, A., Serghini-Caid, H., Elamrani, A., Hano, C., & Addi, M. (2022). Phenotypic Comparison of Three Populations of Juniperus turbinata Guss. in North-Eastern Morocco. Forests, 13(2), 287. https://doi.org/10.3390/f13020287







