Changes within a South Carolina Coastal Wetland Forest in the Face of Rising Sea Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Depth and Salinity
2.3. Woody Growth
2.4. Litterfall and Aboveground Net Primary Production
2.5. Vascular Plant Collection
2.6. Statistical Analysis
3. Results
3.1. Water Depth and Salinity
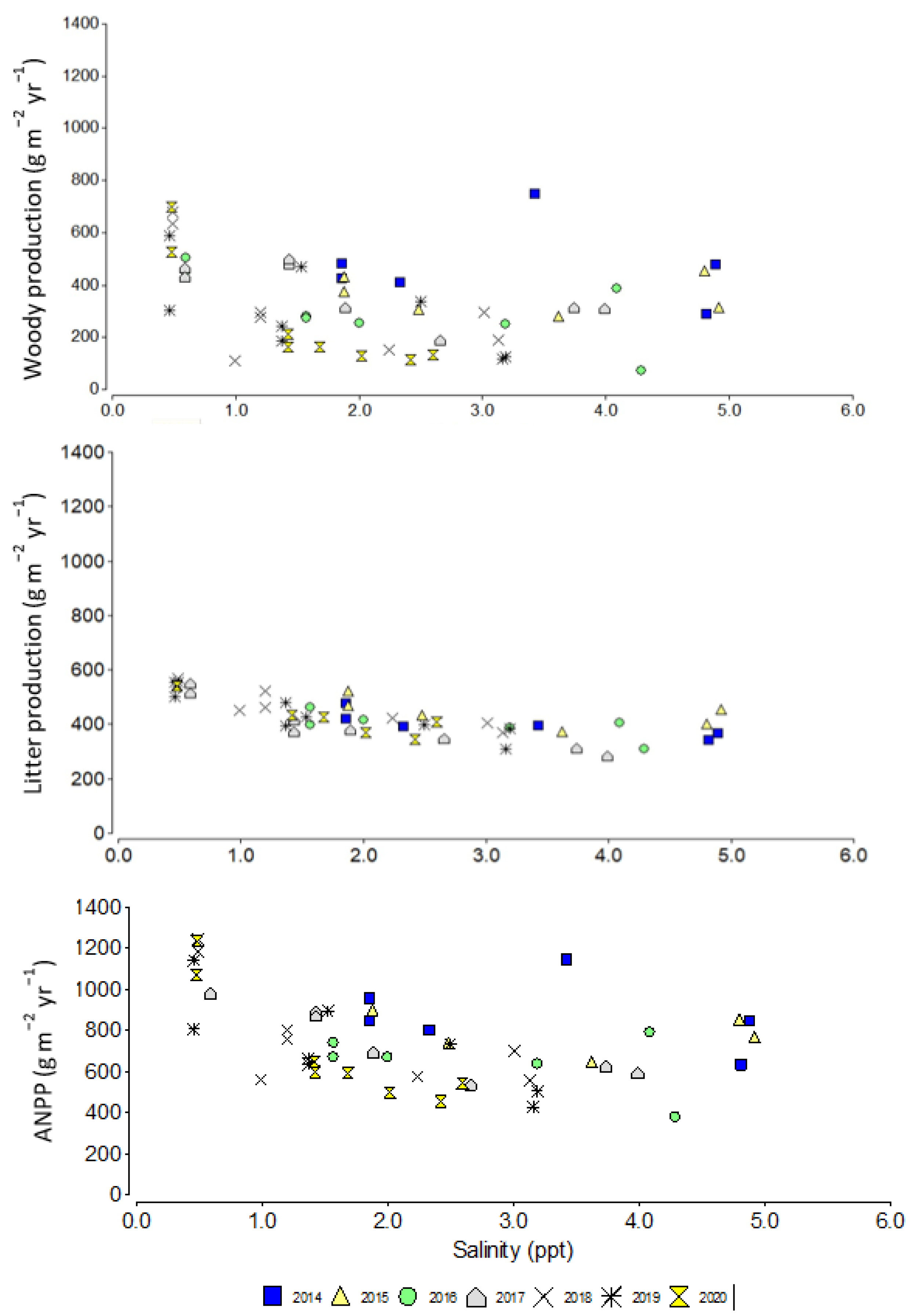
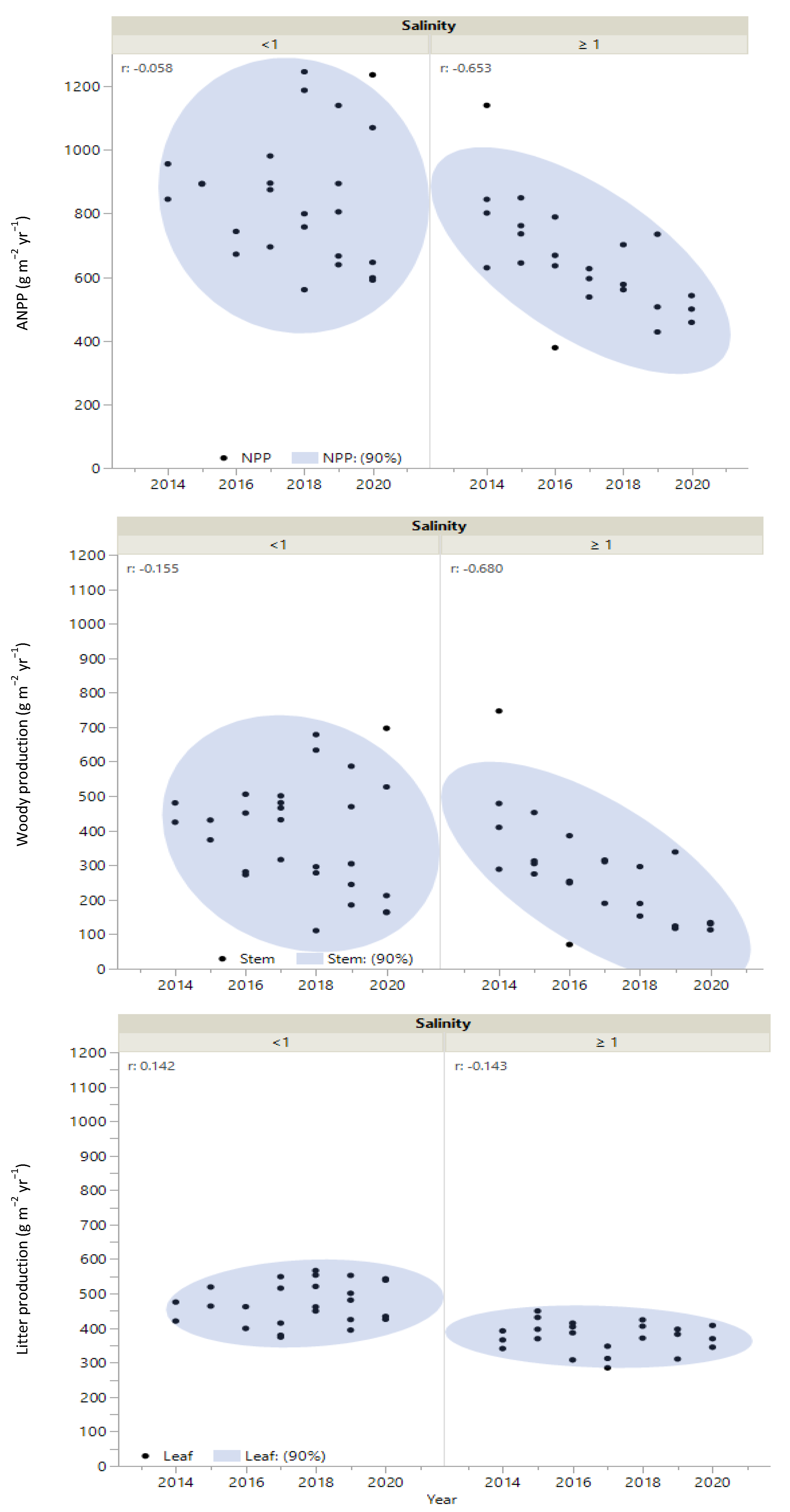
3.2. Woody Growth, Litterfall, and Aboveground Net Primary Production
3.3. Vascular Plant Collection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, K.; Ewel, K.C.; Stumpf, R.P.; Putz, F.E.; Workman, T.W. Sea-level rise and coastal forest retreat on the west coast of Florida, USA. Ecology 1999, 80, 2045–2063. [Google Scholar] [CrossRef]
- Morris, J.T.; Sundareshwar, P.V.; Nietch, C.T.; Kjerfve, B.; Cahoon, D.R. Responses of coastal wetlands to rising sea level. Ecology 2002, 83, 2869–2877. [Google Scholar] [CrossRef]
- Craft, C.; Clough, J.; Ehman, J.; Jove, S.; Park, R.; Pennings, S.; Guo, H.; Machmuller, M. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front. Ecol. Environ. 2009, 7, 73–78. [Google Scholar] [CrossRef]
- Anderson, C.J.; Lockaby, B.G.; Click, N. Changes in wetland forest structure, basal growth, and composition across a tidal gradient. Am. Midl. Nat. 2013, 170, 1–13. [Google Scholar] [CrossRef]
- Conner, W.H.; Day, J.W. Productivity and composition of a baldcypress-water tupelo site and a bottomland hardwood site in a Louisiana Swamp. Am. J. Bot. 1976, 63, 1354–1364. [Google Scholar] [CrossRef]
- Conner, W.H.; Day, J.W. Diameter growth of Taxodium distichum (L.) Rich. and Nyssa aquatica L. from 1979–1985 in 4 Louisiana swamp stands. Am. Midl. Nat. 1992, 127, 290–299. [Google Scholar] [CrossRef]
- Ratard, M.A. Factors Affecting Growth and Regeneration of Baldcypress in a South Carolina Tidal Freshwater Swamp. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2004. [Google Scholar]
- Duberstein, J.A.; Kitchens, W.M. Community composition of select areas of tidal freshwater forest along the Savannah River. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 321–348. [Google Scholar]
- Ensign, S.H.; Noe, G.B. Tidal extension and sea-level rise: Recommendations for a research agenda. Front. Ecol. Environ. 2018, 16, 37–43. [Google Scholar] [CrossRef]
- Duberstein, J.A.; Conner, W.H.; Krauss, K.W. Woody vegetation communities of tidal freshwater swamps in South Carolina, Georgia, and Florida (US) with comparisons to similar systems in the US and South America. J. Veg. Sci. 2014, 25, 848–862. [Google Scholar] [CrossRef]
- Krauss, K.W.; Noe, G.B.; Duberstein, J.A.; Conner, W.H.; Stagg, C.L.; Cormier, N.; Jones, M.C.; Bernhardt, C.J.; Lockaby, B.G.; From, A.S.; et al. The role of the upper tidal estuary in wetland blue carbon storage and flux. Glob. Biogeochem. Cycles 2018, 32, 817–839. [Google Scholar] [CrossRef]
- Ungar, I.A. Ecophysiology of Vascular Halophyte; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Schile, L.M.; Callaway, J.C.; Parker, V.T.; Vasey, M.C. Salinity and inundation influence productivity of the halophytic plant Sarcocornia pacifica. Wetlands 2011, 31, 1165–1174. [Google Scholar] [CrossRef]
- Doyle, T.W.; Conner, W.H.; Ratard, M.; Inabinette, L.W. Assessing the impacts of tidal flooding and salinity on long-term growth of baldcypress under changing climate and riverflow. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 441–445. [Google Scholar]
- DeLaune, R.D.; Lindau, C.W. δ13C signature of organic carbon in estuarine bottom sediment as an indicator of carbon export from adjacent marshes. Biogeochemistry 1987, 4, 225–230. [Google Scholar] [CrossRef]
- Conner, W.H.; Brody, M. Rising water levels and the future of southeastern Louisiana swamp forests. Estuaries 1989, 12, 318–323. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Delaune, R.D.; Patrick, W.H. Flooding and saltwater intrusion: Potential effects on survival and productivity of wetland forests along the U.S. Gulf Coast. For. Ecol. Manag. 1990, 33–34, 287–301. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; DeLaune, R.D.; Patrick, W.H. Response of Spartina patterns to increasing levels of salinity in rapidly subsiding marshes of the Mississippi River Deltaic Plain. Estuar. Coast. Shelf Sci. 1987, 24, 389–399. [Google Scholar] [CrossRef]
- Hackney, C.T.; Avery, G.B.; Leonard, L.A.; Posey, M.; Alphin, T. Biological, chemical, and physical characteristics of tidal freshwater swamp forests of the Lower Cape Fear River/Estuary, North Carolina. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 183–221. [Google Scholar]
- Krauss, K.W.; Duberstein, J.A.; Doyle, T.W.; Conner, W.H.; Day, R.H.; Inabinette, L.W.; Whitbeck, J.L. Site condition, structure, and growth of baldcypress along tidal/non-tidal salinity gradients. Wetlands 2009, 29, 505–519. [Google Scholar] [CrossRef]
- Krauss, K.W.; Whitbeck, J.L.; Howard, R.J. On the relative roles of hydrology, salinity, temperature, and root productivity in controlling soil respiration from coastal swamps (freshwater). Plant Soil 2012, 358, 265–274. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Lugo, A.E.; Alber, M.; Covich, A.; Bloem, S.V. Understanding and forecasting the effects of sea level rise and intense windstorms on coastal and upland ecosystems: The need for a continental-scale network of observatories. Front. Ecol. Environ. 2008, 6, 255–263. [Google Scholar] [CrossRef][Green Version]
- Nicholls, R.J.; Cazenave, A. Sea-level rise and its impact on coastal zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef]
- Conner, W.H.; Krauss, K.W.; Doyle, T.W. Ecology of tidal freshwater forests in coastal deltaic Louisiana and northeastern South Carolina. In Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Conner, W.H., Doyle, T.W., Krauss, K.W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 223–254. [Google Scholar]
- Raabe, E.A.; Stumpf, R.P. Expansion of tidal marsh in response to sea-level rise: Gulf Coast of Florida, USA. Estuaries Coasts 2016, 39, 145–157. [Google Scholar] [CrossRef]
- Light, H.M.; Darst, M.R.; Lewis, L.J.; Howell, D.A. Hydrology, Vegetation, and Soils of Riverine and Tidal Floodplain Forests of the Lower Suwannee River, Florida, and Potential Impacts of Flow Reductions; USGS Professional Paper: Denver, CO, USA, 2002; p. 1656-A.
- Krauss, K.W.; Duberstein, J.A.; Conner, W.H. Assessing stand water use in four coastal wetland forests using sapflow techniques: Annual estimates, errors and associated uncertainties. Hydrol. Processes 2015, 29, 112–127. [Google Scholar] [CrossRef]
- Kearney, J.B. Report on a botanical survey of the Great Dismal Swamp region. Contrib. U.S. Natl. Herb. 1901, 5, 321–585. [Google Scholar]
- Wright, A.H.; Wright, A.A. The habitats and composition of the vegetation of the Okefenokee Swamp, Georgia. Ecol. Monogr. 1932, 2, 190–232. [Google Scholar] [CrossRef]
- Beaven, G.F.; Oosting, H.J. Pocomoke Swamp: A study of a cypress swamp on the eastern shore of Maryland. Bull. Torrey Bot. Club 1939, 66, 367–389. [Google Scholar] [CrossRef]
- Hall, T.F.; Penfound, W.T. Cypress-gum communities in the Blue Girth Swamp near Selma, Alabama. Ecology 1943, 24, 208–217. [Google Scholar] [CrossRef]
- Oosting, H.J. The Study of Plant Communities; W.H. Freeman and Company: San Francisco, CA, USA, 1956. [Google Scholar]
- Messina, M.G.; Conner, W.H. (Eds.) Southern Forested Wetlands: Ecology and Management; Lewis Publishers/CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Baden, J.; Batson, W.T.; Stalter, R. Factors affecting the distribution of vegetation of abandoned rice fields, Georgetown County, South Carolina. Castanea 1975, 40, 171–184. [Google Scholar]
- Stalter, R.; Rachlin, J.; Baden, J. A forty-seven year comparison of the vascular flora at three abandoned rice fields, Georgetown, South Carolina, U.S.A. J. Bot. Res. Inst. Tex. 2021, 15, 271–282. [Google Scholar] [CrossRef]
- Barry, J.M. A Survey of the Native Vascular Plants of the Baruch Plantation, Georgetown, South Carolina. Master’s Thesis, University of South Carolina, Columbia, SC, USA, 1968. [Google Scholar]
- Baden, J. Tidal marsh vegetation on abandoned rice fields, Winyah Bay, Georgetown, South Carolina. Master’s Thesis, University of South Carolina, Columbia, SC, USA, 1971. [Google Scholar]
- Conner, W.H.; Inabinette, L.W. Tree growth in three South Carolina (USA) swamps after Hurricane Hugo: 1991–2001. For. Ecol. Manag. 2003, 182, 371–380. [Google Scholar] [CrossRef]
- Liu, X.; Conner, W.H.; Song, B.; Jayakaran, A.D. Forest composition and growth in a freshwater forested wetland community in response to increased soil salinity in South Carolina, USA. For. Ecol. Manag. 2017, 389, 211–219. [Google Scholar] [CrossRef]
- Williams, T.M.; Chow, A.T.; Song, B. Historical visualization evidence on forest-salt marsh transition in Winyah Bay, South Carolina: A retrospective study in sea level rise. Wetl. Sci. Pract. 2012, 20, 5–17. [Google Scholar]
- Jayakaran, A.D.; Williams, T.M.; Conner, W.H.; Hitchcock, D.R.; Song, B.; Chow, A.T.; Smith, E.M. Monitoring water quality changes in a forested freshwater wetland threatened by salinity. In Proceedings of the South Carolina Water Resources Conference, Columbia Metropolitan Convention Center, Columbia, SC, USA, 15–16 October 2014. [Google Scholar]
- Stuckey, B.N. Soil Survey of Georgetown County South Carolina; Report Prepared for the Soil Conservation Service; USDA: Washington, DC, USA, 1982.
- NOAA. National Centers for Environmental Information Data Tools: Find a Station. Available online: https://www.ncdc.noaa.gov/cdo-web/datatools/findstation (accessed on 1 April 2021).
- Clark, A., III; Phillips, D.R.; Frederick, D.J. Weight, Volume, and Physical Properties of Major Hardwood Species in the Gulf and Atlantic Coastal Plains; Research Paper SE-250; USDA Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1985.
- Megonigal, J.P.; Conner, W.H.; Kroeger, S.; Sharitz, R.R. Aboveground production in Southeastern floodplain forests: A test of the subsidy-stress hypothesis. Ecology 1997, 78, 370–384. [Google Scholar]
- Busbee, W.S.; Conner, W.H.; Allen, D.M.; Lanham, J.D. Composition and aboveground productivity of three seasonally flooded depressional forested wetlands in coastal South Carolina. Southeast. Nat. 2003, 2, 335–346. [Google Scholar] [CrossRef]
- Clawson, R.G.; Lockaby, B.G.; Rummer, B. Changes in production and nutrient cycling across a wetness gradient within a floodplain forest. Ecosystems 2001, 4, 126–138. [Google Scholar] [CrossRef]
- Schilling, E.B.; Lockaby, B.G. Relationships between productivity and nutrient circulation within two contrasting southeastern U.S. floodplain forests. Wetlands 2006, 26, 181–192. [Google Scholar] [CrossRef]
- Shaffer, G.P.; Wood, W.B.; Hoeppner, S.S.; Perkins, T.E.; Zoller, J.; Kandalepas, D. Degradation of baldcypress–water tupelo swamp to marsh and open water in Southeastern Louisiana, U.S.A.: An irreversible trajectory? J. Coast. Res. 2009, 10054, 152–165. [Google Scholar] [CrossRef]
- Cormier, N.; Krauss, K.W.; Conner, W.H. Periodicity in stem growth and litterfall in tidal freshwater forested wetlands: Influence of salinity and drought on nitrogen recycling. Estuaries Coasts 2013, 36, 533–546. [Google Scholar] [CrossRef]
- Catchpole, W.R.; Wheeler, C.J. Estimating plant biomass: A review of techniques. Aust. Ecol. 1992, 17, 121–131. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Taylor, J.R.; Benson, K.B. Estimating primary productivity of forested wetland communities in different hydrologic landscapes. Landsc. Ecol. 1991, 5, 75–92. [Google Scholar] [CrossRef]
- Weakley, A.S. Flora of the Southern and Mid-Atlantic States. 2015. Available online: http://herbarium.unc.edu/flora.htm (accessed on 28 April 2020).
- Statistical Analysis System. SAS Version 9.4 for Windows; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Hackney, C.T.; Avery, G.B. Tidal wetland community response to varying levels of flooding by saline water. Wetlands 2015, 35, 227–236. [Google Scholar] [CrossRef]
- Stalter, R. Phragmites communis, Trinius in South Carolina. Rhodora 1975, 77, 809. [Google Scholar]
- Celik, S.; Anderson, C.J.; Kalin, L.; Rezaeianzadeh, M. Long-term salinity, hydrology, and forested wetlands along a tidal freshwater gradient. Estuaries Coasts 2021, 44, 1816–1830. [Google Scholar] [CrossRef]
- Stahl, M.; Widney, S.; Craft, C. Tidal freshwater forests: Sentinels for climate change. Ecol. Eng. 2018, 116, 104–109. [Google Scholar] [CrossRef]
- Jayakaran, A.D.; Williams, T.M.; Conner, W.H. Tracking salinity intrusions in a coastal forested freshwater wetland system. In Proceedings of the Fifth Interagency Conference on Research in the Watersheds, North Charleston, SC, USA, 2–5 March 2015. [Google Scholar]
- Powell, A.M.; Jackson, L.; Ardon, M. Disentangling the effects of drought and salinity on growth of bald cypress trees at different life stages. Restor. Ecol. 2016, 24, 548–557. [Google Scholar] [CrossRef]
- Bartram, W. Travels of William Bartram; van Doren, M., Ed.; Reprint edition; Dover Publications: New York, NY, USA, 1928. [Google Scholar]
- Mattoon, W.R. The Southern Cypress; Bulletin 272; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1915.
- Penfound, W.T.; Hathaway, E.S. Plant communities in the marshlands of southeastern Louisiana. Ecol. Monogr. 1938, 8, 1–56. [Google Scholar] [CrossRef]
- Conner, W.H.; Doyle, T.W.; Krauss, K.W. (Eds.) Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Springer: Dordrecht, SC, USA, 2007. [Google Scholar]
- Brinson, M.M.; Bradshaw, H.D.; Jones, M.N. Transitions in forested wetlands along gradients of salinity and hydroperiod. J. Elisha Mitchell Sci. Soc. 1985, 101, 76–94. [Google Scholar]
- Ardon, M.; Helton, A.M.; Bernhardt, E. Drought and saltwater incursion synergistically reduce dissolved organic carbon export from coastal freshwater wetlands. Biogeochemistry 2016, 127, 411–426. [Google Scholar] [CrossRef]
- Smart, L.S.; Taillie, P.J.; Poulter, B.P.; Vukomanovic, J.; Singh, K.K.; Swenson, J.J.; Mitasova, H.; Smith, J.W.; Meentemeyer, R.K. Aboveground carbon loss associated with the spread of ghost forests as sea levels rise. Environ. Res. Lett. 2020, 15, 104028. [Google Scholar] [CrossRef]
- Pierfelice, K.N.; Lockaby, B.G.; Krauss, K.W.; Conner, W.H.; Noe, G.B.; Ricker, M.C. Salinity influences on aboveground and belowground net primary productivity in tidal wetlands. J. Hydrol. Eng. 2017, 22. [Google Scholar] [CrossRef]
- Yanosky, T.; Hupp, C.; Hackney, C. Chloride concentrations in growth rings of Taxodium disticum in a saltwater-intruded estuary. Ecol. Appl. 1995, 5, 785–792. [Google Scholar] [CrossRef]
- Krauss, K.W.; Duberstein, J.A. Sapflow and water use of freshwater wetland trees exposed to saltwater incursion in a tidally influenced South Carolina watershed. Can. J. For. Res. 2010, 40, 525–535. [Google Scholar] [CrossRef]
- Thomas, B.L.; Doyle, T.W.; Krauss, K.W. Annual growth patterns of baldcypress (Taxodium distichum) along salinity gradients. Wetlands 2015, 35, 831–839. [Google Scholar] [CrossRef]
- Cuffney, T.F. Input, movement and exchange of organic matter within a subtropical coastal blackwater river-floodplain system. Freshw. Biol. 1988, 19, 305–320. [Google Scholar] [CrossRef]
- Watt, K.M.; Golladay, S.W. Organic matter dynamics in seasonally inundated, forested wetlands of the Gulf Coastal Plain. Wetlands 1999, 19, 139–148. [Google Scholar] [CrossRef]
- Gomez, M.M.; Day, F.P., Jr. Litter nutrient content and production in the Great Dismal Swamp. Am. J. Bot. 1982, 69, 1314–1321. [Google Scholar] [CrossRef]
- Conner, W.H.; Mihalia, I.; Wolfe, J. Tree community structure and changes from 1987 to 1999 in three Louisiana and three South Carolina forested wetlands. Wetlands 2002, 22, 58–70. [Google Scholar] [CrossRef]


| Mean DBH (cm) | Density (# ha−1) | BA (m2 ha−1) | |||||
|---|---|---|---|---|---|---|---|
| Site | Species | 2014 | 2020 | 2014 | 2020 | 2014 | 2020 |
| Fresh | Ash | 21.5 | 22.4 | 380 | 370 | 15.6 | 16.67 |
| (0.1 ppt) | Baldcypress | 26.3 | 27.2 | 290 | 290 | 18.41 | 19.58 |
| Elm | 31.5 | 31.5 | 10 | 10 | 0.78 | 0.78 | |
| Red maple | 22.8 | 23.3 | 230 | 190 | 10.61 | 9.2 | |
| Swamp blackgum | 46.4 | 46.4 | 20 | 20 | 3.68 | 3.68 | |
| Sweetgum | 16.2 | 16.7 | 150 | 150 | 3.56 | 3.78 | |
| TOTAL | 1080 | 1030 | 52.64 | 53.69 | |||
| Low-salt | Ash | 25.5 | 27.1 | 60 | 50 | 3.54 | 3.3 |
| (0.8 ppt) | Baldcypress | 28 | 26 | 290 | 320 | 23.58 | 25.12 |
| Swamp blackgum | 24.7 | 25.1 | 250 | 240 | 13.34 | 13.23 | |
| Water tupelo | 36.8 | 37.2 | 140 | 140 | 16.5 | 16.8 | |
| Waxmyrtle | -- | 11.6 | -- | 20 | -- | 0.21 | |
| Dahoon holly | -- | 14.9 | -- | 10 | -- | 0.2 | |
| TOTAL | 740 | 780 | 56.96 | 58.86 | |||
| Mid-salt | Ash | 54.4 | -- | 10 | -- | 2.32 | -- |
| (2.6 ppt) | Baldcypress | 45.4 | 46.5 | 260 | 240 | 42.74 | 42.73 |
| Swamp blackgum | 20.2 | 20.6 | 170 | 160 | 5.81 | 5.7 | |
| Water tupelo | 34.6 | 33.6 | 130 | 110 | 15.43 | 12.76 | |
| Waxmyrtle | 12.2 | 12.9 | 30 | 20 | 0.39 | 0.2 | |
| Loblolly pine | 11.5 | 13.5 | 10 | 10 | 0.1 | 0.14 | |
| Red maple | 23 | 23.3 | 10 | 10 | 0.42 | 0.43 | |
| TOTAL | 620 | 550 | 67.21 | 61.96 | |||
| High-salt | Ash | 10.15 | 11 | 10 | 10 | 0.08 | 0.1 |
| (4.6 ppt) | Baldcypress | 47.45 | 47.38 | 250 | 230 | 46.99 | 43.08 |
| Swamp blackgum | 17.49 | 18.32 | 130 | 130 | 3.49 | 3.78 | |
| Water tupelo | 23.9 | 16.3 | 140 | 40 | 7.42 | 0.86 | |
| Waxmyrtle | 10.95 | 11.65 | 70 | 30 | 0.73 | 0.31 | |
| Loblolly pine | -- | 11.14 | -- | 20 | 0.1 | 0.2 | |
| TOTAL | 600 | 460 | 58.81 | 48.33 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conner, W.; Whitmire, S.; Duberstein, J.; Stalter, R.; Baden, J. Changes within a South Carolina Coastal Wetland Forest in the Face of Rising Sea Level. Forests 2022, 13, 414. https://doi.org/10.3390/f13030414
Conner W, Whitmire S, Duberstein J, Stalter R, Baden J. Changes within a South Carolina Coastal Wetland Forest in the Face of Rising Sea Level. Forests. 2022; 13(3):414. https://doi.org/10.3390/f13030414
Chicago/Turabian StyleConner, William, Stefanie Whitmire, Jamie Duberstein, Richard Stalter, and John Baden. 2022. "Changes within a South Carolina Coastal Wetland Forest in the Face of Rising Sea Level" Forests 13, no. 3: 414. https://doi.org/10.3390/f13030414
APA StyleConner, W., Whitmire, S., Duberstein, J., Stalter, R., & Baden, J. (2022). Changes within a South Carolina Coastal Wetland Forest in the Face of Rising Sea Level. Forests, 13(3), 414. https://doi.org/10.3390/f13030414







