Abstract
Current widely used climate envelope approaches, i.e., correlations between climatic variables and the presence of a species, simulate responses for the whole species and predict future ranges based mainly on climatic suitability. However, short-term tree responses to climate change will take place within current populations, and these populations, acclimated to their local environments, are not likely to respond similarly to climate change. Thus, to develop reliable forecasts of forest responses to climate change, this variability among populations needs to be considered. In this study, we tested the effect of environmental conditions on the growth of two common maple species (Acer rubrum L. and A. saccharum Marshall) at two different latitudes within their northern distributional ranges. We collected increment cores, and analyzed year to year variabilities in tree growth as a function of temperature and precipitation. The results suggest divergent responses between species and between populations of the same species. Predicted growth under different climate scenarios for the region suggested that the growth of southern populations might decrease, while northern populations might still be able to retain their current growth. These results document the population-level responses to environmental conditions of these two species, providing latitude-specific guidance for future forest distribution prediction.
1. Introduction
Global climate patterns have rapidly changed in the past century with increasing temperatures, more intense precipitation events and a higher frequency of extreme drought [1]. These changes will shape forest ecosystems worldwide, as their composition, structure and function are strongly influenced by local environmental conditions [2,3,4]. Shifts in environmental variables will likely result in the alteration of plant physiology, population demography, community assemblage and species distributional ranges [5,6,7,8]. However, the effects of these changes will be heterogeneous across the distributional range of a species [9], with populations at higher latitudes likely benefiting from warming and populations at lower latitudes mostly being negatively affected [10]. Within these broad patterns of climate change, it is not clear which populations will be most affected. Thus, to understand how individual populations will cope with current environmental trends, we need to assess the impacts of climate change in different locations within the species distributional range.
Climate envelope approaches are the most common methods used to predict future changes in the distributional ranges of tree species. They use correlations between the occurrence of a species and environmental conditions at those sites [11,12]. These predictions work well when looking at dynamics that may take place on the order of centuries. However, to make predictions on finer time scales, i.e., decades, information about performance at the population level is needed, and these responses are likely to differ across the distributional range of a species [13]. Increases in temperature at higher latitudes will result in a longer growing season, which might benefit the growth of local tree populations since they could be operating below their temperature optima [14,15]; however, for populations at lower latitudes, an increase in temperature might result in a shift beyond their optimal temperature range and affect growth negatively [16]. Tree species with wide distributions are likely to show physiological acclimation at the population level, determined by the temperature and precipitation of the site, and this would likely result in different response patterns under similar environmental changes [17,18]. Each population’s susceptibility to changing conditions is likely going to be different as a function of both the population’s acclimation ability [19] and the direction of the change with respect to the population optimum [20]. Thus, with these location-specific environment-induced growth responses [21], multi-site demographic studies can help us to better understand the variability among populations’ responses to climate change.
The two most important climatic variables affecting tree growth are growing season temperature and precipitation [22,23,24]. Temperature affects growth by conditioning cell division, photosynthesis, and respiration [25]. Temperatures from late winter to spring can initiate cambial cell division and xylem differentiation [26,27], and cumulative elevated temperatures from late winter to spring can extend the growth period by advancing cambial reactivation and xylem differentiation [28]. At the same time, warmer night temperature during the growing season is a critical factor that enhances radial growth [29]. Temperature also regulates photosynthesis by affecting the plastid apparatus and gas exchange [30]. However, high temperatures exceeding photosynthetic temperature optima deactivate Rubisco, inhibit the activity of stromal enzymes, reduce assimilation and cause an increase in respiration in general [31,32], inhibiting carbohydrate accumulation. Furthermore, increasing temperatures will exacerbate water stress by increasing soil water deficits through enhanced evapotranspiration [33]. Reductions in local precipitation would further exacerbate water deficits [34]. Water scarcity during the growing season can cause the partial or complete loss of xylem function due to embolism, which increases stem hydraulic failure [35,36,37]. Meanwhile, leaves under increased water stress will close their stomata more often, which will reduce their carbon dioxide uptake, possibly even causing carbon starvation within plant cells [37,38].
When studying the effects of temperature as well as precipitation on tree growth, legacy effects are also relevant [39]; climatic events that occurred in the previous year can have a large influence on tree growth in the current year [39,40,41]. When there are drought events, trees adjust their anatomy and physiology, and growth in subsequent years is affected [42]. Additionally, a continuous gain in resources in one year can be allocated to growth in the following year [43]. Thus, these legacy effects should be considered in any environment-related analyses of tree growth responses to climate change.
To investigate how current trends in temperature and precipitation may affect future tree growth across populations, and whether growth responses differ among closely related species, we studied the growth patterns of two tree species over 21 years (from 1997 to 2017) at two geographic locations that differ in growing season length by more than 50 days. We analyzed tree growth as a function of spring temperature, summer temperature and summer precipitation. Spring temperature is a good proxy for the beginning of the growing season and determines its length. Summer temperature and precipitation are good proxies for water demand and water availability [15,44]. The aim of this study was to evaluate the effect of year-to-year variability in environmental conditions on the annual growth of these two species, and assess how these effects may vary between latitudes. We asked the following questions: (1) Do populations at higher latitudes respond more positively to increasing temperature? (2) Does increasing precipitation always benefit tree growth under all scenarios? (3) Are these effects species-specific? Answers to these questions will help us to assess possible forest changes under anticipated changes in the future climate.
2. Materials and Methods
2.1. Study Area
We collected tree core samples from forest stands located at two different latitudes in Michigan’s Lower Peninsula, USA (Figure 1), which encompass two different Ecological Provinces. The northern part is categorized as Laurentian Mixed Forest and the southern part as Midwest Broadleaf Forest [45]. At the southern location, the climate is usually continental with warm to hot summers and frequent growing season water deficits. The average growing season length is around 173 days. At the northern site winters are moderately long, and snow usually stays on the ground throughout the winter. The average growing season is much shorter at about 122 days [7]. The northern stands used in this study were located on the properties of University of Michigan Biological Station, Pellston, MI (Table 1). All stands we sampled were secondary forests belonging to the University of Michigan as field sites for research and recreation purposes. The average minimum temperature in January is −12.1 °C, the average maximum temperature in July is 26.2 °C, and the annual precipitation is 735.076 mm. The southern stands are located around Ann Arbor, MI (Table 1). The average minimum temperature in January is −7.4 °C, the average maximum temperature in July is 28.8 °C, and the annual average precipitation is 981.202 mm [46].
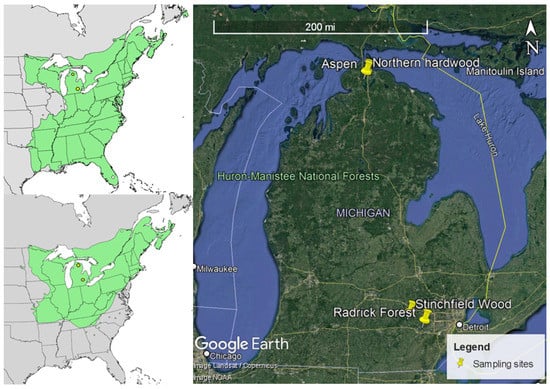
Figure 1.
Distributional range of Acer rubrum L. (left top) and Acer saccharum Marshall (left bottom) and locations of study sites at two latitudes in Lower peninsula, Michigan (Right). (Map retrieved from https://www.usgs.gov/centers/geosciences-and-environmental-change-science-center, accessed on 10 December 2021 and Google Earth, accessed on 3 March 2022).

Table 1.
Description of four sampling sites at the two latitudes.
2.2. Studied Species
The two target species chosen for this study were red maple (Acer rubrum L.) and sugar maple (Acer saccharum Marshall), which are common tree species widely distributed across eastern North America. A. rubrum is one of the most popular ornamental trees worldwide for its flaming fall foliage. A. saccharum has very high economic importance for three of its features: maple syrup, maple timber and fall foliage. They both prefer a mesic environment, but A. saccharum is usually associated with well-drained soil, while A. rubrum can survive in poorly drained swamps [47]. The sampled populations are located within the northern range of both species (Figure 1).
Previous research has suggested that A. rubrum and A. saccharum respond differently to environmental conditions at different life stages because of their physiological differences [20]. For example, one study found that increasing temperatures negatively impact the coarse root respiration and root mass of A. rubrum seedlings, but not A. saccharum seedlings [48]. One researcher pointed out that compared to environmental variability, site history such as logging events or other natural disasters have a bigger impact on the growth of A. rubrum [49], which means we might not expect to see a big effect of climatic variables on their growth. These studies provide evidence that these two species might respond to environmental variability differently.
2.3. Field and Laboratory Methods
Field sample collection took place in May and July of 2019. At each stand, all tagged A. rubrum and A. saccharum trees with a diameter at breast height (DBH) greater than 10 cm were sampled. Two increment cores were extracted from the east and west sides of each tree using a Haglof 3 threaded 4.3 mm increment borer. All tree cores were stored in paper straws and air dried by spreading the cores out on a table for 24–48 h before processing [50].
The preparation of the tree cores followed standard protocols [50,51,52]. All air-dried tree cores were placed on wooden mounts prior to being sanded with P220 sandpaper to provide a flat core surface. The samples were then sanded with increasingly finer grit sandpaper (P320, P600, and for A. saccharum, up to P1500) until the individual growth rings of the cross-sectional view could be seen clearly under a microscope. Among all of the collected samples, only those increment cores containing distinct growth rings were selected for further scanning and analysis. We ended up with data from 20 A. saccharum individuals and 19 A. rubrum individuals from the northern stands, and 26 A. saccharum as well as 22 A. rubrum individuals from the southern sites.
The cores were scanned using a flatbed scanner at 1200 dpi resolution. The width of each growth ring in the tree core was measured using the program CooRecorder (version 9.3.1). Growth ring measurements were taken along a predetermined radius in a straight line, and were generally perpendicular to the growth ring boundaries. All the tree cores collected from the same site were cross-dated using the software program CDendro (Version 9.3.1). After the cross-dating process, growth ring width measurements of the two tree cores that had been collected from the same individual tree were averaged to calculate the annual radial growth. The subsequent analysis only utilized the tree core samples that could be correctly cross-dated for at least 10 years.
2.4. Climate Data
All the climate data used in this study were retrieved from the National Oceanic and Atmospheric Administration (NOAA) national weather station database [46]. The climate data obtained included the average monthly temperature and monthly precipitation from 1997 to 2017. The climate data used for northern sites were retrieved from the Pellston regional airport (GHCND: USW00014841) weather station (45°55′ N, 84°78′ W; Figure 1). The climate data used for southern sites were retrieved from the University of Michigan (GHCND: USC00200230) weather station located in Ann Arbor, Michigan (42°17′ N, 83°39′ W; Figure 1).
2.5. Tree Growth Data Analysis
We performed extensive exploratory data analysis to determine which monthly climate variables showed the strongest association with tree growth. The climate variables that displayed the highest correlation with growth were selected for the final analyses of tree growth, those being spring temperature, summer temperature and summer precipitation. To account for growth variation as a function of tree size, the natural log of DBH was included in the model [52]. To account for the previous years’ effect on current growth, growth in each of the two previous years (Growthi,y−1, Growthi,y−2) was included as the lag effect [39,40,53].
Growth for tree i in year y () was modeled using normal likelihood:
The process model is:
Parameter is the growth without any influence of temperature, precipitation and lag effect. Parameter represents the coefficient of that particular component in the model. Parameters ω1 and ω2 represent the weight of each year’s effect, . To account for changes in growth variability with tree size [54], we estimated the variance as a function of DBH:
In the southern sites, there were some individuals with missing growth data in certain years that prevented us from directly estimating DBH in those years. We treated these missing DBH values as latent variables to be estimated as:
Parameter d represents the average increase in diameter each year. Parameter α was estimated by using a slightly informative prior distribution, via α~LogNormal (1, 0.001), since α as the base growth should have a positive value. The other parameters were estimated by using non-informative prior distributions as follows: β*,b,d~Normal (0, 0.0001), a~LogNormal (1, 0.001), σd2~Gamma (0.0001, 0.001), and ω~Dirichlet (1). Each species and latitude were analyzed independently.
2.6. Simulation Modeling
The results obtained from this model were used to estimate future tree growth under forecasted changes of temperature and precipitation for this region. These forecasts were generated by the “Shared socio-economic pathway” (SSP) scenario using Coupled Model Intercomparison Project Phase 6 (CMIP6) [1]. The SSP1-2.6 scenario, also called the “sustainable development” model, predicts that spring temperatures will increase by 1.8 °C, summer temperatures will increase by 1.8 °C and summer precipitation will increase by 3.5% in the Midwest region by the end of this century [1]. The SSP5-8.5 scenario, also called “fossil-fuel based development”, with the highest emissions, predicts spring temperatures will increase by 5 °C, summer temperatures will increase by 5.6 °C and summer precipitation will decrease by 2.9% in the Midwest region by the end of this century [1]. To estimate growth under these forecasts, we ran three simulations for each scenario: Scenario 1 (S1), an increase in both spring and summer temperature; Scenario 2 (S2), a decrease in summer precipitation; and Scenario 3 (S3), an increase in spring and summer temperature, as well as a decrease in summer precipitation.
Analysis and simulations were conducted using OpenBUGS (version 3.2.3) [55]; for the analysis, we ran three MCMC chains for 10,000 iterations until convergence was reached. The posterior parameter means, standard deviations, and 95% credible intervals were then estimated across 20,000 iterations.
3. Results
The DBH range of the A. rubrum samples collected from the southern and northern sites were 13.9–37.6 cm and 11.6–30.6 cm, respectively. The DBH range of A. saccharum samples collected from the southern and northern sites were 10.9–52.8 cm and 18.4–39.3 cm, respectively. The average growth rate for A. saccharum in the southern sites was 1.518 ± 0.983 mm/y and it was 0.999 ± 0.405 mm/y in the north. The average growth rate for A. rubrum in southern sites was 1.273 ± 0.761 mm/y and 0.938 ± 0.446 mm/y in the north.
3.1. Model Selection and Model Fit
An exploratory data analysis indicated that using the April mean temperature as the spring temperature, the August mean temperature as the summer temperature and the July total precipitation as the summer precipitation had the highest association with tree growth in the southern sites. For the northern sites, we used May mean temperature as spring temperature, July mean temperature as summer temperature, and June total precipitation as summer precipitation. The goodness of fit (predicted vs. observed; R2) was 0.60 and 0.69 for A. rubrum at the southern and northern sites, respectively, and it was 0.50 and 0.47 for A. saccharum at the southern and northern sites, respectively.
Tree ring growth was positively related to DBH for almost all of the sample groups, with the exception of A. saccharum in the southern site (Table S1). All parameter values are provided in Table S1. In the description that follows, the 95% credible intervals for estimates did not cross zero, unless otherwise specified.
3.2. Effect of Climate Variables
For A. rubrum in the southern population, the effect of August temperature was (mean ± SD) −0.037 ± 0.021, which means summer temperature was negatively associated with tree growth. The other variables did not significantly affect tree growth. In the northern population, none of the climate variables significantly affected tree growth.
For A. saccharum in the south, the effect of August temperature was −0.101 ± 0.032, which means summer temperature negatively affected growth, whereas the effect of July precipitation was 0.002 ± 0.001, which means summer precipitation positively affected growth (Figure 2b). The other variables did not significantly affect tree growth. In the northern population, none of the climate variables had any statistically significant effect on growth.
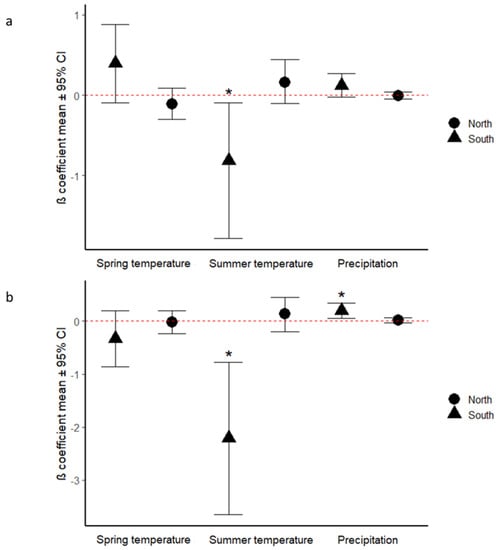
Figure 2.
Parameter estimates (mean ± 95% CI) showing the effects of spring temperature (April for south and May for north), summer temperature (June for south and July for north) and summer precipitation (July for southern sites, August for northern sites) on the growth of (a) Acer rubrum and (b) Acer saccharum. Parameters were standardized by multiplying each one by the covariate mean. * outlier.
3.3. Lag Effect
For both species at both latitudes, previous years’ growth had a significantly positive influence on the current year’s growth (Table S1), and this effect was mainly attributed to growth at y–1 (Table S1). The lag effects for A. rubrum in the south and north were 0.698 ± 0.036 and 0.914 ± 0.035. The lag effects for A. saccharum in the south and north were 0.771 ± 0.041 and 0.670 ± 0.038.
3.4. Simulations
3.4.1. Simulations under SSP1-2.6 Scenario
For both species, trees growing in the southern sample area were predicted to be negatively affected by changing climate variables compared to northern individuals (Figure 3). Although it was not statistically different, the predicted growth in the north slightly increased for both species under all changing climate conditions. For A. rubrum located in the northern area, the predicted growth under the current climate condition is 0.952 (±0.062) mm/y, and the predicted growth rates under S1, S2 and S3 are 0.952 (±0.062) mm/y, 0.953 (±0.062) mm/y and 0.951 (±0.062) mm/y, respectively, indicating that the predicted growth under S1, S2 and S3 would probably remain almost the same compared to the current climate condition. For A. saccharum located in the northern area, the predicted growth under the current condition is 0.973 (±0.065) mm/y, while the predicted growth rates under S1, S2 and S3 are 0.981 (±0.067) mm/y, 0.973 (±0.066) mm/y and 0.982 (±0.068) mm/y, indicating the predicted growth would probably remain almost the same under S1, S2 and S3 compared to growth under the current climate condition.
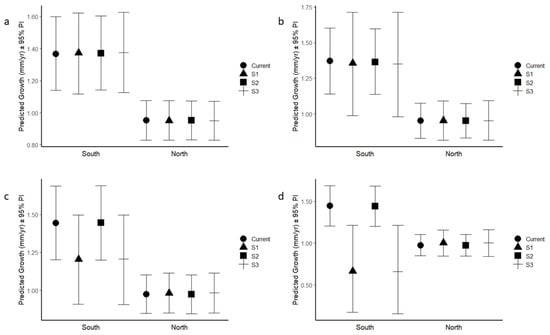
Figure 3.
Simulated growth under current conditions, S1, S2 and S3 in north and south for (a) Acer rubrum under SSP1-2.6 scenario, (b) Acer rubrum under SSP5-8.5 scenario, (c) Acer saccharum under SSP1-2.6 scenario, and (d) Acer saccharum under SSP5-8.5 scenario. Predicted mean + 95%PI.
The predicted growth in the south for two species varied. For A. rubrum, the predicted growth under the current climate condition is 1.370 (±0.117) mm/y, and the predicted growth under S1, S2 and S3 is 1.373 (±0.129) mm/y, 1.370 (±0.117) mm/y and 1.376 (±0.129) mm/y, indicating that predicted growth under S1, S2 and S3 would probably remain almost the same compared to current climate condition. For A. saccharum, the predicted growth under the current climate condition is 1.444 (±0.125) mm/y, and the predicted growth rates under S1, S2 and S3 are 1.203 (±0.150) mm/y, 1.447 (±0.124) mm/y and 1.207 (±0.151) mm/y, indicating a decrease of 16.7% and 16.4% under S1 and S3, respectively, while growth remains almost the same under S2 compared to the current climate condition.
3.4.2. Simulations under SSP5-8.5 Scenario
For both species, trees growing in the south sample area were predicted to be relatively more negatively influenced by changing climate variables compared to northern individuals (Figure 3). For A. rubrum located in the northern area, the predicted growth rates under S1, S2 and S3 are 0.953 (±0.071) mm/y, 0.953 (±0.061) mm/y and 0.953 (±0.071) mm/y, respectively, indicating that the predicted growth would probably remain almost the same under S1, S2 and S3 compared to the current climate condition, which is 0.952 (±0.062) mm/y. Although not statistically different, the predicted growth in the north slightly increases for A. saccharum under the S1 and S3 changing climate conditions, which are the scenarios with increasing temperatures. For A. saccharum located in the northern area, the predicted growth values under S1, S2 and S3 are 1.001 (±0.080) mm/y, 0.972 (±0.066) mm/y and 1.001 (±0.081) mm/y, indicating an increase of 2.8% under S1 and S3, while the growth remains almost the same under S2 compared to the current climate condition.
In contrast, predicted growth in the south decreases for both species under the S1 and S3 changing climate conditions, while it remains almost the same under S2. For A. rubrum, the predicted growth rates under S1, S2, S3 are 1.356 (±0.192) mm/y, 1.364 (±0.117) mm/y and 1.350 (±0.193) mm/y, indicating a decrease of 1% and 1.5% under S1 and S3, respectively, compared to the current climate conditions. For A. saccharum, the predicted growth rates under S1, S2 and S3 are 0.712 (±0.288) mm/y, 1.438 (±0.124) mm/y and 0.708 (±0.290) mm/y, indicating a decrease of 50.7% and 51.0% under S1 and S3, respectively, compared to current climate conditions.
4. Discussion
To understand how tree populations across their distributional range respond to environmental changes differently, we studied location-specific environment-induced growth responses. In this study, we identified how spring and summer temperature and summer precipitation affected the growth of two common maple species in eastern North America. We analyzed tree growth patterns at two latitudes to assess what climatic cues each tree species and population responded to. We then used these results to forecast how each species might respond to future climate conditions. Our results suggest that, within each species, the climatic variables influencing tree growth differed between latitudes. In general, in the northern locations, tree growth was not affected by changes in temperature, while in the southern location, tree growth decreased as temperatures increased. Precipitation had a relatively positive effect on tree growth for both species at both latitudes, but to different extents.
The general expectation for climate change is that forests at higher latitudes operate at growing season temperatures below their optimum, thus they will respond positively to warming [15,56]. In this study, we observed that the trees growing in these two locations, within the higher latitudes of their ranges (Figure 1), varied in physiological acclimation to temperature, and did not always respond positively to higher temperatures. A recent study [57] found that A. saccharum populations in the western part of their distributional range may be more vulnerable to increasing temperature and drought compared to populations in the eastern part of their distributional range, which also indicated population-level variations in their response to environmental conditions. In our study, neither species, or population, responded to variabilities in spring temperature, our proxy for longer growing seasons (Figure 2), which indicates that an extended growing season for their early growth might not benefit trees of these species, although it could benefit seedlings [58,59]. In the southern populations, summer temperature had a negative effect on tree growth for both species, while in the north the effect was relatively positive but not significant, which suggests that the current temperature might have already exceeded the temperature optimum for individuals of southern populations with the current water availability, especially for A. saccharum. An increase in temperature can cause an increase in stomatal conductance, which will lead to water loss. In this case, plants will be threatened, and their growth will be adversely affected under water-deficient conditions [60]. A. saccharum has a broad temperature range of positive photosynthetic performance, and the optimum temperature for photosynthesis does not vary a lot among populations, but that for respiration can vary a lot under different conditions [61]. Thus, the different responses to increasing temperature for the northern and southern populations might be caused by their different acclimations of respiration. A. rubrum has a wide environmental tolerance [62], so warming may, in general, directly enhance photosynthesis, but it may indirectly reduce tree growth by exacerbating abiotic and biotic stresses such as drought and herbivory [63]. In our case, the negative effect of increasing temperature on the southern A. rubrum population might be caused by insufficient water availability and the consequent closure of the stomata.
A. rubrum and A. saccharum are both shade-tolerant species, and this characteristic further shapes the hydraulic conductivity in their roots, since shade-tolerant species have low plasticity in root conduit numbers, as well as root-specific hydraulic conductance among growth rings that allows them to perform well in environments with fluctuating water status [64,65]. Both A. rubrum and A. saccharum are tree species associated with mesic environments [47], thus our results corroborate their dependence on moist conditions, especially at the southern sites, where we documented a positive growth response to higher water availability. The fact that precipitation was only significant at the southern site likely indicates a higher water demand due to higher temperature [66]. Even though the southern populations we sampled were also in the relatively northern distributional range of both species (Figure 1), the results of this study suggest these individuals are already water-limited, since they respond positively to increasing precipitation, which makes them sensitive to both precipitation decline and global warming.
The lag effect of previous season growth was positive in this study, and this relationship has also been reported for A. saccharum in these regions [40]. This positive effect indicates a continuous increase in resources from previous years that can be allocated for growth in the following year [67]. The significant effect showed that the lag effect needs to be included when we were looking at the relationship between growth and environmental conditions [40].
Our SSP1-2.6 simulations suggest that both southern A. saccharum populations and northern A. saccharum populations will be negatively affected under two hotter conditions: only hotter (S1) as well as hotter and drier (S3) (Figure 3). Additionally, for the SSP5-8.5 simulations, southern A. rubrum populations under the two hotter conditions could be slightly negatively impacted. The future will likely lie between these two conditions, indicating that these populations, even if located in the northern parts of their distributional ranges (Figure 1), are at risk of being negatively affected by warming. Any increase in temperature and/or decrease in precipitation will cause a decline in their growth. For A. saccharum, the effects of increasing temperature and decreasing precipitation will be considerably more negative in the southern population (Figure 3). The decline in A. saccharum, mainly characterized as reduced radial increment and loss of crown vigor, has been recorded broadly across North American eastern deciduous forests [68,69]. Our predicted growth of northern populations suggests that their growth will increase slightly under the two hotter SSP5-8.5 simulations. This indicates that low temperatures during the growing season are still a limiting factor in this area, while the summers are still moist enough to provide positive growth conditions.
For both species, the northern populations might be able to maintain or increase their growth rates, while the southern populations are likely to experience lower growth rates for A. saccharum but not for A. rubrum under any scenarios. In this case, A. rubrum might be more successful than A. saccharum in the south under climate change conditions, which means the population of A. saccharum is likely to shrink in the future, while the population of A. rubrum will probably not be affected that much. These predicted results are consistent with the future species distribution changes forecasted by the United States Department of Agriculture (USDA), which indicates that populations in these two areas are going to decline in the future—A. saccharum under warmer and drier conditions [70], while A. rubrum will be more successful compared to A. saccharum when facing climate change.
5. Conclusions
Our study presents evidence of performance variations among populations in response to temperature and precipitation changes between different species. Although located in the northern range of their distribution, with a relatively small latitudinal difference between two sites, these populations showed varying responses to climate variables. Even if we expected individuals from these two populations to benefit from the longer growing seasons associated with warming, we found that this might not be the case at least for the southern A. saccharum populations in this study. We found that the most northern population is not expected to experience big changes in growth, while the southern population will likely decline. Incorporating these differences in vegetation models will be critical to ensuring accurate predictions of future forest growth patterns with a finer resolution.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f13030429/s1, Figure S1: Annual spring temperature, summer temperature and summer precipitation for southern and northern sites from 1997 to 2017. Table S1: Posterior parameter estimates at two latitudes for A. rubrum and A. saccharum, mean ± SD and 95% CI. Bold indicates statistically significant coefficients 95% CI does not include zero.
Author Contributions
I.I. mainly designed the study with X.W. X.W. collected the samples. X.W. prepared and processed the tree cores. X.W. analyzed the data with the help of I.I. X.W. wrote the manuscript and I.I. edited it. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Schrank Research fellowship in School of Environment and Sustainability (SEAS), University of Michigan and partly funded by the National Science Foundation-Division of Environmental Biology of the U.S., Grant Nos. 1252664.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the first author upon reasonable request.
Acknowledgments
We are grateful to Kirk Acharya for helping with the sampling, and to University of Michigan Biological Station for providing necessary facilities and accommodation during the field season. We are also grateful to Ivan Eastin, Richard Corlett and Kyle Tomlinson for reading the manuscript and giving helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2021: The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; WMO: Geneva, Switzerland; IPCC Secretariat: Geneva, Switzerland, 2021. [Google Scholar]
- Cramer, W.; Bondeau, A.; Woodward, F.I.; Prentice, I.C.; Betts, R.A.; Brovkin, V.; Cox, P.M.; Fisher, V.; Foley, J.A.; Friend, A.D.; et al. Global Response of Terrestrial Ecosystem Structure and Function to CO2 and Climate Change: Results from Six Dynamic Global Vegetation Models. Glob. Chang. Biol. 2001, 7, 357–373. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G.; Bond, W.J. Alternative Biome States in Terrestrial Ecosystems. Trends Plant Sci. 2020, 25, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; He, H.S.; Thompson, F.R., III; Fraser, J.S.; Dijak, W.D. Changes in Forest Biomass and Tree Species Distribution under Climate Change in the Northeastern United States. Landsc. Ecol. 2017, 32, 1399–1413. [Google Scholar] [CrossRef]
- Ackerly, D.D. Community Assembly, Niche Conservatism, and Adaptive Evolution in Changing Environments. Int. J. Plant Sci. 2003, 164, S165–S184. [Google Scholar] [CrossRef]
- Clark, J.S.; Bell, D.M.; Hersh, M.H.; Nichols, L. Climate Change Vulnerability of Forest Biodiversity: Climate and Competition Tracking of Demographic Rates. Glob. Chang. Biol. 2011, 17, 1834–1849. [Google Scholar] [CrossRef]
- Hatfield, J.; Lead, M.H.; Swanston, C.; Lead, N.; Janowiak, M.; Hub, N.F.S.; Steele, R.F.; Hub, M.; Cole, A.; Sharon Hestvik, R.M.A.; et al. USDA Midwest and Northern Forests Regional Climate Hub: Assessment of Climate Change Vulnerability and Adaptation and Mitigation Strategies; U.S. Department of Agriculture: Washington, DC, USA, 2015; p. 55. [Google Scholar]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global Trends in Phenotypic Plasticity of Plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Lamy, J.-B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and Plastic Responses of Quercus petraea Populations to Climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.B.; Shaw, R.G. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Watling, J.I.; Brandt, L.A.; Mazzotti, F.J.; Romanach, S.S. Use and Interpretation of Climate Envelope Models: A Practical Guide; University of Florida: Gainesville, FL, USA, 2013. [Google Scholar]
- Fernández-Pérez, L.; Zavala, M.A.; Villar -Salvador, P.; Madrigal-González, J. Divergent Last Century Tree Growth along an Altitudinal Gradient in a Pinus sylvestris L. Dry-Edge Population. For. Trees Livelihoods 2019, 10, 532. [Google Scholar] [CrossRef] [Green Version]
- Tucker, C.J.; Slayback, D.A.; Pinzon, J.E.; Los, S.O.; Myneni, R.B.; Taylor, M.G. Higher Northern Latitude Normalized Difference Vegetation Index and Growing Season Trends from 1982 to 1999. Int. J. Biometeorol. 2001, 45, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Oren, R. Differential Responses to Changes in Growth Temperature between Trees from Different Functional Groups and Biomes: A Review and Synthesis of Data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feeley, K.J.; Joseph Wright, S.; Nur Supardi, M.N.; Kassim, A.R.; Davies, S.J. Decelerating Growth in Tropical Forest Trees. Ecol. Lett. 2007, 10, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Venegas-González, A.; Roig, F.A.; Peña-Rojas, K.; Hadad, M.A.; Aguilera-Betti, I.; Muñoz, A.A. Recent Consequences of Climate Change Have Affected Tree Growth in Distinct Nothofagus macrocarpa (DC.) FM Vaz & Rodr Age Classes in Central Chile. For. Trees Livelihoods 2019, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.G.; Dukes, J.S. Plant Respiration and Photosynthesis in Global-Scale Models: Incorporating Acclimation to Temperature and CO2. Glob. Chang. Biol. 2013, 19, 45–63. [Google Scholar] [CrossRef]
- Repo, T.; Mononen, K.; Alvila, L.; Pakkanen, T.T.; Hänninen, H. Cold Acclimation of Pedunculate Oak (Quercus robur L.) at Its Northernmost Distribution Range. Environ. Exp. Bot. 2008, 63, 59–70. [Google Scholar] [CrossRef]
- Ibáñez, I.; Katz, D.S.W.; Lee, B.R. The Contrasting Effects of Short-Term Climate Change on the Early Recruitment of Tree Species. Oecologia 2017, 184, 701–713. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Hewison, A.J.M.; Klein, F.; Plard, F.; Douhard, M.; Davison, R.; Bonenfant, C. How Does Climate Change Influence Demographic Processes of Widespread Species? Lessons from the Comparative Analysis of Contrasted Populations of Roe Deer. Ecol. Lett. 2013, 16 (Suppl. S1), 48–57. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On Underestimation of Global Vulnerability to Tree Mortality and Forest Die-off from Hotter Drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth Century Redistribution in Climatic Drivers of Global Tree Growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. Responses of Forest Trees to Single and Multiple Environmental Stresses from Seedlings to Mature Plants: Past Stress History, Stress Interactions, Tolerance and Acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Ludlow, A. Some Factors Influencing the Increment of Forests. Forestry 1997, 70, 381–388. [Google Scholar] [CrossRef]
- Oribe, Y.; Funada, R.; Shibagaki, M.; Kubo, T. Cambial Reactivation in Locally Heated Stems of the Evergreen Conifer Abies Sachalinensis (Schmidt) Masters. Planta 2001, 212, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Oribe, Y.; Kubo, T. Effect of Heat on Cambial Reactivation during Winter Dormancy in Evergreen and Deciduous Conifers. Tree Physiol. 1997, 17, 81–87. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of Cambial Activity in Relation to Environmental Conditions: Understanding the Role of Temperature in Wood Formation of Trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Balducci, L.; Cuny, H.E.; Rathgeber, C.B.K.; Deslauriers, A.; Giovannelli, A.; Rossi, S. Compensatory Mechanisms Mitigate the Effect of Warming and Drought on Wood Formation. Plant Cell Environ. 2016, 39, 1338–1352. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Law, R.D.; Crafts-Brandner, S.J. Inhibition and Acclimation of Photosynthesis to Heat Stress Is Closely Correlated with Activation of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Physiol. 1999, 120, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; pp. 662–710. ISBN 9780132279383. [Google Scholar]
- McDowell, N.G.; Williams, A.P.; Xu, C.; Pockman, W.T.; Dickman, L.T.; Sevanto, S.; Pangle, R.; Limousin, J.; Plaut, J.; Mackay, D.S.; et al. Multi-Scale Predictions of Massive Conifer Mortality due to Chronic Temperature Rise. Nat. Clim. Chang. 2016, 6, 295–300. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How Do Trees Die? A Test of the Hydraulic Failure and Carbon Starvation Hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature Sensitivity of Drought-Induced Tree Mortality Portends Increased Regional Die-off under Global-Change-Type Drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltier, D.M.P.; Fell, M.; Ogle, K. Legacy Effects of Drought in the Southwestern United States: A Multi-species Synthesis. Ecol. Monogr. 2016, 86, 312–326. [Google Scholar] [CrossRef]
- Ibáñez, I.; Zak, D.R.; Burton, A.J.; Pregitzer, K.S. Anthropogenic Nitrogen Deposition Ameliorates the Decline in Tree Growth Caused by a Drier Climate. Ecology 2018, 99, 411–420. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Peng, S. Global Analysis of Time-Lag and -Accumulation Effects of Climate on Vegetation Growth. Int. J. Appl. Earth Obs. Geoinf. 2020, 92, 102179. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of Drought at Different Time Scales on Forest Growth across a Wide Climatic Gradient in North-Eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- McCollum, C.; Ibáñez, I. Soil Moisture Gradients and Climate Change: Predicting Growth of a Critical Boreal Tree Species. Can. J. For. Res. 2020, 50, 1074–1080. [Google Scholar] [CrossRef]
- Tardif, J.; Brisson, J.; Bergeron, Y. Dendroclimatic Analysis of Acer saccharum, Fagus grandifolia, and Tsuga canadensis from an Old-Growth Forest, Southwestern Quebec. Can. J. For. Res. 2001, 31, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- McNab, W.H.; Cleland, D.T.; Freeouf, J.A.; Keys, J.E.; Nowacki, G.J.; Carpenter, C. Description of Ecological Subregions: Sections of the Conterminous United States; General Technical Report WO-76B; Washington Office: Washington, DC, USA, 2007; Volume 76, pp. 1–82. [Google Scholar] [CrossRef] [Green Version]
- National Centers for Environmental Information, Climate Data Online: Station Details. Available online: https://www.ncdc.noaa.gov/cdo-web/datasets (accessed on 5 November 2019).
- Barnes, B.V.; Wagner, W.H. Michigan Trees, Revised and Updated: A Guide to the Trees of the Great Lakes Region; University of Michigan Press: Ann Arbor, MI, USA, 2004; ISBN 0472089218. [Google Scholar]
- Edwards, N.T.; Norby, R.J. Below-ground respiratory responses of sugar maple and red maple saplings to atmospheric CO2 enrichment and elevated air temperature. Plant Soil 1998, 206, 85–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Bergeron, Y.; Zhao, X.-H.; Drobyshev, I. Stand History Is More Important than Climate in Controlling Red Maple (Acer rubrum L.) Growth at Its Northern Distribution Limit in Western Quebec, Canada. J. Plant Ecol. 2014, 8, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Phipps, R.L. Collecting, Preparing, Crossdating, and Measuring Tree Increment Cores; U.S. Department of the Interior, Geological Survey: Reston, VA, USA, 1985. [Google Scholar]
- Stokes, M.A. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996; ISBN 9780816516803. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010; ISBN 9780816526840. [Google Scholar]
- Ogle, K.; Barber, J.J.; Barron-Gafford, G.A.; Bentley, L.P.; Young, J.M.; Huxman, T.E.; Loik, M.E.; Tissue, D.T. Quantifying Ecological Memory in Plant and Ecosystem Processes. Ecol. Lett. 2015, 18, 221–235. [Google Scholar] [CrossRef] [Green Version]
- Lines, E.R.; Zavala, M.A.; Purves, D.W.; Coomes, D.A. Predictable Changes in Aboveground Allometry of Trees along Gradients of Temperature, Aridity and Competition. Glob. Ecol. Biogeogr. 2012, 21, 1017–1028. [Google Scholar] [CrossRef]
- Thomas, A.; O’Hara, R.; Ligges, U.; Sturtz, S. Making BUGS Open. R News 2006, 6, 12–17. [Google Scholar]
- Zeng, H.; Jia, G.; Epstein, H. Recent changes in phenology over the northern high latitudes detected from multi-satellite data. Environ. Res. Lett. 2011, 6, 045508. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, A.; LeBlanc, D. Growth-Climate Relationships of Acer saccharum (Aceraceae) along a Latitudinal Climate Gradient in Its Western range1. J. Torrey Bot. Soc. 2020, 147, 232–242. [Google Scholar] [CrossRef]
- Lee, B.R.; Ibáñez, I. Improved Phenological Escape Can Help Temperate Tree Seedlings Maintain Demographic Performance under Climate Change Conditions. Glob. Chang. Biol. 2021, 27, 3883–3897. [Google Scholar] [CrossRef]
- Lee, B.R.; Ibáñez, I. Spring Phenological Escape Is Critical for the Survival of Temperate Tree Seedlings. Funct. Ecol. 2021, 35, 1848–1861. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal Conductance Increases with Rising Temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, C.A.; Norby, R.J.; Wullschleger, S.D. Acclimation of Photosynthesis and Respiration to Simulated Climatic Warming in Northern and Southern Populations of Acer saccharum: Laboratory and Field Evidence. Tree Physiol. 2000, 20, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, M.D. The Red Maple Paradox. Bioscience 1998, 48, 355–364. [Google Scholar] [CrossRef]
- Lahr, E.C.; Dunn, R.R.; Frank, S.D. Variation in Photosynthesis and Stomatal Conductance among Red Maple (Acer rubrum) Urban Planted Cultivars and Wildtype Trees in the Southeastern United States. PLoS ONE 2018, 13, e0197866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maherali, H.; DeLucia, E.H.; Sipe, T.W. Hydraulic Adjustment of Maple Saplings to Canopy Gap Formation. Oecologia 1997, 112, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Zadworny, M.; Comas, L.H.; Eissenstat, D.M. Linking Fine Root Morphology, Hydraulic Functioning and Shade Tolerance of Trees. Ann. Bot. 2018, 122, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.S.A.; Lawson, T. Climate Change and Stomatal Physiology. Annu. Plant Rev. Online 2019, 2, 713–752. [Google Scholar] [CrossRef]
- Babst, F.; Poulter, B.; Trouet, V.; Tan, K.; Neuwirth, B.; Wilson, R.; Carrer, M.; Grabner, M.; Tegel, W.; Levanic, T.; et al. Site- and Species-Specific Responses of Forest Growth to Climate across the European Continent. Glob. Ecol. Biogeogr. 2013, 22, 706–717. [Google Scholar] [CrossRef]
- Bishop, D.A.; Beier, C.M.; Pederson, N.; Lawrence, G.B.; Stella, J.C.; Sullivan, T.J. Regional Growth Decline of Sugar Maple (Acer saccharum) and Its Potential Causes. Ecosphere 2015, 6, art179. [Google Scholar] [CrossRef] [Green Version]
- Horsley, S.B.; Long, R.P.; Bailey, S.W.; Hallett, R.A.; Wargo, P.M. Health of eastern North American sugar maple forests and factors affecting decline. North. J. Appl. For. 2002, 19, 34–44. [Google Scholar] [CrossRef]
- Climate Change Tree Atlas, Version 4. 2019. Available online: https://doi.org/10.2737/climate-change-tree-atlas-v4 (accessed on 1 December 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).