Predictors of the Success of Natural Regeneration in a Himalayan Treeline Ecotone
Abstract
:1. Introduction
2. Materials and Methods
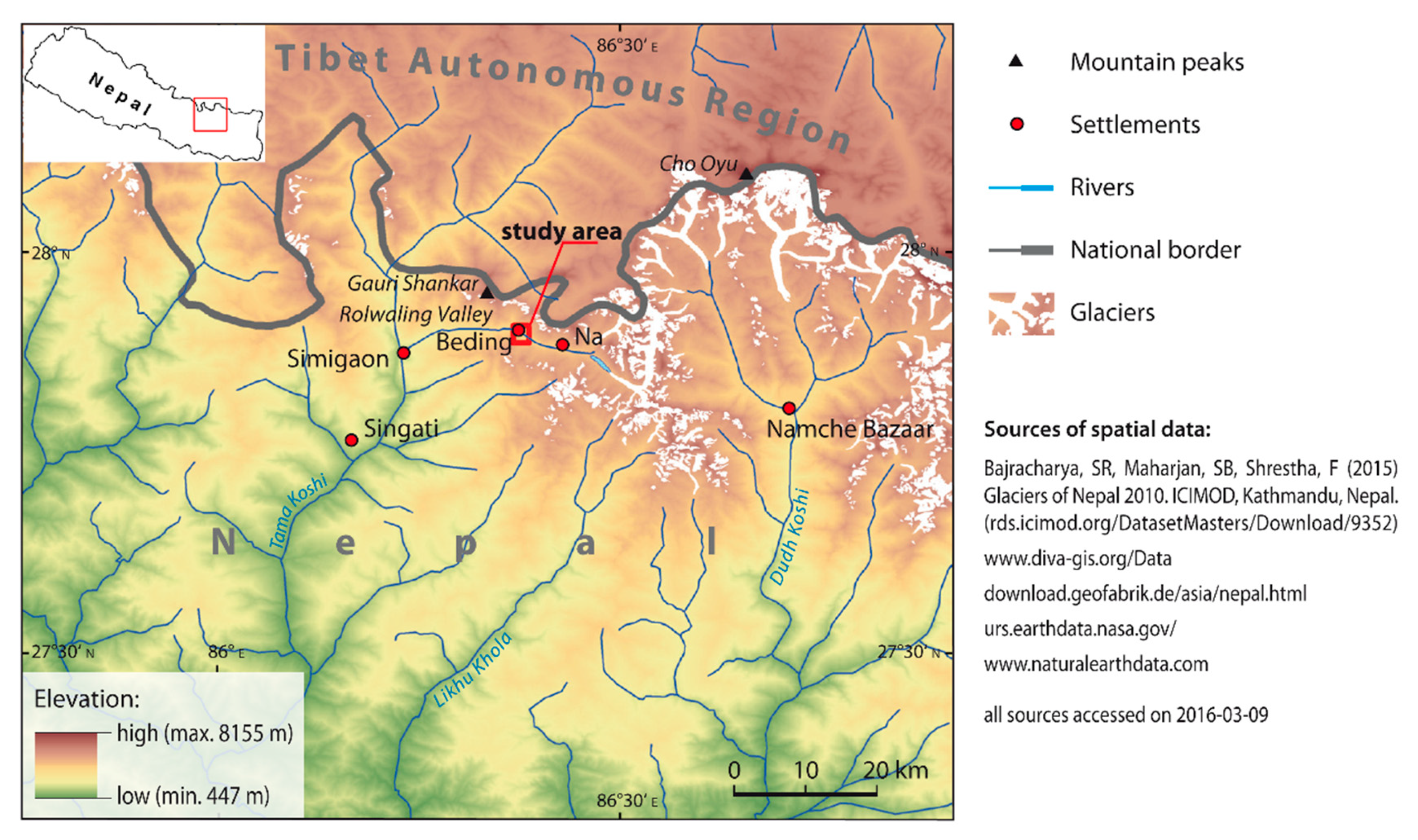
2.1. Study Site
2.2. Data Collection, Processing and Analyses
3. Results
4. Discussion
4.1. Abies spectabilis
4.2. Betula utilis
4.3. Rhododendron campanulatum
4.4. Cross-Species Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtmeier, F.-K. Mountain Timberlines; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-9704-1. [Google Scholar]
- Körner, C. Climatic controls of global high elevation treelines. In Encyclopedia of the World’s Biomes; Goldstein, M.I., DellaSala, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 275–281. [Google Scholar]
- Körner, C. Alpine treelines. In Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems, 3rd ed.; Körner, C., Ed.; Springer: Cham, Switzerland, 2021; pp. 141–173. [Google Scholar]
- Schickhoff, U.; Bobrowski, M.; Mal, S.; Schwab, N.; Singh, R.B. The world’s mountains in the Anthropocene. In Mountain Landscapes in Transition: Effects of Land Use and Climate Change; Schickhoff, U., Singh, R.B., Mal, S., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–144. ISBN 978-3-030-70238-0. [Google Scholar]
- Schwörer, C.; Kaltenrieder, P.; Glur, L.; Berlinger, M.; Elbert, J.; Frei, S.; Gilli, A.; Hafner, A.; Anselmetti, F.S.; Grosjean, M.; et al. Holocene climate, fire and vegetation dynamics at the treeline in the northwestern Swiss alps. Veg. Hist. Archaeobotany 2014, 23, 479–496. [Google Scholar] [CrossRef]
- Vincze, I.; Orbán, I.; Birks, H.H.; Pál, I.; Finsinger, W.; Hubay, K.; Marinova, E.; Jakab, G.; Braun, M.; Biró, T.; et al. Holocene treeline and timberline changes in the south Carpathians (Romania): Climatic and anthropogenic drivers on the southern slopes of the Retezat Mountains. Holocene 2017, 27, 1613–1630. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Liao, M.; Ni, J.; Liu, X.; Wang, Y. Treeline composition and biodiversity change on the southeastern Tibetan plateau during the past millennium, inferred from a high-resolution alpine pollen record. Quat. Sci. Rev. 2019, 206, 44–55. [Google Scholar] [CrossRef]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liang, E.; Wang, Y.; Babst, F.; Camarero, J.J. Mountain treelines climb slowly despite rapid climate warming. Glob. Ecol. Biogeogr. 2021, 30, 305–315. [Google Scholar] [CrossRef]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhary, R.P.; Müller, M.; Scholten, T.; Schwab, N.; Weidinger, J. The treeline ecotone in Rolwaling Himal, Nepal: Pattern-process relationships and treeline shift potential. In Ecology of Himalayan Treeline Ecotone, Singh, S.P., Reshi, Z.A., Joshi, R., Eds.; Springer: Singapore, 2022; in press. [Google Scholar]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhary, R.P.; Gerlitz, L.; Heyken, H.; Lange, J.; Müller, M.; Scholten, T.; et al. Do Himalayan treelines respond to recent climate change? An evaluation of sensitivity indicators. Earth Syst. Dynam. 2015, 6, 245–265. [Google Scholar] [CrossRef]
- Schwab, N.; Janecka, K.; Kaczka, R.J.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Schickhoff, U. Ecological relationships at a near-natural treeline, Rolwaling Valley, Nepal Himalaya: Implications for the sensitivity to climate change. Erdkunde 2020, 74, 14–55. [Google Scholar] [CrossRef]
- Lv, L.-X.; Zhang, Q.-B. Asynchronous recruitment history of Abies spectabilis along an altitudinal gradient in the Mt. Everest region. J. Plant. Ecol. 2012, 5, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhary, R.P.; Gerlitz, L.; Lange, J.; Müller, M.; Scholten, T.; Schwab, N. Climate change and treeline dynamics in the Himalaya. In Climate Change, Glacier Response, and Vegetation Dynamics in the Himalaya; Singh, R.B., Schickhoff, U., Mal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 271–306. ISBN 978-3-319-28975-5. [Google Scholar]
- Tiwari, A.; Fan, Z.-X.; Jump, A.S.; Li, S.-F.; Zhou, Z.-K. Gradual expansion of moisture sensitive Abies spectabilis forest in the Trans-Himalayan zone of central Nepal associated with climate change. Dendrochronologia 2017, 41, 34–43. [Google Scholar] [CrossRef]
- Bürzle, B.; Schickhoff, U.; Schwab, N.; Wernicke, L.M.; Müller, Y.K.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Oldeland, J. Seedling recruitment and facilitation dependence on safe site characteristics in a Himalayan treeline ecotone. Plant Ecol. 2018, 219, 115–132. [Google Scholar] [CrossRef]
- Mainali, K.; Shrestha, B.B.; Sharma, R.K.; Adhikari, A.; Gurarie, E.; Singer, M.; Parmesan, C. Contrasting responses to climate change at Himalayan treelines revealed by population demographics of two dominant species. Ecol. Evol. 2020, 10, 1209–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.K.; Tiwari, A.; Shrestha, B.B. Changes in regeneration and leaf traits of Rhododendron campanulatum along a treeline ecotone in central Nepal. J. Mt. Sci. 2020, 17, 602–613. [Google Scholar] [CrossRef]
- Schwab, N.; Kaczka, R.J.; Janecka, K.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Schickhoff, U. Climate change-induced shift of tree growth sensitivity at a central Himalayan treeline ecotone. Forests 2018, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Schickhoff, U. The upper timberline in the Himalayas, Hindu Kush and Karakorum: A review of geographical and ecological aspects. In Mountain Ecosystems: Studies in Treeline Ecology; Broll, G., Keplin, B., Eds.; Springer: Berlin, Germany, 2005; pp. 275–354. [Google Scholar]
- Miehe, G.; Miehe, S.; Böhner, J.; Ghimire, S.K.; Bhattarai, K.; Chaudhary, R.P.; Subedi, M.; Jha, P.K.; Pendry, C. Vegetation ecology. In Nepal: An Introduction to the Natural History, Ecology and Human Environment in the Himalayas; Miehe, G., Pendry, C., Chaudhary, R.P., Eds.; Royal Botanic Garden Edinburgh: Edinburgh, UK, 2015; pp. 385–472. ISBN 978-1-910877-02-9. [Google Scholar]
- Bobrowski, M.; Gerlitz, L.; Schickhoff, U. Modelling the potential distribution of Betula utilis in the Himalaya. Glob. Ecol. Conserv. 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Frei, E.R.; Bianchi, E.; Bernareggi, G.; Bebi, P.; Dawes, M.A.; Brown, C.D.; Trant, A.J.; Mamet, S.D.; Rixen, C. Biotic and abiotic drivers of tree seedling recruitment across an alpine treeline ecotone. Sci. Rep. 2018, 8, 10894. [Google Scholar] [CrossRef]
- Johnson, A.C.; Yeakley, J.A. Microsites and climate zones: Seedling regeneration in the alpine treeline ecotone worldwide. Forests 2019, 10, 864. [Google Scholar] [CrossRef] [Green Version]
- Schwab, N.; Schickhoff, U.; Bürzle, B.; Müller, M.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Oldeland, J. Implications of tree species—environment relationships for the responsiveness of Himalayan krummholz treelines to climate change. J. Mt. Sci. 2017, 14, 453–473. [Google Scholar] [CrossRef]
- Wang, Y.; Pederson, N.; Ellison, A.M.; Buckley, H.L.; Case, B.S.; Liang, E.; Camarero, J.J. Increased stem density and competition may diminish the positive effects of warming at alpine treeline. Ecology 2016, 97, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.B.; Ghimire, B.; Lekhak, H.D.; Jha, P.K. Regeneration of treeline birch (Betula utilis D. Don) forest in a trans-Himalayan dry valley in Central Nepal. Mt. Res. Dev. 2007, 27, 259–267. [Google Scholar] [CrossRef]
- Kambo, D.; Danby, R.K. Factors influencing the establishment and growth of tree seedlings at subarctic alpine treelines. Ecosphere 2018, 9, e02176. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R. Farewell to Yak and Yeti? The Sherpas of Rolwaling Facing a Globalised World; Vajra Books: Kathmandu, Nepal, 2015; ISBN 978-9937-623-43-8. [Google Scholar]
- Schwab, N.; Schickhoff, U.; Müller, M.; Gerlitz, L.; Bürzle, B.; Böhner, J.; Chaudhary, R.P.; Scholten, T. Treeline responsiveness to climate warming: Insights from a krummholz treeline in Rolwaling Himal, Nepal. In Climate Change, Glacier Response, and Vegetation Dynamics in the Himalaya; Singh, R.B., Schickhoff, U., Mal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 307–345. ISBN 978-3-319-28975-5. [Google Scholar]
- Bürzle, B.; Schickhoff, U.; Schickhoff, U.; Schwab, N.; Oldeland, J.; Müller, M.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Dickoré, W.B. Phytosociology and ecology of treeline ecotone vegetation in Rolwaling Himal, Nepal. Phytocoenologia 2017, 47, 197–220. [Google Scholar] [CrossRef]
- Schwab, N.; Bürzle, B.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Schickhoff, U. Environmental drivers of species composition and tree species density of a near-natural central Himalayan treeline ecotone: Consequences for the response to climate change. In Mountain Landscapes in Transition: Effects of Land Use and Climate Change; Schickhoff, U., Singh, R.B., Mal, S., Eds.; Springer: Cham, Switzerland, 2022; pp. 349–370. ISBN 978-3-030-70238-0. [Google Scholar] [CrossRef]
- Müller, M.; Schickhoff, U.; Scholten, T.; Drollinger, S.; Böhner, J.; Chaudhary, R.P. How do soil properties affect alpine treelines? General principles in a global perspective and novel findings from Rolwaling Himal, Nepal. Prog. Phys. Geogr. 2016, 40, 135–160. [Google Scholar] [CrossRef] [Green Version]
- Böhner, J.; Miehe, G.; Miehe, S.; Nagy, L. Climate and weather. In Nepal: An Introduction to the Natural History, Ecology and Human Environment in the Himalayas; Miehe, G., Pendry, C., Chaudhary, R.P., Eds.; Royal Botanic Garden Edinburgh: Edinburgh, UK, 2015; pp. 23–90. ISBN 978-1-910877-02-9. [Google Scholar]
- Gerlitz, L.; Bechtel, B.; Böhner, J.; Bobrowski, M.; Bürzle, B.; Müller, M.; Scholten, T.; Schickhoff, U.; Schwab, N.; Weidinger, J. Analytic comparison of temperature lapse rates and precipitation gradients in a Himalayan treeline environment: Implications for statistical downscaling. In Climate Change, Glacier Response, and Vegetation Dynamics in the Himalaya; Singh, R.B., Schickhoff, U., Mal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 49–64. ISBN 978-3-319-28975-5. [Google Scholar]
- Karki, R.; Talchabhadel, R.; Aalto, J.; Baidya, S.K. New climatic classification of Nepal. Appl. Clim. 2016, 125, 799–808. [Google Scholar] [CrossRef]
- Weidinger, J.; Gerlitz, L.; Bobrowski, M.; Böhner, J.; Chaudhary, R.P.; Schickhoff, U.; Schwab, N.; Scholten, T. TREELINE—Longterm atmospheric and pedo-climatic observations along an upper treeline ecotone in the Himalayas, Nepal [Data Set]. 2021. Available online: https://www.fdr.uni-hamburg.de/record/9563#.Yi6spDURWUl (accessed on 15 November 2021).
- Press, J.R.; Shrestha, K.K.; Sutton, D.A. Annotated Checklist of the Flowering Plants of Nepal (Updated Online Version 2014); Natural History Museum: London, UK, 2000; ISBN 0-565-09154-9. [Google Scholar]
- Watson, M.F.; Akiyama, S.; Ikeda, H.; Pendry, C.A.; Rajbhandari, K.R.; Shrestha, K.K. (Eds.) Flora of Nepal: Magnoliaceae to Rosaceae; Royal Botanic Garden Edinburgh: Edinburgh, UK, 2011; Volume 3, ISBN 978-1-906129-78-1. [Google Scholar]
- Rich, P.M.; Wood, J.; Vieglais, D.A.; Burek, K.; Webb, N. HemiView User Manual, Version 2.1; Helios Environmental Modelling Institute, LLC & Delta-T Devices Ltd.: Cambridge, UK, 1999. [Google Scholar]
- Newton, A.C. Forest Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2007; ISBN 978-0-19-856745-5. [Google Scholar]
- Müller, M.; Schwab, N.; Schickhoff, U.; Böhner, J.; Scholten, T. Soil temperature and soil moisture patterns in a Himalayan alpine treeline ecotone. Arct. Antarct. Alp. Res. 2016, 48, 501–521. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Kursa, M.; Jankowski, A.; Rudnicki, W. Boruta—A system for feature selection. Fundam. Inform. 2010, 101, 271–285. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration patterns of European oak species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in dependence of environment and neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar] [CrossRef]
- Friendly, M. Visualizing Categorical Data; SAS Institute: Cary, NC, USA, 2000; ISBN 978-1-59047-497-6. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer New York: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Zuur, A.F.; Hilbe, J.M.; Leno, E.N. A Beginner’s Guide to GLM and GLMM with R: A Frequentist and Bayesian Perspective for Ecologists; Highland Statistics Ltd.: Newburgh, UK, 2013; ISBN 978-0-9571741-3-9. [Google Scholar]
- Walker, J.A. Applied Statistics for Experimental Biology. 2018. Available online: https://www.middleprofessor.com/files/applied-biostatistics_bookdown/_book/ (accessed on 20 December 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE: Los Angeles, CA, USA, 2019; ISBN 978-1-5443-3647-3. [Google Scholar]
- Dormann, C. Environmental Data Analysis: An Introduction with Examples in R.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-55019-6. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.3.3.0. 2020. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 20 September 2021).
- Monteiro-Henriques, T.; Fernandes, P.M. Regeneration of native forest species in mainland Portugal: Identifying main drivers. Forests 2018, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Barton, K. MuMIn: MuMIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 20 September 2021).
- Lüdecke, D. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Hilbe, J.M. Negative Binomial Regression; Cambridge Univ. Press: Cambridge, UK, 2011; ISBN 978-0-521-19815-8. [Google Scholar]
- Leeper, T.J. Margins: Marginal Effects for Model Objects. R Package Version 0.3.26. 2021. Available online: https://CRAN.R-project.org/package=margins (accessed on 20 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 June 2021).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 10 September 2021).
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J. Stat. Softw. 2018, 87, 1–27. [Google Scholar] [CrossRef]
- Patil, I. ggstatsplot: “Ggplot2” Based Plots with Statistical Details. CRAN. 2018. Available online: https://CRAN.R-project.org/web/packages/ggstatsplot/index.html (accessed on 18 September 2021).
- Wilke, C.O. Ggtext: Improved Text Rendering Support for “ggplot2”. R Package Version 0.1.1. 2020. Available online: https://CRAN.R-project.org/package=ggtext (accessed on 20 September 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Fernihough, A. Mfx: Marginal Effects, Odds Ratios and Incidence Rate Ratios for GLMs. R Package Version 1.2-2. 2019. Available online: https://CRAN.R-project.org/package=mfx (accessed on 30 September 2021).
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2020; Available online: https://cran.r-project.org/web/packages/psych/index.html (accessed on 12 September 2021).
- Pedersen, T.L.; Shemanarev, M. Ragg: Graphic Devices Based on AGG. R Package Version 1.2.1. 2021. Available online: https://CRAN.R-project.org/package=ragg (accessed on 21 November 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Meyer, D.; Zeileis, A.; Hornik, K. Vcd: Visualizing Categorical Data. R Package Version 1.4-9. 2021. Available online: https://CRAN.R-project.org/package=vcd (accessed on 10 September 2021).
- Germino, M.J.; Smith, W.K.; Resor, A.C. Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecol. 2002, 162, 157–168. [Google Scholar] [CrossRef]
- Tingstad, L.; Olsen, S.L.; Klanderud, K.; Vandvik, V.; Ohlson, M. Temperature, precipitation and biotic interactions as determinants of tree seedling recruitment across the tree line ecotone. Oecologia 2015, 179, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrowolska, D.; Veblen, T.T. Treefall-gap structure and regeneration in mixed Abies alba stands in central Poland. For. Ecol. Manag. 2008, 255, 3469–3476. [Google Scholar] [CrossRef]
- Dobrowolska, D. Structure of silver fir (Abies alba Mill.) natural regeneration in the ‘Jata’ reserve in Poland. For. Ecol. Manag. 1998, 110, 237–247. [Google Scholar] [CrossRef]
- Johnson, A.C.; Yeakley, J.A. Seedling regeneration in the alpine treeline ecotone: Comparison of wood microsites and adjacent soil substrates. Mt. Res. Dev. 2016, 36, 443–451. [Google Scholar] [CrossRef]
- Lei, T.T.; Semones, S.W.; Walker, J.F.; Clinton, B.D.; Nilsen, E.T. Effects of Rhododendron maximum thickets on tree seed dispersal, seedling morphology, and survivorship. Int. J. Plant Sci. 2002, 163, 991–1000. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Piao, S.; Lu, X.; Camarero, J.J.; Zhu, H.; Zhu, L.; Ellison, A.M.; Ciais, P.; Peñuelas, J. Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loranger, H.; Zotz, G.; Bader, M.Y. Competitor or facilitator? The ambiguous role of alpine grassland for the early establishment of tree seedlings at treeline. Oikos 2017, 126, 1625–1636. [Google Scholar] [CrossRef]
- Gaire, N.P.; Koirala, M.; Bhuju, D.R.; Carrer, M. Site- and species-specific treeline responses to climatic variability in eastern Nepal Himalaya. Dendrochronologia 2017, 41, 44–56. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Wind as an ecological agent at treelines in north America, the Alps, and the European Subarctic. Phys. Geogr. 2010, 31, 203–233. [Google Scholar] [CrossRef]
- Parish, R.; Antos, J.A. Advanced regeneration and seedling establishment in small cutblocks in high-elevation spruce-fir forest at Sicamous Creek, southern British Columbia. Can. J. For. Res. 2005, 35, 1877–1888. [Google Scholar] [CrossRef]
- Huth, F.; Wagner, S. Gap structure and establishment of silver birch regeneration (Betula pendula Roth.) in Norway spruce stands (Picea abies L. Karst.). For. Ecol. Manag. 2006, 229, 314–324. [Google Scholar] [CrossRef]
- Gratzer, G.; Darabant, A.; Chhetri, P.B.; Rai, P.B.; Eckmüllner, O. Interspecific variation in the response of growth, crown morphology, and survivorship to light of six tree species in the conifer belt of the Bhutan Himalayas. Can. J. For. Res. 2004, 34, 1093–1107. [Google Scholar] [CrossRef]
- Hughes, N.M.; Johnson, D.M.; Akhalkatsi, M.; Abdaladze, O. Characterizing Betula litwinowii seedling microsites at the alpine-treeline ecotone, central greater Caucasus mountains, Georgia. Arct. Antarct. Alp. Res. 2009, 41, 112–118. [Google Scholar] [CrossRef]
- Wright, E.F.; Coates, K.D.; Bartemucci, P. Regeneration from seed of six tree species in the interior cedar-hemlock forests of British Columbia as affected by substrate and canopy gap position. Can. J. For. Res. 1998, 28, 1352–1364. [Google Scholar] [CrossRef]
- Anschlag, K.; Broll, G.; Holtmeier, F.-K. Mountain birch seedlings in the treeline ecotone, subarctic Finland: Variation in above- and below-ground growth depending on microtopography. Arct. Antarct. Alp. Res. 2008, 40, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Pröll, G.; Darabant, A.; Gratzer, G.; Katzensteiner, K. Unfavourable microsites, competing vegetation and browsing restrict post-disturbance tree regeneration on extreme sites in the Northern Calcareous Alps. Eur. J. For. Res. 2015, 134, 293–308. [Google Scholar] [CrossRef]
- Kubota, Y.; Hara, T. Recruitment processes and species coexistence in a sub-boreal forest in northern Japan. Ann. Bot. 1996, 78, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Götmark, F.; Kiffer, C. Regeneration of oaks (Quercus robur/Q. petraea) and three other tree species during long-term succession after catastrophic disturbance (windthrow). Plant Ecol. 2014, 215, 1067–1080. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Fan, Z.-X.; Jump, A.S.; Zhou, Z.-K. Warming induced growth decline of Himalayan birch at its lower range edge in a semi-arid region of Trans-Himalaya, central Nepal. Plant Ecol. 2017, 218, 621–633. [Google Scholar] [CrossRef]
- Drollinger, S.; Müller, M.; Kobl, T.; Schwab, N.; Böhner, J.; Schickhoff, U.; Scholten, T. Decreasing nutrient concentrations in soils and trees with increasing elevation across a treeline ecotone in Rolwaling Himal, Nepal. J. Mt. Sci. 2017, 14, 843–858. [Google Scholar] [CrossRef]
- Holtmeier, F.; Broll, G. Feedback effects of clonal groups and tree clusters on site conditions at the treeline: Implications for treeline dynamics. Clim. Res. 2017, 73, 85–96. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Liang, E.; Wang, Y.; Dawadi, B.; Camarero, J.J. Tree-to-tree interactions slow down Himalayan treeline shifts as inferred from tree spatial patterns. J. Biogeogr. 2020, 47, 1816–1826. [Google Scholar] [CrossRef]
- Chhetri, P.K.; Bista, R.; Shrestha, K.B. How does the stand structure of treeline-forming species shape the treeline ecotone in different regions of the Nepal Himalayas? J. Mt. Sci. 2020, 17, 2354–2368. [Google Scholar] [CrossRef]


| Development Stage | Height [cm] | Diameter 130 cm above Ground [cm] | Description |
|---|---|---|---|
| 1 | 0–10 | <7 | smallest seedlings |
| 2 | >10–50 | <7 | large seedlings |
| 3 | >50–130 | <7 | small saplings |
| 4 | >130 | <7 | large saplings |
| 5 | >130 | ≥7 | mature/adult trees |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwab, N.; Bürzle, B.; Bobrowski, M.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Weidinger, J.; Schickhoff, U. Predictors of the Success of Natural Regeneration in a Himalayan Treeline Ecotone. Forests 2022, 13, 454. https://doi.org/10.3390/f13030454
Schwab N, Bürzle B, Bobrowski M, Böhner J, Chaudhary RP, Scholten T, Weidinger J, Schickhoff U. Predictors of the Success of Natural Regeneration in a Himalayan Treeline Ecotone. Forests. 2022; 13(3):454. https://doi.org/10.3390/f13030454
Chicago/Turabian StyleSchwab, Niels, Birgit Bürzle, Maria Bobrowski, Jürgen Böhner, Ram Prasad Chaudhary, Thomas Scholten, Johannes Weidinger, and Udo Schickhoff. 2022. "Predictors of the Success of Natural Regeneration in a Himalayan Treeline Ecotone" Forests 13, no. 3: 454. https://doi.org/10.3390/f13030454






