The Effects of Environmental Changes on Plant Species and Forest Dependent Communities in the Amazon Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Literature Selection
2.1.1. Building Tables to Demonstrate Reducing and Increasing Populations of Plant
Species in Deforested Amazon Lands
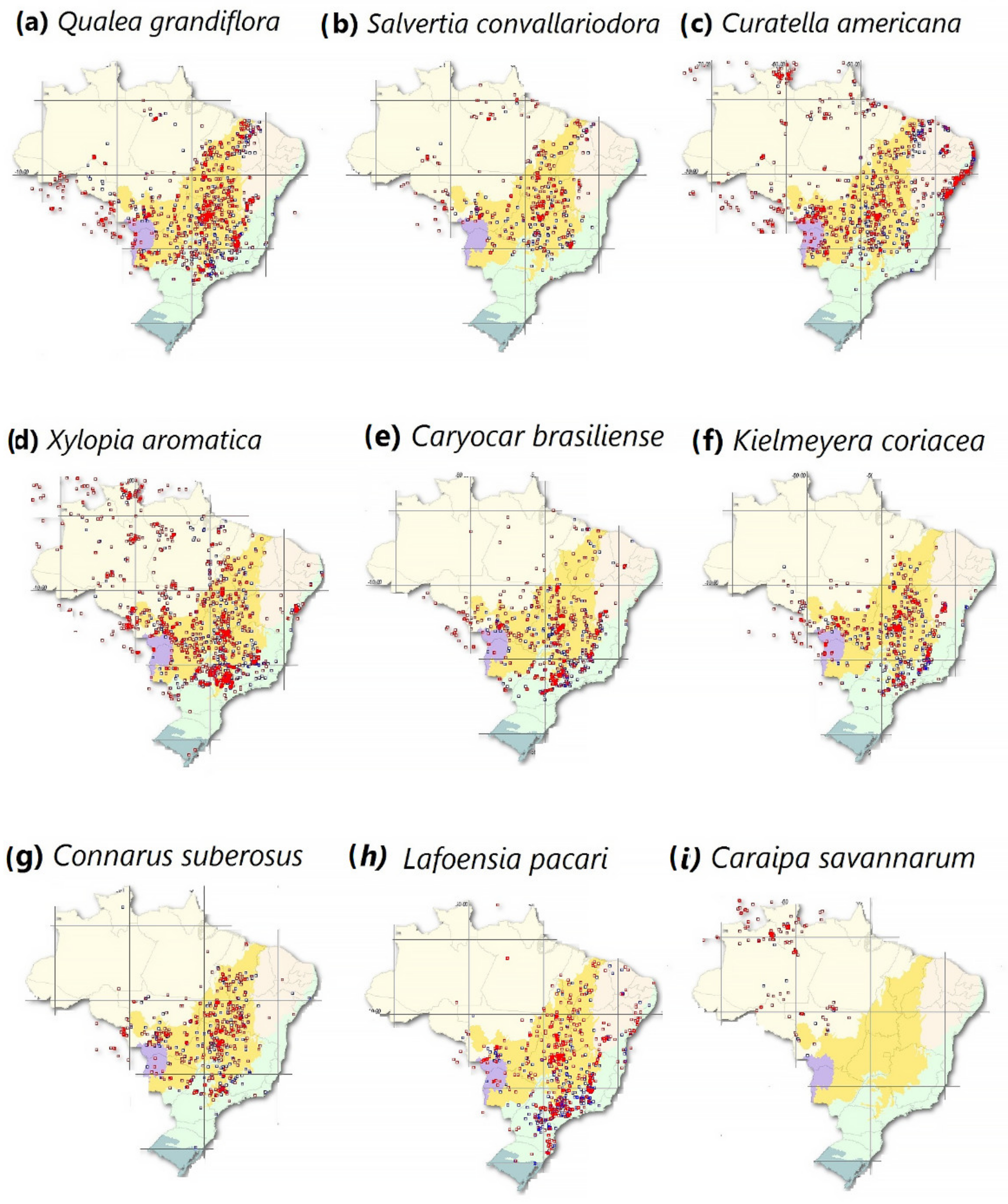
2.1.2. Maps Concerning Typical Amazon Savannah Species
2.1.3. Annual Range of Economic Importance Losses Caused by Deforestation
2.1.4. Potential for Agroforestry System Strategy Improvement
3. Results
3.1. Deforestation Has Significantly Reduced Both Seed and Fruit Production and Altered Species Composition
3.2. Forest Fragmentation Has Reduced Seed and Fruit Diversity and Density
3.3. Selective Logging Reduces the Wood Density and Availability of Non-Timber
Forest Products
3.4. Forest Fires Destroy Trees and Strongly Affect Species Composition
3.5. Droughts Increase the Mortality of Plant Species
3.6. Global Warming Has Led to Changes in Vegetation
3.7. Changes to Degraded Savanna-like Vegetation
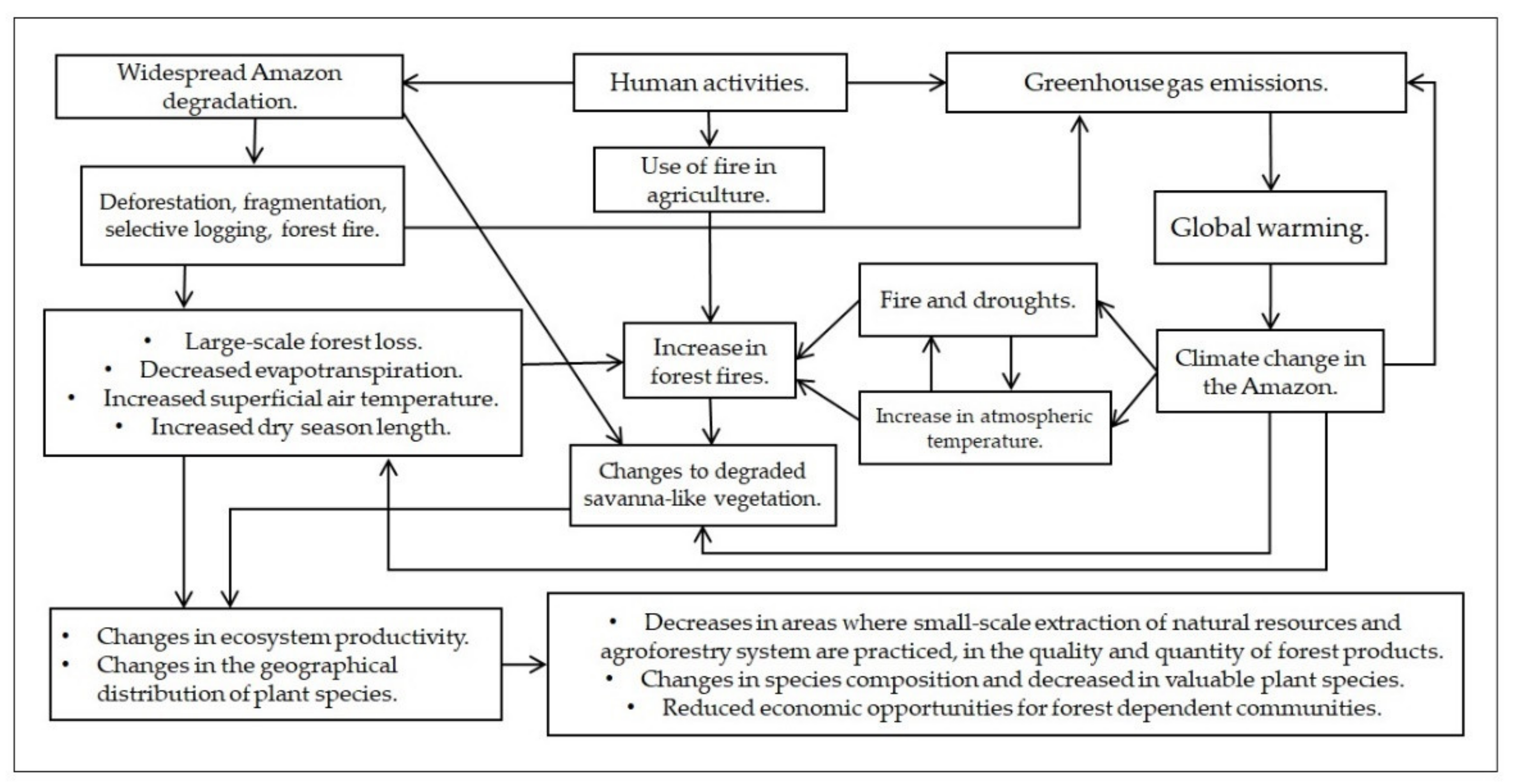
4. Discussion
4.1. Deforestation and Forest Degradation Implications to Forest Dependent Communities
4.2. The Effects of Climate Change on Plant Species
4.3. Potential Reduction of Native Amazonian Plants and Annual Range of Economic Losses
4.4. Potential for the Improvement of Agroforestry Systems Strategies and Advancement of Stakeholder Engagement Approaches
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobre, C.A.; Sellers, P.J.; Shukla, J. Amazonian Deforestation and Regional Climate Change. J. Clim. 1991, 4, 957–988. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; 32p. [Google Scholar]
- IPCC. 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Feeley, K.J.; Malhi, Y.; Zelazowski, P.; Silman, M.R. The relative importance of deforestation, precipitation change, and temperature sensitivity in determining the future distributions and diversity of Amazonian plant species. Glob. Chang. Biol. 2012, 18, 2636–2647. [Google Scholar] [CrossRef]
- Fearnside, P.M. Deforestation in Brazilian Amazonia: History, Rates, and Consequences. Conserv. Biol. 2005, 19, 680–688. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; Vieira, I.C.G.; Salomão, R.P.; ter Steege, H. Amazonian tree species threatened by deforestation and climate chance. Nat. Clim. Chang. 2019, 9, 547–553. [Google Scholar] [CrossRef]
- Barata, L.E.S. A economia verde: Amazônia. Cienc. Cult. 2012, 64, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Brandão, D.O.; Barata, L.E.S.; Nobre, I.; Nobre, C.A. The effects of Amazon deforestation on non-timber forest products. Reg. Environ. Chang. 2021, 21, 122. [Google Scholar] [CrossRef]
- Shanley, P.; Luz, L. The Impacts of Forest Degradation on Medicinal Plant Use and Implications for Health Care in Eastern Amazonia. Bioscience 2003, 53, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Rist, L.; Shanley, P.; Sunderland, T.; Sheil, D.; Ndoye, O.; Liswanti, N.; Tieguhong, J. The impacts of selective logging on non-timber forest products of livelihood importance. For. Ecol. Manag. 2012, 268, 57–69. [Google Scholar] [CrossRef]
- Scoles, R.; Canto, M.S.; Almeida, R.G.; Vieira, D.P. Sobrevivência e Frutificação de Bertholletia excelsa Bonpl. em Áreas Desmatadas em Oriximiná, Pará. Floresta Ambient. 2016, 23, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J. Environmental Security. In International Encyclopedia of Human Geography, 1st ed.; Kitchin, R., Thrift, N., Eds.; Elsevier: London, UK, 2009; Volume 1, pp. 553–557. [Google Scholar] [CrossRef]
- Nobre, C.A.; Sampaio, G.; Borma, L.S.; Castilla-Rubio, J.C.; Silva, J.S.; Cardoso, M. Land-use and climate change risks in the Amazon and the need of a novel sustainable development paradigm. Proc. Natl. Acad. Sci. USA 2016, 113, 10759–10768. [Google Scholar] [CrossRef] [Green Version]
- Soriano, M.; Zuidema, P.A.; Barber, C.; Mohren, F.; Ascarrunz, N.; Licona, J.C.; Peña-Claros, M. Commercial Logging of Timber Species Enhances Amazon (Brazil) Nut Populations: Insights from Bolivian Managed Forests. Forests 2021, 12, 1059. [Google Scholar] [CrossRef]
- Milheiras, S.G.; Mace, G.M. Assessing ecosystem service provision in a tropical region with high forest cover: Spatial overlap and the impact of land use change in Amapá, Brazil. Ecol. Indic. 2019, 99, 12–18. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.W.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Hawes, J.E.; Vieira, I.C.G.; Magnago, L.F.S.; Berenguer, E.; Ferreira, J.; Aragão, L.E.O.C.; Cardoso, A.; Lees, A.C.; Lennox, G.D.; Tobias, J.A.; et al. A large-scale assessment of plant dispersal mode and seed traits across human-modified Amazonian forests. J. Ecol. 2020, 108, 1373–1385. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Marengo, J.A.; Alves, L.M.; Sulca, J.C.; Takahashi, K.; Ferrett, S.; Collins, M. The role of ENSO flavours and TNA on recent droughts over Amazon forests and the Northeast Brazil region. Int. J. Climatol. 2021, 41, 3761–3780. [Google Scholar] [CrossRef]
- Aragão, L.E.O.C.; Anderson, L.O.; Fonseca, M.G.; Rosan, T.M.; Vedovato, L.B.; Wagner, F.H.; Silva, C.V.J.; Silva Junior, C.H.L.; Arai, E.; Aguiar, A.P.; et al. 21st Century drought-related fires counteract the decline of Amazon deforestation carbon emissions. Nat. Commun. 2018, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Fontes, C.G.; Dawson, T.E.; Jardine, K.; McDowell, N.; Gimenez, B.O.; Anderegg, L.; Negrón-Juárez, R.; Higuchi, N.; Fine, P.V.A.; Araújo, A.C.; et al. Dry and hot: The hydraulic consequences of a climate change–type drought for Amazonian trees. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20180209. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.N.; Taylor, T.C.; van Haren, J.; Rosolem, R.; Restrepo-Coupe, N.; Adams, J.; Wu, J.; Oliveira, R.C.; Silva, R.; Araujo, A.C.; et al. Empirical evidence for resilience of tropical forest photosynthesis in a warmer world. Nat. Plants 2020, 6, 1225–1230. [Google Scholar] [CrossRef]
- Berenguer, E.; Lennox, G.D.; Ferreira, J.; Malhi, Y.; Aragão, L.E.O.C.; Barreto, J.R.; Espírito-Santo, F.D.B.; Figueiredo, A.E.S.; França, F.; Gardner, T.A.; et al. Tracking the impacts of El Niño drought and fire in human-modified Amazonian forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2019377118. [Google Scholar] [CrossRef]
- Evangelista-Vale, J.C.; Weihs, M.; José-Silva, L.; Arruda, R.; Sander, N.L.; Gomides, S.C.; Machado, T.M.; Pires-Oliveira, J.C.; Barros-Rosa, L.; Castuera-Oliveira, L.; et al. Climate change may affect the future of extractivism in the Brazilian Amazon. Biol. Conserv. 2021, 257, 109093. [Google Scholar] [CrossRef]
- ter Steege, H.; Pitman, N.C.A.; Killeen, T.J.; Laurance, W.F.; Peres, C.A.; Guevara, J.E.; Salomão, R.P.; Castilho, C.V.; Amaral, I.L.; de Almeida Matos, F.D.; et al. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 2015, 1, e1500936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanley, P.; Pierce, A.R.; Laird, S.A.; Binnqüist, C.L.; Guariguata, M.R. From Lifelines to Livelihoods: Non-timber Forest Products into the Twenty-First Century. In Tropical Forestry Handbook; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–50. [Google Scholar]
- Lima, M.; Vale, J.C.E.; Costa, G.M.; Santos, R.C.; Correia Filho, W.L.F.; Gois, G.; Oliveira-Junior, J.F.; Teodoro, P.E.; Rossi, F.S.; Silva Junior, C.A. The forests in the indigenous lands in Brazil in peril. Land Use Policy 2020, 90, 104258. [Google Scholar] [CrossRef]
- Peters, C.M.; Gentry, A.H.; Mendelsohn, R.O. Valuation of an Amazonian rainforest. Nature 1989, 339, 655–656. [Google Scholar] [CrossRef]
- Groot, R.; Brander, L.; van der Ploeg, S.; Costanza, R.; Bernard, F.; Braat, L.; Christie, M.; Crossman, N.; Ghermandi, A.; Hein, L.; et al. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 2012, 1, 50–61. [Google Scholar] [CrossRef]
- Mataveli, G.A.V.; Oliveira, G.; Seixas, H.T.; Pereira, G.; Stark, S.C.; Gatti, L.V.; Basso, L.S.; Tejada, G.; Cassol, H.L.G.; Anderson, L.O.; et al. Relationship between Biomass Burning Emissions and Deforestation in Amazonia over the Last Two Decades. Forests 2021, 12, 1217. [Google Scholar] [CrossRef]
- Forest Resources Assessment. Global Ecological Zoning for the Global Forest Resources Assessment 2000, 1st ed.; Food and Agriculture Organization (FAO): Rome, Italy, 2001; pp. 1–56. [Google Scholar]
- Sales, L.P.; Rodrigues, L.; Masiero, R. Climate change drives spatial mismatch and threatens the biotic interactions of the Brazil nut. Glob. Ecol. Biogeogr. 2021, 30, 117–127. [Google Scholar] [CrossRef]
- Parrotta, J.A.; Wildburger, C.; Mansourian, S. Understanding Relationships between Biodiversity, Carbon, Forests and People: The Key to Achieving REDD+ Objectives, 1st ed.; IUFRO World Series: Vienna, Austria, 2012; 161p. [Google Scholar]
- Gash, J.H.C.; Nobre, C.A. Climatic Effects of Amazonian Deforestation: Some Results from ABRACOS. Bull. Am. Meteorol. Soc. 1997, 78, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, R.D.C.G.; Massoca, P.E.D.S.; Jakovac, C.C.; Bentos, T.V.; Williamson, G.B. Amazon Rain Forest Succession: Stochasticity or Land-Use Legacy? Bioscience 2015, 65, 849–861. [Google Scholar] [CrossRef]
- Hooper, E.R.; Ashton, M.S. Fragmentation reduces community-wide taxonomic and functional diversity of dispersed tree seeds in the Central Amazon. Ecol. Appl. 2020, 30, e02093. [Google Scholar] [CrossRef]
- Barlow, J.; Berenguer, E.; Carmenta, R.; França, F. Clarifying Amazonia’s burning crisis. Glob. Chang. Biol. 2020, 26, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, W.D.; Mustin, K.; Hilário, R.R.; Vasconcelos, I.M.; Eilers, V.; Fearnside, P.M. Deforestation control in the Brazilian Amazon: A conservation struggle being lost as agreements and regulations are subverted and bypassed. Perspect. Ecol. Conserv. 2019, 17, 122–130. [Google Scholar] [CrossRef]
- Almeida, C.A.; Coutinho, A.C.; Esquerdo, J.C.D.M.; Adami, M.; Venturieri, A.; Diniz, C.G.; Dessay, N.; Durieux, L.; Gomes, A.R. High spatial resolution land use and land cover mapping of the Brazilian legal Amazon in 2008 using Landsat-5/TM and MODIS data. Acta Amaz. 2016, 46, 291–302. [Google Scholar] [CrossRef]
- Santos, A.M.; Silva, C.F.A.; Almeida Junior, P.M.; Rudke, A.P.; Melo, S.N. Deforestation drivers in the Brazilian Amazon: Assessing new spatial predictors. J. Environ. Manag. 2021, 294, 113020. [Google Scholar] [CrossRef] [PubMed]
- INPE. Taxas de Desmatamento Amazonia Legal Estados. Available online: http://terrabrasilis.dpi.inpe.br/app/dashboard/deforestation/biomes/legal_amazon/rates (accessed on 1 February 2020).
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-term forest degradation surpasses deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Bullock, E.L.; Woodcock, C.E.; Souza, C.; Olofsson, P. Satellite-based estimates reveal widespread forest degradation in the Amazon. Glob. Chang. Biol. 2020, 26, 2956–2969. [Google Scholar] [CrossRef]
- INPE. Instituto Nacional de Pesquisas Espaciais. Monitoramento do Uso e Cobertura da Terra nas Áreas Desflorestadas da Amazônia Legal—TerraClass Amazônia; São José dos Campos: São Paulo, Brazil, 2021. [Google Scholar]
- Saatchi, S.; Houghton, R.A.; Santos Alvalá, R.C.; Soares, J.V.; Yu, Y. Distribution of aboveground live biomass in the Amazon basin. Glob. Chang. Biol. 2007, 13, 816–837. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Junqueira, A.B.; Crouzeilles, R.; Peña-Claros, M.; Mesquita, R.C.G.; Bongers, F. The role of land-use history in driving successional pathways and its implications for the restoration of tropical forests. Biol. Rev. 2021, 96, 1114–1134. [Google Scholar] [CrossRef]
- Feigl, B.; Cerri, C.; Piccolo, M.; Noronha, N.; Augusti, K.; Melillo, J.; Eschenbrenner, V.; Melo, L. Biological Survey of a Low-Productivity Pasture in Rondônia State, Brazil. Outlook Agric. 2006, 35, 199–208. [Google Scholar] [CrossRef]
- Soares-Filho, B.S.; Nepstad, D.C.; Curran, L.M.; Cerqueira, G.C.; Garcia, R.A.; Ramos, C.A.; Voll, E.; McDonald, A.; Lefebvre, P.; Schlesinger, P. Modelling conservation in the Amazon basin. Nature 2006, 440, 520–523. [Google Scholar] [CrossRef]
- Vieira, I.; Toledo, P.; Silva, J.; Higuchi, H. Deforestation and threats to the biodiversity of Amazonia. Braz. J. Biol. 2008, 68, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Stropp, J.; Umbelino, B.; Correia, R.A.; Campos-Silva, J.V.; Ladle, R.J.; Malhado, A.C.M. The ghosts of forests past and future: Deforestation and botanical sampling in the Brazilian Amazon. Ecography 2020, 43, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Oyama, M.D.; Nobre, C.A. A new climate-vegetation equilibrium state for Tropical South America. Geophys. Res. Lett. 2003, 30, 23. [Google Scholar] [CrossRef] [Green Version]
- Salazar, L.F.; Nobre, C.A.; Oyama, M.D. Climate change consequences on the biome distribution in tropical South America. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef] [Green Version]
- Laurance, W.F.; Camargo, J.L.C.; Fearnside, P.M.; Lovejoy, T.E.; Williamson, G.B.; Mesquita, R.C.G.; Meyer, C.F.J.; Bobrowiec, P.E.D.; Laurance, S.G.W. An Amazonian rainforest and its fragments as a laboratory of global change. Biol. Rev. 2018, 93, 223–247. [Google Scholar] [CrossRef]
- Brinck, K.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Paula, M.D.; Pütz, S.; Sexton, J.O.; Song, D.; Huth, A. High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 2017, 8, 14855. [Google Scholar] [CrossRef] [Green Version]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [Green Version]
- Aragão, L.E.O.C.; Poulter, B.; Barlow, J.B.; Anderson, L.O.; Malhi, Y.; Saatchi, S.; Phillips, O.L.; Gloor, E. Environmental change and the carbon balance of Amazonian forests. Biol. Rev. 2014, 89, 913–931. [Google Scholar] [CrossRef]
- Laurance, W.F.; Delamonica, P.; Laurance, S.G.; Vasconcelos, H.L.; Lovejoy, T.E. Rainforest fragmentation kills big trees. Nature 2000, 404, 836. [Google Scholar] [CrossRef]
- D’Angelo, S.A.; Andrade, A.C.S.; Laurance, S.G.; Laurance, W.F.; Mesquita, R.C.G. Inferred causes of tree mortality in fragmented and intact Amazonian forests. J. Trop. Ecol. 2004, 20, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Meza-Elizalde, M.C.; Armenteras-Pascual, D. Edge influence on the microclimate and vegetation of fragments of a north Amazonian forest. For. Ecol. Manag. 2021, 498, 119546. [Google Scholar] [CrossRef]
- Laurance, W.F.; Curran, T.J. Impacts of wind disturbance on fragmented tropical forests: A review and synthesis. Austral Ecol. 2008, 33, 399–408. [Google Scholar] [CrossRef]
- Bayma, M.M.A.; Malavazi, F.W.; Sá, C.P.; Fonseca, F.L.; Andrade, E.P.; Wadt, L.H.C. Aspectos da cadeia produtiva da castanha-do-brasil no estado do Acre. Bol. Mus. Para. Emílio Goeldi. Ciênc. Nat. 2014, 9, 417–426. [Google Scholar] [CrossRef]
- Strand, J.; Soares-Filho, B.; Costa, M.H.; Oliveira, U.; Ribeiro, S.C.; Pires, G.F.; Oliveira, A.; Rajão, R.; May, P.; van der Hoff, R.; et al. Spatially explicit valuation of the Brazilian Amazon Forest’s Ecosystem Services. Nat. Sustain. 2018, 1, 657–664. [Google Scholar] [CrossRef]
- Batista, A.P.B.; Scolforo, H.F.; Mello, J.M.; Guedes, M.C.; Terra, M.C.N.S.; Scalon, J.D.; Gomide, L.R.; Scolforo, P.G.V.; Cook, R.L. Spatial association of fruit yield of Bertholletia excelsa Bonpl. trees in eastern Amazon. For. Ecol. Manag. 2019, 441, 99–105. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais No Brasil: Nativas e Exóticas, 2nd ed.; Plantarum, I., Ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Homma, A.K.O.; Carvalho, R.A.; Ferreira, C.A.P.; Júnior, J.D.B.N. A Destruição de Recursos Naturais: O Caso da Castanha-do-Pará No Sudeste Paraense; Embrapa Amazônia Oriental: Belém, Brazil, 2000. [Google Scholar]
- Homma, A.K.O.; Menezes, A.J.E.A.; Maués, M.M. Brazil nut tree: The challenges of extractivism for agricultural plantations. Bol. Mus. Para. Emílio Goeldi. Ciênc. Nat. 2014, 9, 293–306. [Google Scholar] [CrossRef]
- IBGE. Produção da Extração Vegetal e da Silvicultura (PEVS); Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2019; pp. 1–16. [Google Scholar]
- Ferraz, G.; Nichols, J.D.; Hines, J.E.; Stouffer, P.C.; Bierregaard, R.O.; Lovejoy, T.E. A large-scale deforestation experiment: Effects of patch area and isolation on Amazon birds. Science 2007, 315, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Peres, C.A.; Gardner, T.A.; Barlow, J.; Zuanon, J.; Michalski, F.; Lees, A.C.; Vieira, I.C.G.; Moreira, F.M.S.; Feeley, K.J. Biodiversity conservation in human-modified Amazonian forest landscapes. Biol. Conserv. 2010, 143, 2314–2327. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Almeida, D.R.A.; Stark, S.C.; Schietti, J.; Camargo, J.L.C.; Amazonas, N.T.; Gorgens, E.B.; Rosa, D.M.; Smith, M.N.; Valbuena, R.; Saleska, S.; et al. Persistent effects of fragmentation on tropical rainforest canopy structure after 20 yr of isolation. Ecol. Appl. 2019, 29, e01952. [Google Scholar] [CrossRef]
- May, P.H.; Barata, L.E.S. Rosewood exploitation in the Brazilian Amazon: Options for sustainable production. Econ. Bot. 2004, 58, 257–265. [Google Scholar] [CrossRef]
- Amusant, N.; Digeon, A.; Descroix, L.; Bruneau, O.; Bezard, V.; Beauchène, J. Planting rosewood for sustainable essential oil production: Influence of surrounding forest and seed provenance on tree growth and essential oil yields. Bois For. Trop. 2015, 326, 57. [Google Scholar] [CrossRef]
- Lara, C.S.; Costa, C.R.; Sampaio, P.D.T.B. O mercado de sementes e mudas de pau-rosa (Aniba spp.) no Estado do Amazonas. Rev. Econ. Sociol. Rural 2021, 59, e221035. [Google Scholar] [CrossRef]
- Chantraine, J.M.; Dhénin, J.M.; Moretti, C. Chemical Variability of Rosewood (Aniba rosaeodora Ducke) Essential Oil in French Guiana. J. Essent. Oil Res. 2009, 21, 486–495. [Google Scholar] [CrossRef]
- Krainovic, P.M.; Almeida, D.R.A.; Veiga Junior, V.F.; Sampaio, P.T.B. Changes in rosewood (Aniba rosaeodora Ducke) essential oil in response to management of commercial plantations in Central Amazonia. For. Ecol. Manag. 2018, 429, 143–157. [Google Scholar] [CrossRef]
- Antunes, A.; Simmons, C.S.; Veiga, J.P. Non-timber forest products and the cosmetic industry: An econometric assessment of contributions to income in the Brazilian Amazon. Land 2021, 10, 588. [Google Scholar] [CrossRef]
- Vidal, T.C.S.; Simão, M.O.A.R.; Almeida, V.F. A sustentabilidade da produção de óleos e manteigas vegetais em comunidade amazônica- RESEX Médio Juruá. Res. Soc. Dev. 2021, 10, e32710313478. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tripathi, Y.C.; Kumar, A. Non Timber Forest Products (NTFPs) for Sustained Livelihood: Challenges and Strategies. Res. J. For. 2016, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Uhl, C.; Kauffman, J.B. Deforestation, Fire Susceptibility, and Potential Tree Responses to Fire in the Eastern Amazon. Ecology 1990, 71, 437–449. [Google Scholar] [CrossRef]
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragão, L.E.O.C.; Camargo, P.B.; Cerri, C.E.; Durigan, M.; Oliveira, R.C.; Vieira, I.C.G.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef] [Green Version]
- Barlow, J.; Peres, C.A. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1787–1794. [Google Scholar] [CrossRef] [Green Version]
- Martini, A.M.Z.; Rosa, N.A.; Uhl, C. An Attempt to predict which Amazonian tree species may be threatened by logging activities. Environ. Conserv. 1994, 21, 152–162. [Google Scholar] [CrossRef]
- Richardson, V.A.; Peres, C.A. Temporal Decay in Timber Species Composition and Value in Amazonian Logging Concessions. PLoS ONE 2016, 11, e0159035. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Lopes, A.; Silva, C.V.J.; Barlow, J.; Rincón, L.M.; Campanharo, W.A.; Nunes, C.A.; Almeida, C.T.; Silva Júnior, C.H.L.; Cassol, H.L.G.; Dalagnol, R.; et al. Drought-driven wildfire impacts on structure and dynamics in a wet Central Amazonian forest. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210094. [Google Scholar] [CrossRef] [PubMed]
- Arruda, D.M.; Fernandes-Filho, E.I.; Solar, R.R.C.; Schaefer, C.E.G.R. Combining climatic and soil properties better predicts covers of Brazilian biomes. Sci. Nat. 2017, 104, 32. [Google Scholar] [CrossRef]
- De Oliveira, G.; Chen, J.M.; Mataveli, G.A.V.; Chaves, M.E.D.; Seixas, H.T.; Cardozo, F.d.S.; Shimabukuro, Y.E.; He, L.; Stark, S.C.; dos Santos, C.A.C. Rapid Recent Deforestation Incursion in a Vulnerable Indigenous Land in the Brazilian Amazon and Fire-Driven Emissions of Fine Particulate Aerosol Pollutants. Forests 2020, 11, 829. [Google Scholar] [CrossRef]
- Chen, Y.; Randerson, J.T.; Morton, D.C.; DeFries, R.S.; Collatz, G.J.; Kasibhatla, P.S.; Giglio, L.; Jin, Y.; Marlier, M.E. Forecasting fire season severity in South America using sea surface temperature anomalies. Science 2011, 334, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Michaletz, S.T.; Johnson, E.A. How forest fires kill trees: A review of the fundamental biophysical processes. Scand. J. For. Res. 2007, 22, 500–515. [Google Scholar] [CrossRef]
- Brando, P.; Macedo, M.; Silvério, D.; Rattis, L.; Paolucci, L.; Alencar, A.; Coe, M.; Amorim, C. Amazon wildfires: Scenes from a foreseeable disaster. Flora 2020, 268, 151609. [Google Scholar] [CrossRef]
- Liesenfeld, M.V.A.; Vieira, G. Brote posfuego de la palma en el bosque amazónico: ¿son los tallos subterráneos una ventaja? Perspect. Rural Nueva Época 2018, 16, 11–23. [Google Scholar] [CrossRef]
- Falcão, M.A.; Clement, C.R. Fenologia e produtividade do Abiu (Pouteria caimito) na Amazônia Central. Acta Amaz. 1999, 29, 3. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, P.N.; Machado, M.I.L.; Cavalcanti, F.S.; Lemos, T.L.G. Essential Oil Composition of Leaves, Fruits and Resin of Protium heptaphyllum (Aubl.) March. J. Essent. Oil Res. 2011, 13, 33–34. [Google Scholar] [CrossRef]
- Bandeira, P.N.; Pessoa, O.D.L.; Trevisan, M.T.S.; Lemos, T.L.G. Metabólitos secundários de Protium heptaphyllum march. Quim. Nova 2002, 25, 1078–1080. [Google Scholar] [CrossRef] [Green Version]
- Noblick, L.; Wintergerst, S.; Noblick, D.; Lima, J.T. Syagrus coronata (Arecaceae) phenology and the impact of fire on survival and reproduction of the licuri palm. SITIENTIBUS Sér. Ciênc. Biol. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Castilho, C.V.; Magnusson, W.E.; Araújo, R.N.O.; Luizão, R.C.C.; Luizão, F.J.; Lima, A.P.; Higuchi, N. Variation in aboveground tree live biomass in a central Amazonian Forest: Effects of soil and topography. For. Ecol. Manag. 2006, 234, 85–96. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A. Effects of Single and Recurrent Wildfires on Fruit Production and Large Vertebrate Abundance in a Central Amazonian Forest. Biodivers. Conserv. 2006, 15, 985–1012. [Google Scholar] [CrossRef]
- Uhl, C.; Buschbacher, R.; Serrao, E.A.S. Abandoned Pastures in Eastern Amazonia. I. Patterns of Plant Succession. J. Ecol. 1988, 76, 663. [Google Scholar] [CrossRef]
- Longworth, J.B.; Mesquita, R.C.; Bentos, T.V.; Moreira, M.P.; Massoca, P.E.; Williamson, G.B. Shifts in Dominance and Species Assemblages over Two Decades in Alternative Successions in Central Amazonia. Biotropica 2014, 46, 529–537. [Google Scholar] [CrossRef]
- Marengo, J.A.; Williams, E.R.; Alves, L.M.; Soares, W.R.; Rodriguez, D.A. Extreme Seasonal Climate Variations in the Amazon Basin: Droughts and Floods. In Interactions between Biosphere, Atmosphere and Human Land Use in the Amazon Basin, 1st ed.; Nagy, L., Forsberg, B., Artaxo, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 227, pp. 55–76. [Google Scholar]
- Borma, L.S.; Nobre, C.A. Secas na Amazônia: Causas e Consequências; Oficina de Texto: São Paulo, Brazil, 2013; p. 367. [Google Scholar]
- Panisset, J.S.; Libonati, R.; Gouveia, C.M.P.; Machado-Silva, F.; França, D.A.; França, J.R.A.; Peres, L.F. Contrasting patterns of the extreme drought episodes of 2005, 2010 and 2015 in the Amazon Basin. Int. J. Climatol. 2018, 38, 1096–1104. [Google Scholar] [CrossRef]
- Lewis, S.L.; Brando, P.M.; Phillips, O.L.; van der Heijden, G.M.F.; Nepstad, D. The 2010 Amazon Drought. Science 2011, 331, 554. [Google Scholar] [CrossRef]
- Anderson, L.O.; Aragão, L.E.O.C.; Valeriano, D.M.; Cardoso, M.; Shimabukuro, Y.E.; Lima, A. Impactos de secas nas florestas amazônicas. In Secas na Amazônia: Causas e Consequências; Borma, L.S., Nobre, C.A., Eds.; Oficina de Texto: São Paulo, Brazil, 2013; pp. 147–164. [Google Scholar]
- Brando, P.M.; Balch, J.K.; Nepstad, D.C.; Morton, D.C.; Putz, F.E.; Coe, M.T.; Silvério, D.; Macedo, M.N.; Davidson, E.A.; Nóbrega, C.C.; et al. Abrupt increases in Amazonian tree mortality due to drought-fire interactions. Proc. Natl. Acad. Sci. USA 2014, 111, 6347–6352. [Google Scholar] [CrossRef] [Green Version]
- Meir, P.; Brando, P.M.; Nepstad, D.; Vasconcelos, S.; Costa, A.C.L.; Davidson, E.; Almeida, S.; Fisher, R.A.; Sotta, E.D.; Zarin, D.; et al. The effects of drought on Amazonian rain forests. Geophys. Monogr. Ser. 2009, 186, 429–449. [Google Scholar] [CrossRef]
- Sousa, T.R.; Schietti, J.; Souza, F.C.; Esquivel-Muelbert, A.; Ribeiro, I.O.; Emílio, T.; Pequeno, P.A.C.L.; Phillips, O.; Costa, F.R.C. Palms and trees resist extreme drought in Amazon forests with shallow water tables. J. Ecol. 2020, 108, 2070–2082. [Google Scholar] [CrossRef]
- Wagner, F.H.; Hérault, B.; Bonal, D.; Stahl, C.; Anderson, L.O.; Baker, T.R.; Becker, G.S.; Beeckman, H.; Boanerges Souza, D.; Botosso, P.C.; et al. Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 2016, 13, 2537–2562. [Google Scholar] [CrossRef] [Green Version]
- Alencar, J.C.; Almeida, R.A.; Fernandes, N.P. Fenologia de espécies florestais em floresta tropical úmida de terra firme na Amazônia Central. Acta Amaz. 1979, 9, 163–199. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.T.; Oliveira-Júnior, J.F.; Delgado, R.C.; Cubo, P.; Ramos, M.C. Spatiotemporal rainfall and temperature trends throughout the Brazilian Legal Amazon, 1973–2013. Int. J. Climatol. 2016, 37, 2013–2026. [Google Scholar] [CrossRef]
- Von Randow, C.; Manzi, A.O.; Kruijt, B.; Oliveira, P.J.; Zanchi, F.B.; Silva, R.L.; Hodnett, M.G.; Gash, J.H.C.; Elbers, J.A.; Waterloo, M.J.; et al. Comparative measurements and seasonal variations in energy and carbon exchange over forest and pasture in South West Amazonia. Theor. Appl. Climatol. 2004, 78, 5–26. [Google Scholar] [CrossRef]
- López, J.; Way, D.A.; Sadok, W. Systemic effects of rising atmospheric vapor pressure deficit on plant physiology and productivity. Glob. Chang. Biol. 2021, 27, 1704–1720. [Google Scholar] [CrossRef]
- Lovejoy, T.E.; Nobre, C. Amazon tipping point. Sci. Adv. 2018, 4, 2340. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Martins, G.; Nobre, C.; von Randow, C.; Chen, T.; Dolman, H. Constraining Amazonian land surface temperature sensitivity to precipitation and the probability of forest dieback. NPJ Clim. Atmos. Sci. 2021, 4, 6. [Google Scholar] [CrossRef]
- Sampaio, G.; Nobre, C.; Costa, M.H.; Satyamurty, P.; Soares-Filho, B.S.; Cardoso, M. Regional climate change over eastern Amazonia caused by pasture and soybean cropland expansion. Geophys. Res. Lett. 2007, 34, L17709. [Google Scholar] [CrossRef] [Green Version]
- Culf, A.D.; Fisch, G.; Hodnett, M.G. The Albedo of Amazonian Forest and Ranch Land. J. Clim. 1995, 8, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, G.; Shimizu, M.H.; Guimarães-Júnior, C.A.; Alexandre, F.; Guatura, M.; Cardoso, M.; Domingues, T.F.; Rammig, A.; von Randow, C.; Rezende, L.F.C.; et al. CO2 physiological effect can cause rainfall decrease as strong as large-scale deforestation in the Amazon. Biogeosciences 2021, 18, 2511–2525. [Google Scholar] [CrossRef]
- Fu, R.; Yin, L.; Li, W.; Arias, P.A.; Dickinson, R.E.; Huang, L.; Chakraborty, S.; Fernandes, K.; Liebmann, B.; Fisher, R.; et al. Increased dry-season length over southern Amazonia in recent decades and its implication for future climate projection. Proc. Natl. Acad. Sci. USA 2013, 110, 18110–18115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghtalab, N.; Moore, N.; Heerspink, B.P.; Hyndman, D.W. Evaluating spatial patterns in precipitation trends across the Amazon basin driven by land cover and global scale forcings. Theor. Appl. Climatol. 2020, 140, 411–427. [Google Scholar] [CrossRef]
- Levis, C.; Souza, P.F.; Schietti, J.; Emilio, T.; Pinto, J.L.P.V.; Clement, C.R.; Costa, F.R.C. Historical Human Footprint on Modern Tree Species Composition in the Purus-Madeira Interfluve, Central Amazonia. PLoS ONE 2012, 7, e48559. [Google Scholar] [CrossRef] [Green Version]
- Junqueira, A.B.; Almekinders, C.J.M.; Stomph, T.J.; Clement, C.R.; Struik, P.C. The role of Amazonian anthropogenic soils in shifting cultivation: Learning from farmers’ rationales. Ecol. Soc. 2016, 21, 12. [Google Scholar] [CrossRef] [Green Version]
- Kubitski, K. Ocorrência de Kielmeyera nos “campos de Humaitá” e a natureza dos “campos”—Flora da Amazônia. Acta Amaz. 1979, 9, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Miranda, I.S.; Almeida, S.S.; Dantas, P.J. Florística e estrutura de comunidades arbóreas em cerrados de Rondônia, Brasil. Acta Amaz. 2006, 36, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, W.E.; Lima, A.P.; Albernaz, A.L.K.M.; Sanaiotti, T.M.; Guillaumet, J.L. Composição florística e cobertura vegetal das savanas na região de Alter do Chão, Santarém—PA. Rev. Bras. Bot. 2008, 31, 165–177. [Google Scholar] [CrossRef] [Green Version]
- SpeciesLink Network. The Geographical Distribution of Typical Savannah Species That Are Found in the Brazilian Amazon. Available online: https://specieslink.net/search/ (accessed on 10 October 2021).
- Benchimol, M.; Peres, C.A. Edge-mediated compositional and functional decay of tree assemblages in Amazonian forest islands after 26 years of isolation. J. Ecol. 2015, 103, 408–420. [Google Scholar] [CrossRef]
- Carneiro, M.S. Da certificação para as concessões florestais: Organizações não governamentais, empresas e a construção de um novo quadro institucional para o desenvolvimento da exploração florestal na Amazônia brasileira. Bol. Mus. Para. Emílio Goeldi. Ciênc. Hum. 2011, 6, 525–541. [Google Scholar] [CrossRef] [Green Version]
- Presidência da República. Brazil Gestão de Florestas Públicas Para a Produção (Lei N 11.284); Casa Civil: Brasilia, Brazil, 2006. [Google Scholar]
- Fearnside, P.M. Biodiversity as an environmental service in Brazil’s Amazonian forests: Risks, value and conservation. Environ. Conserv. 1999, 26, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Guariguata, M.R.; Licona, J.C.; Mostacedo, B.; Cronkleton, P. Damage to Brazil nut trees (Bertholletia excelsa) during selective timber harvesting in Northern Bolivia. For. Ecol. Manag. 2009, 258, 788–793. [Google Scholar] [CrossRef]
- Osborne, T.; Kiker, C. Carbon offsets as an economic alternative to large-scale logging: A case study in Guyana. Ecol. Econ. 2005, 52, 481–496. [Google Scholar] [CrossRef]
- Shanley, P.; Luz, L.; Swingland, I.R. The faint promise of a distant market: A survey of Belém’s trade in non-timber forest products. Biodivers. Conserv. 2002, 11, 615–636. [Google Scholar] [CrossRef]
- Davidson, E.A.; Araújo, A.C.; Artaxo, P.; Balch, J.K.; Brown, I.F.; Mercedes, M.M.; Coe, M.T.; Defries, R.S.; Keller, M.; Longo, M.; et al. The Amazon basin in transition. Nature 2012, 481, 321–328. [Google Scholar] [CrossRef]
- De Faria, B.L.; Brando, P.M.; Macedo, M.N.; Panday, P.K.; Soares-Filho, B.S.; Coe, M.T. Current and future patterns of fire-induced forest degradation in Amazonia. Environ. Res. Lett. 2017, 12, 095005. [Google Scholar] [CrossRef]
- Le Page, Y.; Morton, D.; Hartin, C.; Bond-Lamberty, B.; Pereira, J.M.C.; Hurtt, G.; Asrar, G. Synergy between land use and climate change increases future fire risk in Amazon forests. Earth Syst. Dyn. 2017, 8, 1237–1246. [Google Scholar] [CrossRef] [Green Version]
- Aleixo, I.; Norris, D.; Hemerik, L.; Barbosa, A.; Prata, E.; Costa, F.; Poorter, L. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Chang. 2019, 9, 384–388. [Google Scholar] [CrossRef]
- Herraiz, A.D.; de Alencastro Graça, P.M.L.; Fearnside, P.M. Amazonian flood impacts on managed Brazilnut stands along Brazil’s Madeira River: A sustainable forest management system threatened by climate change. For. Ecol. Manag. 2017, 406, 46–52. [Google Scholar] [CrossRef]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, L.; Culot, L.; Pires, M.M. Climate niche mismatch and the collapse of primate seed dispersal services in the Amazon. Biol. Conserv. 2020, 247, 108628. [Google Scholar] [CrossRef]
- IBGE. Produção Agrícola Municipal: Culturas Temporárias e Permanentes (PAM); Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2019; Volume 66.
- Lopes, E.; Soares-Filho, B.; Souza, F.; Rajão, R.; Merry, F.; Carvalho Ribeiro, S. Mapping the socio-ecology of Non Timber Forest Products (NTFP) extraction in the Brazilian Amazon: The case of açaí (Euterpe precatoria Mart) in Acre. Landsc. Urban Plan. 2019, 188, 110–117. [Google Scholar] [CrossRef]
- Costa, F.A.; Ciasca, B.S.; Castro, E.C.C.; Barreiros, R.M.M.; Folhes, R.T.; Bergamini, L.L.; Solyno Sobrinho, S.A.; Cruz, A.; Costa, J.A.; Simões, J.; et al. Bioeconomia da Sociobiodiversidade no Estado do Pará, 1st ed.; The Nature Conservancy (TNC Brasil), Banco Interamericano de Desenvolvimento (BID), Natura: Brasilia, Brazil, 2021; pp. 1–37. [Google Scholar]
- Oliveira, M.S.L.; Scaramussa, P.H.M.; Santos, A.R.S.; Benjamin, A.M.S. Análise do custo econômico de um sistema agroflorestal na comunidade Nova Betel, município de Tomé-Açu, estado do Pará. In Proceedings of the II Congresso Internacional das Ciências Agrárias, Teresina, Brazil, 19 December 2017. [Google Scholar]
- Castro, F.; Futemma, C. Farm Knowledge Co-Production at an Old Amazonian Frontier: Case of the Agroforestry System in Tomé-Açu, Brazil. Rural Landsc. Soc. Environ. Hist. 2021, 8, 3. [Google Scholar] [CrossRef]
- Matos Filho, J.R.; Moraes, L.L.C.; Freitas, J.L.; Cruz Junior, F.O.; Santos, A.C. Quintais agroflorestais em uma comunidade rural no vale do Rio Araguari, Amazônia Oriental. Rev. Ibero-Am. Ciênc. Ambient. 2021, 12, 47–62. [Google Scholar] [CrossRef]
- Gasparinetti, P.; Brandão, D.O.; Araújo, V.; Araújo, N. Economic Feasibility Study for Forest Landscape Restoration Banking Models: Cases from Southern Amazonas State, 1st ed.; Conservation Strategy Fund: Brasilia, Brazil, 2019; pp. 1–49. [Google Scholar]
- WWF-Brasil. Avaliação Financeira da Restauração Florestal com Agroflorestas na Amazônia, 1st ed.; WWW-Brasil: Brasilia, Brazil, 2020; pp. 1–31. [Google Scholar]
- Bolfe, E.L.; Ferreira, M.C.; Batistella, M. Biomassa Epígea e Estoque de Carbono de Agroflorestas em Tomé-Açu, PA. Rev. Bras. Agroecol. 2009, 4, 2171–2175. [Google Scholar]
- Villa, P.M.; Martins, S.V.; Oliveira Neto, S.N.; Rodrigues, A.C.; Hernández, E.P.; Kim, D.G. Policy forum: Shifting cultivation and agroforestry in the Amazon: Premises for REDD+. For. Policy Econ. 2020, 118, 102217. [Google Scholar] [CrossRef]
- BNDES. Fundo Amazônia—Relatórios Anuais (2010–2019); Banco Nacional de Desenvolvimento Econômico e Social: Brasilia, Brazil, 2019. [Google Scholar]
- Nobre, I.; Nobre, C.A. The Amazonia third way initiative: The role of technology to unveil the potential of a novel tropical biodiversity-based economy. In Land Use—Assessing the Past, Envisioning the Future; IntechOpen: London, UK, 2018; Volume 1, pp. 1–32. [Google Scholar]
- Nobre, I.; Margit, A.; Nobre, C.A.; Weser-Koch, M.; Veríssimo, A.; Neto, A.F. Amazon Creative Labs of the Cupuaçu-Cocoa Chain; Banco Interamericano de Desenvolvimento: São Paulo, Brazil, 2021; Volume 2.01, p. 116. [Google Scholar]
- U.S. Embassy & Consulates in Brazil. United States and Brazil to Partner in Biodiversity-Focused Impact Investment Fund for the Brazilian Amazon. Available online: https://br.usembassy.gov/united-states-and-brazil-to-partner-in-biodiversity-focused-impact-investment-fund-for-the-brazilian-amazon/ (accessed on 25 March 2019).
| Botanical Family | Species Name | Scientific References |
|---|---|---|
| Anacardiaceae | Anacardium spruceanum Benth. ex Engl. | [82] |
| Annonaceae | Xylopia nitida Dunal | [82] |
| Apocymaceae | Aspidosperma album (Vahl) Benoist ex Pichon | [82] |
| Araliaceae | Didymopanax morototoni (Aubl.) Dec. & Pla. | [82] |
| Arecaceae | Euterpeoleracea Mart. | [8] |
| Arecaceae | Euterpe precatoria Mart. | [8] |
| Bignoniaceae | Handroanthus serratifolius (Vahl) S.Grose | [82] |
| Burseraceae | Protium tenuifolium (Engl.) Engl. | [82] |
| Caryocaraceae | Caryocar glabrum (Aubl.) Pers. | [82] |
| Clusiaceae | Caraipagrandifolia Mart. | [82] |
| Combretaceae | Terminalia parvifolia (Ducke) Gere & Boatwr. | [82] |
| Dichapetalaceae | Tapura singularis Ducke | [82] |
| Euphorbiaceae | Alchorneopsis floribunda Müll.Arg. | [82] |
| Fabaceae | Copaifera duckei Dwyer | [82] |
| Fabaceae | Dinizia excelsa Ducke | [82] |
| Fabaceae | Dipteryx odorata (Aubl.) Willd. | [8] |
| Fabaceae | Eperua falcata Aubl. | [10] |
| Goupiaceae | Goupia glabra Aubl. | [82] |
| Humiriaceae | Sacoglottis amazonica Mart. | [82] |
| Lauraceae | Aniba rosiodora Ducke | [71] |
| Lecythidaceae | Bertholletia excelsa Bonpl. | [68] |
| Malpighiaceae | Byrsonimaaerugo Sagot | [82] |
| Meliaceae | Carapaguianensis Aubl. | [8] |
| Moraceae | Brosimum acutifolium Huber | [82] |
| Olacaceae | Minquartia guianensis Aubl. | [82] |
| Proteaceae | Euplassa pinnata (Lam.) I.M.Johnst. | [82] |
| Rutaceae | Euxylophoraparaensis Huber | [82] |
| Sapotaceae | Pouteriapariry (Ducke) Baehni | [82] |
| Sapotaceae | Pouteriamacrophylla (Lam.) Eyma | [82] |
| Vochysiaceae | Qualeacoerulea Aubl. | [82] |
| Botanical Family | Species Name | Scientific References |
|---|---|---|
| Annonaceae | Guatteria punctata (Aubl.) R.A.Howard | [98] |
| Arecaceae | Attalea speciosa Mart. ex Spreng. | [46] |
| Cannabaceae | Trema micrantha (L.) Blume | [97] |
| Dilleniaceae | Curatella americana L. | [46] |
| Euphobiaceae | Croton diasii Pires ex Secco & P.E.Berry | [97] |
| Euphobiaceae | Crotonmatourensis Aubl. | [98] |
| Euphorbiaceae | Sapium marmieri Huber | [46] |
| Fabaceae | Apuleia leiocarpa (Vogel) J.F.Macbr. | [46] |
| Fabaceae | Inga thibaudiana DC. | [98] |
| Hypericaceae | Vismia amazonica Ewan | [34] |
| Hypericaceae | Vismia bemerguii M.E.Berg | [34] |
| Hypericaceae | Vismia cauliflora A.C.Sm. | [34] |
| Hypericaceae | Vismia cayennensis (Jacq.) Pers. | [34] |
| Hypericaceae | Vismia guianensis (Aubl.) Choisy | [34] |
| Hypericaceae | Vismia japurensis Reichardt | [34] |
| Malpighiaceae | Byrsonima duckeana W.R.Anderson | [98] |
| Malpighiaceae | Byrsonima stipulacea A.Juss. | [34] |
| Malvaceae | Eriotheca longipedicellata (Ducke) A.Robyns | [97] |
| Melastomataceae | Bellucia grossularioides (L.) Triana | [98] |
| Melastomataceae | Bellucia imperialis Saldanha & Cogn. | [98] |
| Rubiaceae | Coutarea hexandra (Jacq.) K.Schum. | [46] |
| Rutaceae | Zanthoxylum rhoifolium Lam. | [97] |
| Salicaceae | Banara guianensis Aubl. | [97] |
| Salicaceae | Caseariadecandra Jacq. | [97] |
| Salicaceae | Casearia sylvestris Sw. | [46] |
| Solanaceae | Solanum crinitum Lam. | [97] |
| Urticaceae | Cecropia purpurascens C.C.Berg | [34] |
| Urticaceae | Cecropia sciadophylla Mart. | [98] |
| Urticaceae | Pourouma apiculata Spruce ex Benoist | [98] |
| Vochysiaceae | Erisma uncinatum Warm. | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, D.O.; Barata, L.E.S.; Nobre, C.A. The Effects of Environmental Changes on Plant Species and Forest Dependent Communities in the Amazon Region. Forests 2022, 13, 466. https://doi.org/10.3390/f13030466
Brandão DO, Barata LES, Nobre CA. The Effects of Environmental Changes on Plant Species and Forest Dependent Communities in the Amazon Region. Forests. 2022; 13(3):466. https://doi.org/10.3390/f13030466
Chicago/Turabian StyleBrandão, Diego Oliveira, Lauro Euclides Soares Barata, and Carlos Afonso Nobre. 2022. "The Effects of Environmental Changes on Plant Species and Forest Dependent Communities in the Amazon Region" Forests 13, no. 3: 466. https://doi.org/10.3390/f13030466






