Changes in the Soil Labile Organic Carbon Fractions following Bedrock Exposure Rate in a Karst Context
Abstract
:1. Introduction
2. Materials and Methods
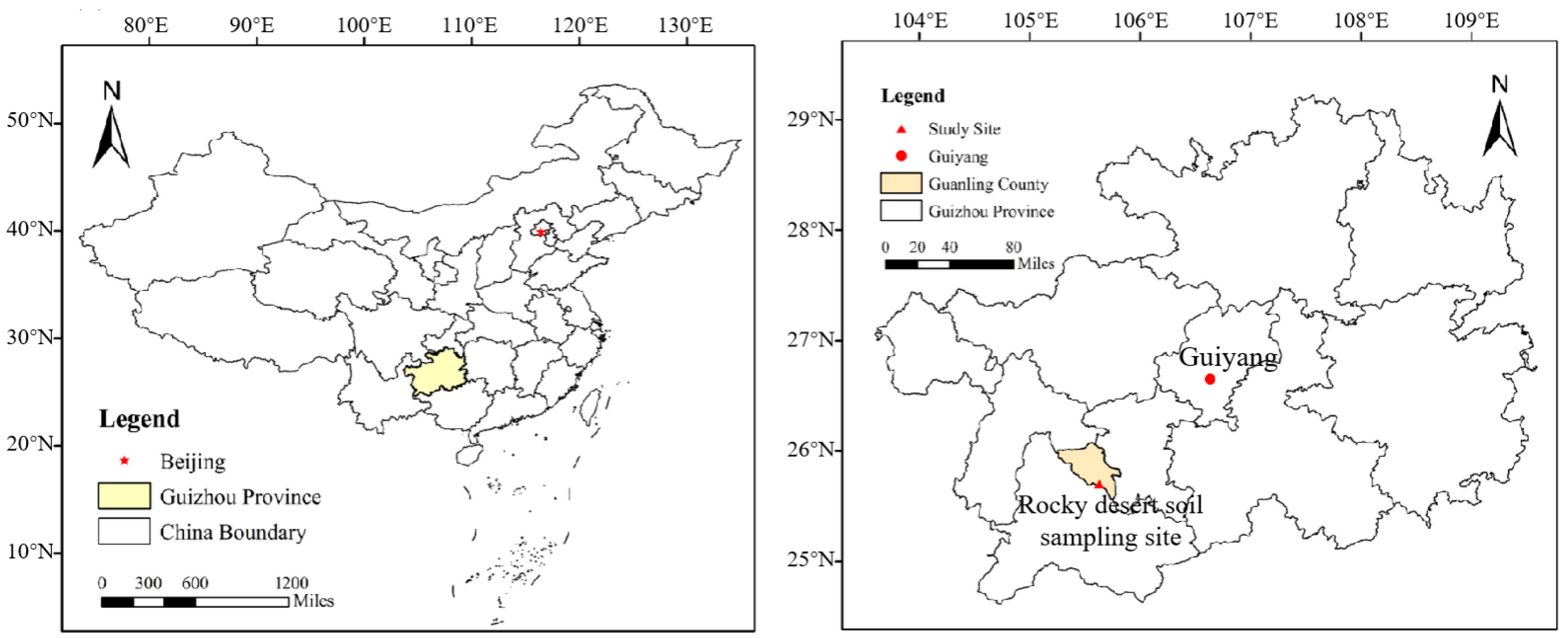
2.1. Study Site
2.2. Soil Sample Collection
2.3. Soil Sample Determination
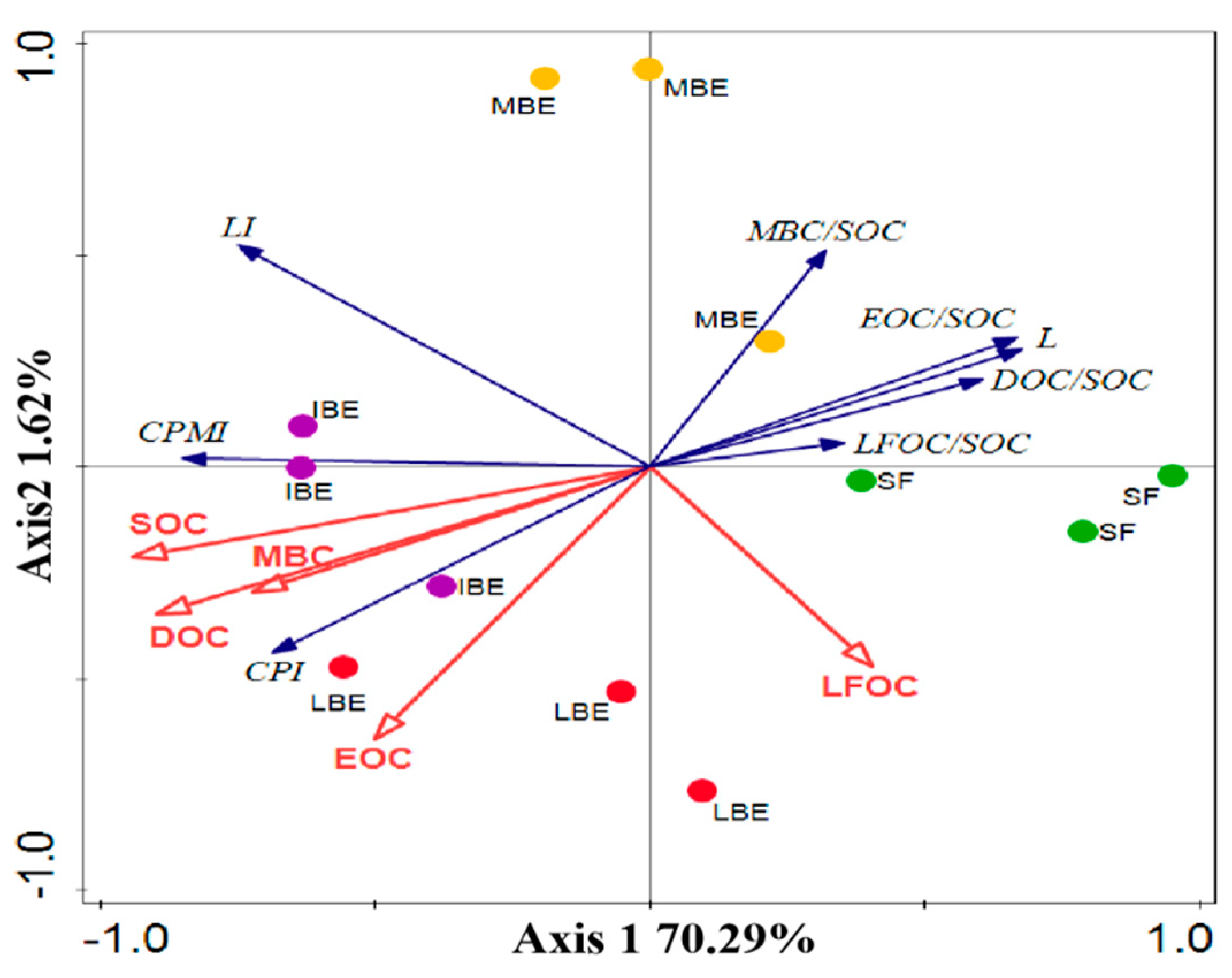
2.4. Data Analysis
3. Results
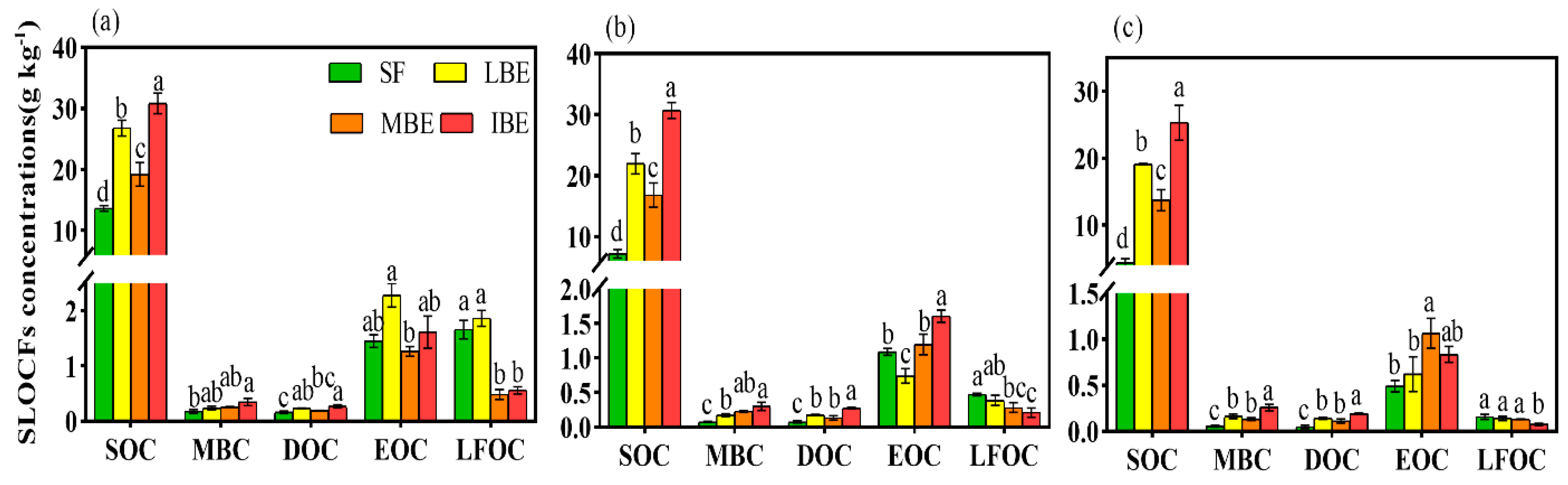
3.1. Soil Labile Organic Carbon Fraction Stocks
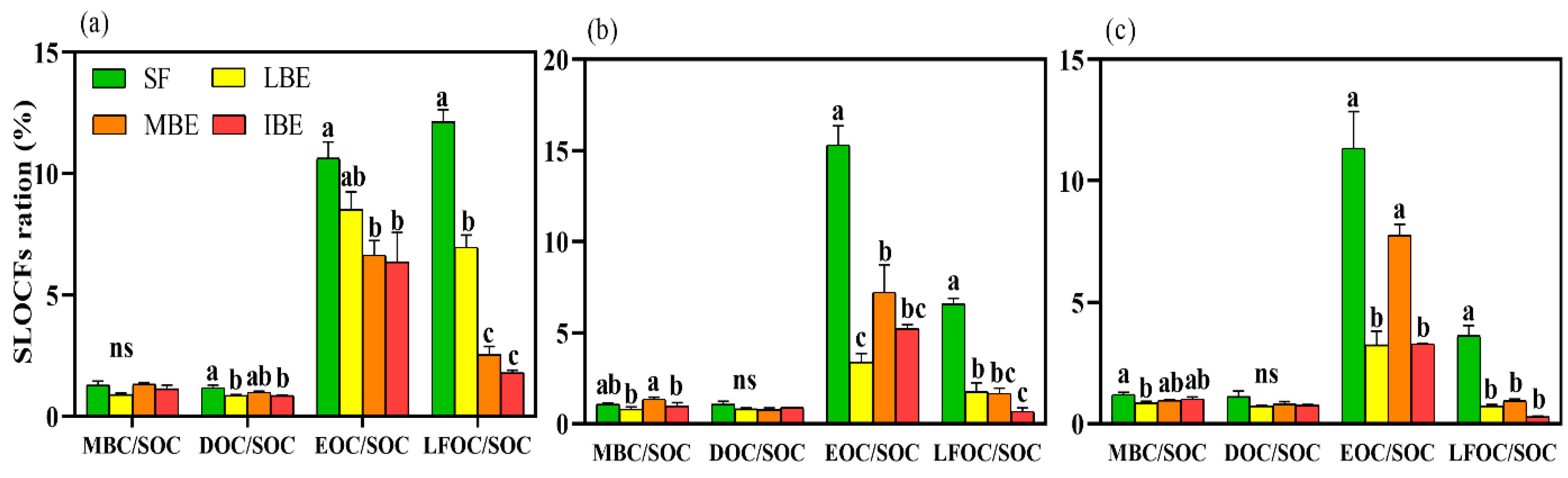
3.2. Proportions of Soil Labile Organic Carbon Fractions
3.3. Sensitivity of Soil Labile Organic Carbon Fractions
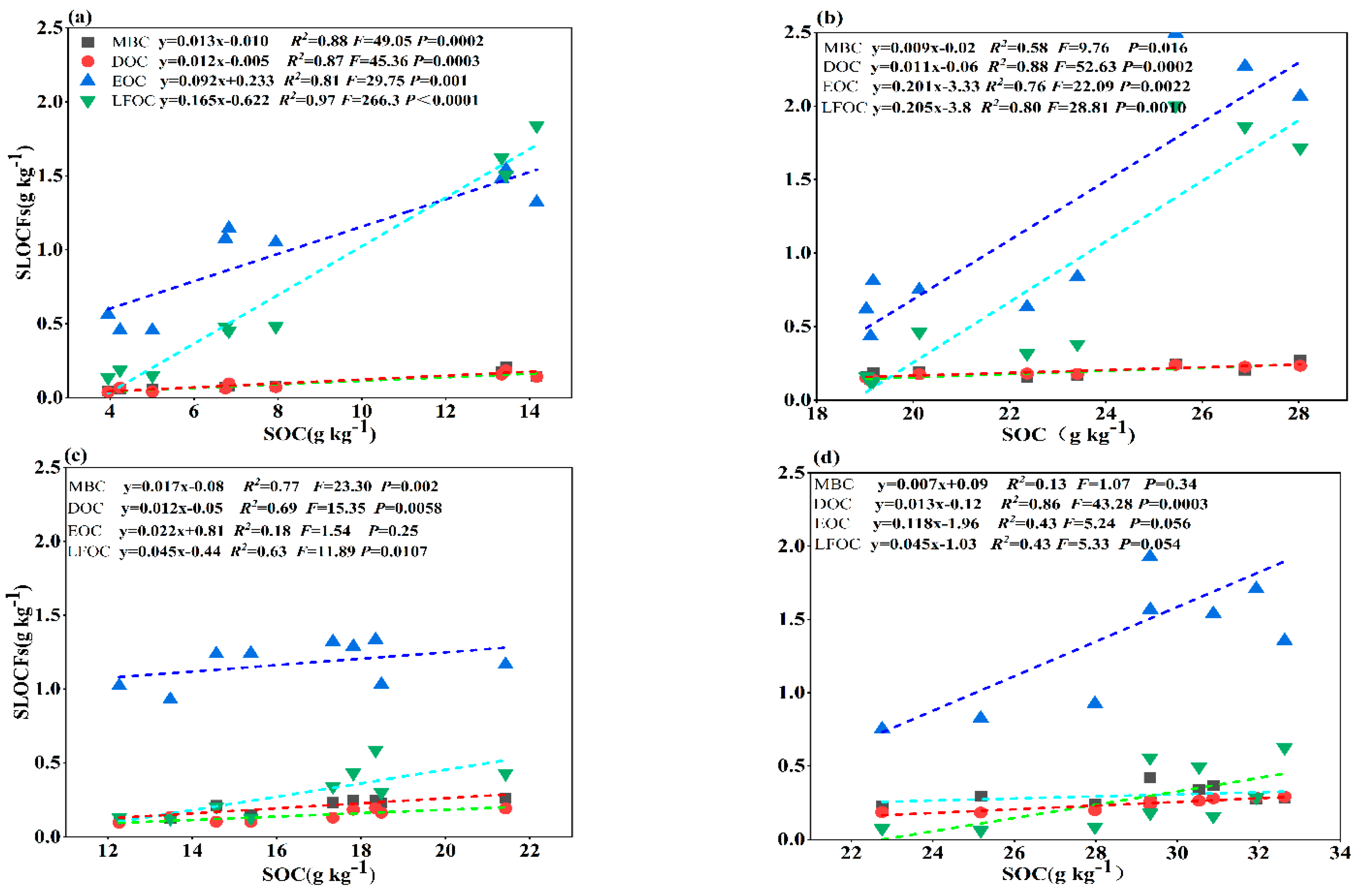
3.4. Relationship between Soil Labile Organic Carbon Fractions and Bedrock Exposure Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prvlie, R. Drylands extent and environmental issues: A global approach. Earth-Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.X.; Qin, Y.M.; He, Q.S.; Yi, Y.; Ji, Z.L. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2018, 649, 1250–1259. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Q.; Yuan, Y.; Peng, X.; Zhao, L. Effects of rainfall intensity on runoff and sediment yields on bare slopes in a karst area, SW China. Geoderma 2018, 330, 30–40. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Huang, Z.S.; Chen, X.; Guo, X.L.; Fang, Y. Suitability of human settlement environment in Buyei traditional villages in rocky desertification area of Guizhou, China. J. Appl. Ecol. 2019, 30, 3203–3214. [Google Scholar]
- Zhang, X.B.; Wang, S.J.; Cao, J.H.; Wang, K.L.; Meng, T.Y.; Bai, X.Y. Characteristics of water loss and soil erosion and some scientific problems on karst rocky desertification in Southwest China karst area. Carsologica Sin. 2010, 29, 274–279. [Google Scholar]
- Zhang, M.; Wang, K.; Liu, H.; Zhang, C.; Yue, Y.; Qi, X. Effect of ecological engineering projects on ecosystem services in a karst region: A case study of northwest Guangxi, China. J. Clean. Prod. 2018, 183, 831–842. [Google Scholar] [CrossRef]
- Qian, M.J.; Fang-Fang, W.U.; Tong, L.Q.; Yuan, C. Causes and Comprehensive Management for Rocky Desertification in Karst Areas of Southwestern China. Resour. Dev. Mark. 2008, 24, 902–904. [Google Scholar]
- Jarecki, M.K.; Lal, R.; James, R. Crop management effects on soil carbon sequestration on selected farmers’ fields in northeastern Ohio. Soil Tillage Res. 2005, 81, 265–276. [Google Scholar] [CrossRef]
- Dong, L.I.; Gao, M. Research Progress of Soil Organic Carbon Recycle. Acta Agric. Jiangxi 2008, 20, 60–63. [Google Scholar]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [Green Version]
- Deng, L. Soil organic carbon dynamics following natural vegetation restoration: Evidence from stable carbon isotopes (δ13C). Agric. Ecosyst. Environ. 2016, 221, 235–244. [Google Scholar] [CrossRef]
- Tie, G.; Wang, K.L.; Song, D.J.; Chen, T.Q.; Peng, H.; Wan, X. Impacts of vegetation restoration strategies on soil organic carbon and nitrogen dynamics in a karst area, southwest China. Ecol. Eng. 2017, 101, 247–254. [Google Scholar]
- Silveira, M.L.; Comerford, N.B.; Reddy, K.R.; Cooper, W.T.; El-Rifai, H. Characterization of soil organic carbon pools by acid hydrolysis. Geoderma 2008, 144, 405–414. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-Associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, 4–9. [Google Scholar] [CrossRef]
- Rolando, J.L.; Dubeux, J.C.; Perez, W.; Ramirez, D.A.; Turin, C.; Ruiz-Moreno, M.; Comerford, N.B.; Mares, V.; Garcia, S.; Quiroz, R. Soil organic carbon stocks and fractionation under different land uses in the Peruvian high-Andean Puna. Geoderma 2017, 307, 65–72. [Google Scholar] [CrossRef]
- Dai, Q.; Peng, X.; Yang, Z.; Zhao, L. Runoff and erosion processes on bare slopes in the Karst Rocky Desertification Area. Catena 2017, 152, 218–226. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, M.; Zhang, Y.; Long, M.; Li, X. Effects of disturbance to moss biocrusts on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. Soil Biol. Biochem. 2021, 152, 108065–108073. [Google Scholar] [CrossRef]
- Peng, X.; Dai, Q.; Ding, G.; Shi, D.; Li, C. Impact of vegetation restoration on soil properties in near-surface fissures located in karst rocky desertification regions. Soil Tillage Res. 2020, 200, 104620–104630. [Google Scholar] [CrossRef]
- Lan, J. Responses of soil organic carbon components and their sensitivity to karst rocky desertification control measures in Southwest China. J. Soils Sediments 2021, 21, 978–989. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ Program: A synthesis. Glob. Change Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Wardle, D.A. A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 1992, 67, 321–358. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Chibuike, G.; Burkitt, L.; Bretherton, M.; Singh, R.; Bishop, P. Dissolved Organic Carbon Concentration and Denitrification Capacity of a New Zealand Hill Country Soil After Forage Crop Establishment. Earth Syst. Environ. 2021, 5, 419–432. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, Z.P.; Hu, C.J. Soil organic carbon fraction methods and their applications in farmland ecosystem: A review. Chin. J. Appl. Ecol. 2011, 22, 1921–1930. [Google Scholar]
- Shen, H.; Cao, Z.H.; Hu, Z.Y. Characteristics and ecological effects of the active organic carbon in soil. Chin. J. Ecol. 1999, 18, 33–39. [Google Scholar]
- Ping, Q.; Shao, J.; Ke, J.; Huang, S.W.; Zhao, S.C.; Zhou, W. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar]
- Dodla, S.K.; Wang, J.J.; Delaune, R.D. Characterization of labile organic carbon in coastal wetland soils of the Mississippi River deltaic Plain: Relationships to carbon functionalities. Sci. Total Environ. 2012, 435–436, 151–158. [Google Scholar] [CrossRef]
- Ma, T.; Deng, X.; Chen, L.; Xiang, W. The soil properties and their effects on plant diversity in different degrees of rocky desertification. Sci. Total Environ. 2020, 736, 139667–139675. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xiao, L.; Yang, R.; Wang, K. Moss-Dominated biological soil crusts modulate soil nitrogen following vegetation restoration in a subtropical karst region. Geoderma 2019, 352, 70–79. [Google Scholar] [CrossRef]
- Cheng, C.; Li, Y.; Long, M.; Gao, M.; Li, X. Moss biocrusts buffer the negative effects of karst rocky desertification on soil properties and soil microbial richness. Plant Soil 2020, 20, 4602–4618. [Google Scholar] [CrossRef]
- Neslon, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis, 1st ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Beare, M.H.; Gregorich, E.G. Physically Uncomplexed Organic Matter. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1262–1268. [Google Scholar]
- Blair, G.J.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Gao, J.; Zhang, X. Dynamics of soil aggregate-associated organic carbon along an afforestation chronosequence. Plant Soil 2015, 391, 237–251. [Google Scholar] [CrossRef]
- Banger, K.; Toor, G.S.; Biswas, A.; Sidhu, S.S.; Sudhir, K. Soil organic carbon fractions after 16-years of applications of fertilizers and organic manure in a Typic Rhodalfs in semi-arid tropics. Nutr. Cycl. Agroecosyst. 2010, 86, 391–399. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, N.; Yang, M.; Zhan, X.; Zhang, Z. Effects of Different Tillage and Straw Return on Soil Organic Carbon in a Rice-Wheat Rotation System. PLoS ONE 2014, 9, e88900. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Xin, Z.; Wang, D.; Xiao, Y. Soil organic carbon storage and its influencing factors in the riparian woodlands of a Chinese karst area. Catena 2017, 153, 21–29. [Google Scholar] [CrossRef]
- Burns, R.G.; Deforest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Z.; Lu, X. Changes of soil labile organic carbon fractions and their relation to soil microbial characteristics in four typical wetlands of Sanjiang Plain, Northeast China. Ecol. Eng. 2015, 82, 381–389. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Q.; Wang, X.; Jin, L.; Mei, L. Response of shallow karst fissure soil quality to secondary succession in a degraded karst area of southwestern China. Geoderma 2019, 348, 76–85. [Google Scholar] [CrossRef]
- Lucas, S.T.; Weil, R.R. Can A Labile Carbon Test Be Used to Predict Crop Responses To Improve Soil Organic Matter Management? Agron. J. 2012, 104, 1160–1170. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Tillage Res. 2014, 139, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Lal, R.; Owens, L.; Izaurralde, R.C. Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res. 2007, 92, 53–59. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, D.; Yao, B.; Liao, Q. Analysis of the karst springs’ supply sources in rocky desertification area of Guanling–Huajiang, Guizhou, China. Carbonates Evaporites 2020, 35, 1–11. [Google Scholar] [CrossRef]
- Yang, L.; Luo, P.; Wen, L.; Li, D. Soil organic carbon accumulation during post-agricultural succession in a karst area, southwest China. Sci. Rep. 2016, 6, 37118–37126. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.; Li, X.; Li, Y.; Yang, X.; Lin, Z.; Song, Z.; Cooper, J.M.; Zhao, B. Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil Tillage Res. 2018, 175, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yue, Y.; Tong, X.; Wang, K.; Brandt, M. Eco-Engineering controls vegetation trends in southwest China karst. Sci. Total Environ. 2021, 770, 145–160. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237–238, 149–158. [Google Scholar] [CrossRef]
- Yue, Y.; Liao, C.; Tong, X.; Wu, Z.; Brandt, M. Large scale reforestation of farmlands on sloping hills in South China karst. Landsc. Ecol. 2020, 35, 1445–1458. [Google Scholar] [CrossRef]
- Hu, Z.L.; Pan, G.X.; Li, L.Q.; Du, Y.X.; Wang, X.Z. Changes in pools and heterogeneity of soil organic carbon, nitrogen and phosphorus under different vegetation types in Karst mountainous area of central Guizhou Province, China. Acta Ecol. Sin. 2009, 8, 4187–4195. [Google Scholar]
- Liu, S.; Wei, Z.; Wang, K.; Pan, F.; Shan, Y.; Shu, S. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total Environ. 2015, 521–522, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Cui, M.; Zhou, Z.; Zhou, J. Effects of secondary succession on soil fungal and bacterial compositions and diversities in a karst area. Plant Soil 2021, 21, 5016–5028. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, K.; He, J.; Yang, Z.; Zhou, Z. Linking rocky desertification to soil erosion by investigating changes in soil magnetic susceptibility profiles on karst slopes. Geoderma 2021, 389, 114949–114960. [Google Scholar] [CrossRef]
- Duval, M.E.; Martinez, J.M.; Galantini, J.A. Assessing soil quality indices based on soil organic carbon fractions in different long-term wheat systems under semiarid conditions. Soil Use Manag. 2019, 36, 71–82. [Google Scholar] [CrossRef]
- Pang, D.; Cui, M.; Liu, Y.; Wang, G.; Zhou, J. Responses of soil labile organic carbon fractions and stocks to different vegetation restoration strategies in degraded karst ecosystems of southwest China. Ecol. Eng. 2019, 138, 391–402. [Google Scholar] [CrossRef]


| Measures | Soil Layers (cm) | CPI | L | LI | CPMI |
|---|---|---|---|---|---|
| SF | 0–10 | − | 0.12 ± 0.01 Aa | − | − |
| 10–20 | − | 0.18 ± 0.03 Aa | − | − | |
| 20–30 | − | 0.13 ± 0.03 Aa | − | − | |
| LBE | 0–10 | 1.48 ± 0.41 Ab | 0.09 ± 0.01 ABa | 0.79 ± 0.14 Aa | 115.5 ±32.1 Aa |
| 10–20 | 2.39 ± 0.44 Ab | 0.03 ± 0.01 Cb | 0.20 ± 0.05 Bb | 47.46 ± 13.33 Ab | |
| 20–30 | 3.74 ± 0.72 Aa | 0.03 ± 0.01 Bb | 0.26 ± 0.02Bb | 97.3 ± 22.8 Aab | |
| MBE | 0–10 | 0.77 ± 0.21 Aa | 0.07 ± 0.01 Ba | 0.61 ± 0.15Aa | 45.09 ± 8.93 Aa |
| 10–20 | 0.76 ±0.06 Ca | 0.08 ± 0.02 Ba | 0.43 ± 0.05Aa | 32.72 ± 4.70 Aa | |
| 20–30 | 0.72 ± 0.19 Ba | 0.08 ± 0.01 Aa | 0.70 ± 0.23Aa | 49.30 ± 18.80 Aa | |
| IBE | 0–10 | 1.65 ± 0.60 Aa | 0.07 ± 0.02 Ba | 0.60 ± 0.28Aa | 96.70 ± 61.80 Aa |
| 10–20 | 1.64 ± 0.19 Ba | 0.06 ± 0.01 BCa | 0.31 ± 0.03 Ba | 50.59 ± 4.17 Aa | |
| 20–30 | 1.61 ± 0.52 Ba | 0.03 ± 0.01 Ba | 0.28 ± 0.07 Ba | 45.90 ± 24.6 Aa |
| Axis | I | II | III | IV | Explains (%) | Pseudo-F | P |
|---|---|---|---|---|---|---|---|
| Eigenvalues | 0.7029 | 0.0162 | 0.0004 | 0.00 | − | − | − |
| Explained variation | 70.29 | 71.91 | 71.95 | 71.95 | − | − | − |
| Pseudo-canonical correlation | 0.8470 | 0.9121 | 0.8963 | 0.3604 | − | − | − |
| Explained fitted variation | 97.69 | 99.94 | 100.00 | 100.00 | − | − | − |
| SOC | − | − | − | − | 62.3 | 16.5 | 0.002 |
| MBC | − | − | − | − | 56.8 | 13.1 | 0.002 |
| DOC | − | − | − | − | 36.9 | 5.9 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Rao, C.; Wu, Q.; Wang, E.; Jiang, X.; Xu, Y.; Hu, L.; Chen, Y.; Liang, X.; Yan, W. Changes in the Soil Labile Organic Carbon Fractions following Bedrock Exposure Rate in a Karst Context. Forests 2022, 13, 516. https://doi.org/10.3390/f13040516
Zheng W, Rao C, Wu Q, Wang E, Jiang X, Xu Y, Hu L, Chen Y, Liang X, Yan W. Changes in the Soil Labile Organic Carbon Fractions following Bedrock Exposure Rate in a Karst Context. Forests. 2022; 13(4):516. https://doi.org/10.3390/f13040516
Chicago/Turabian StyleZheng, Wei, Chengjiao Rao, Qian Wu, Enwen Wang, Xingjian Jiang, Yichen Xu, Lei Hu, Yazhen Chen, Xiaocui Liang, and Wende Yan. 2022. "Changes in the Soil Labile Organic Carbon Fractions following Bedrock Exposure Rate in a Karst Context" Forests 13, no. 4: 516. https://doi.org/10.3390/f13040516
APA StyleZheng, W., Rao, C., Wu, Q., Wang, E., Jiang, X., Xu, Y., Hu, L., Chen, Y., Liang, X., & Yan, W. (2022). Changes in the Soil Labile Organic Carbon Fractions following Bedrock Exposure Rate in a Karst Context. Forests, 13(4), 516. https://doi.org/10.3390/f13040516






