Contribution of Litter Layer to Greenhouse Gas Fluxes between Atmosphere and Soil Varies with Forest Succession
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Litter Manipulation Experiment
2.3. Gas Sampling and Measurements of FCO2, FN2O and FCH4
2.4. Phospholipid Fatty Acid Analysis
2.5. Long-Term Monitoring of Soil CO2 Efflux and Surface Litter Layer Dynamics
2.6. Calculation of the Contributions of fL-L and fL-S to GHG Fluxes
2.7. Statistical Analysis
3. Results
3.1. Soil Temperature and Soil Moisture
3.2. Soil Microbial PLFAs
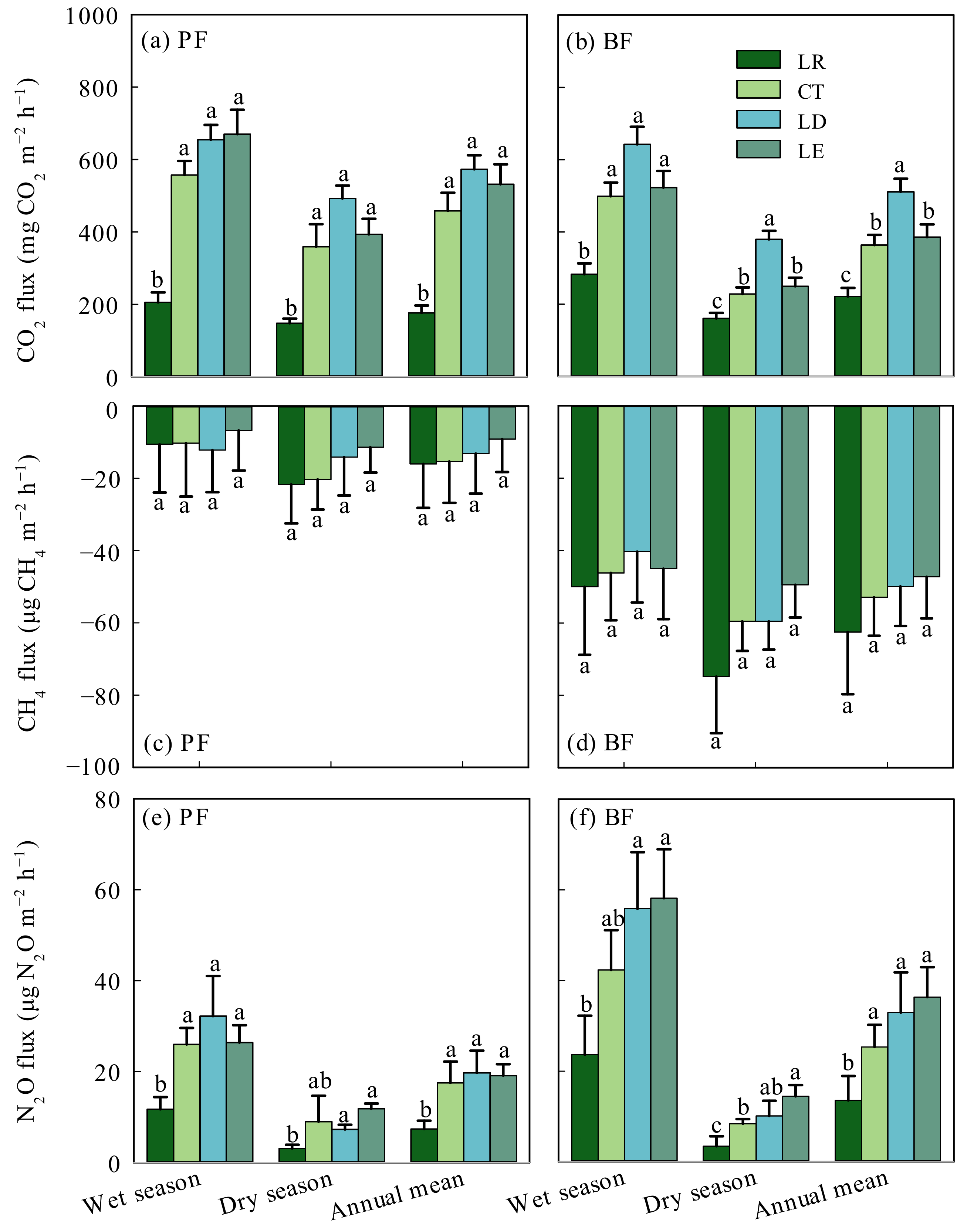
3.3. FCO2, FCH4, and FN2O
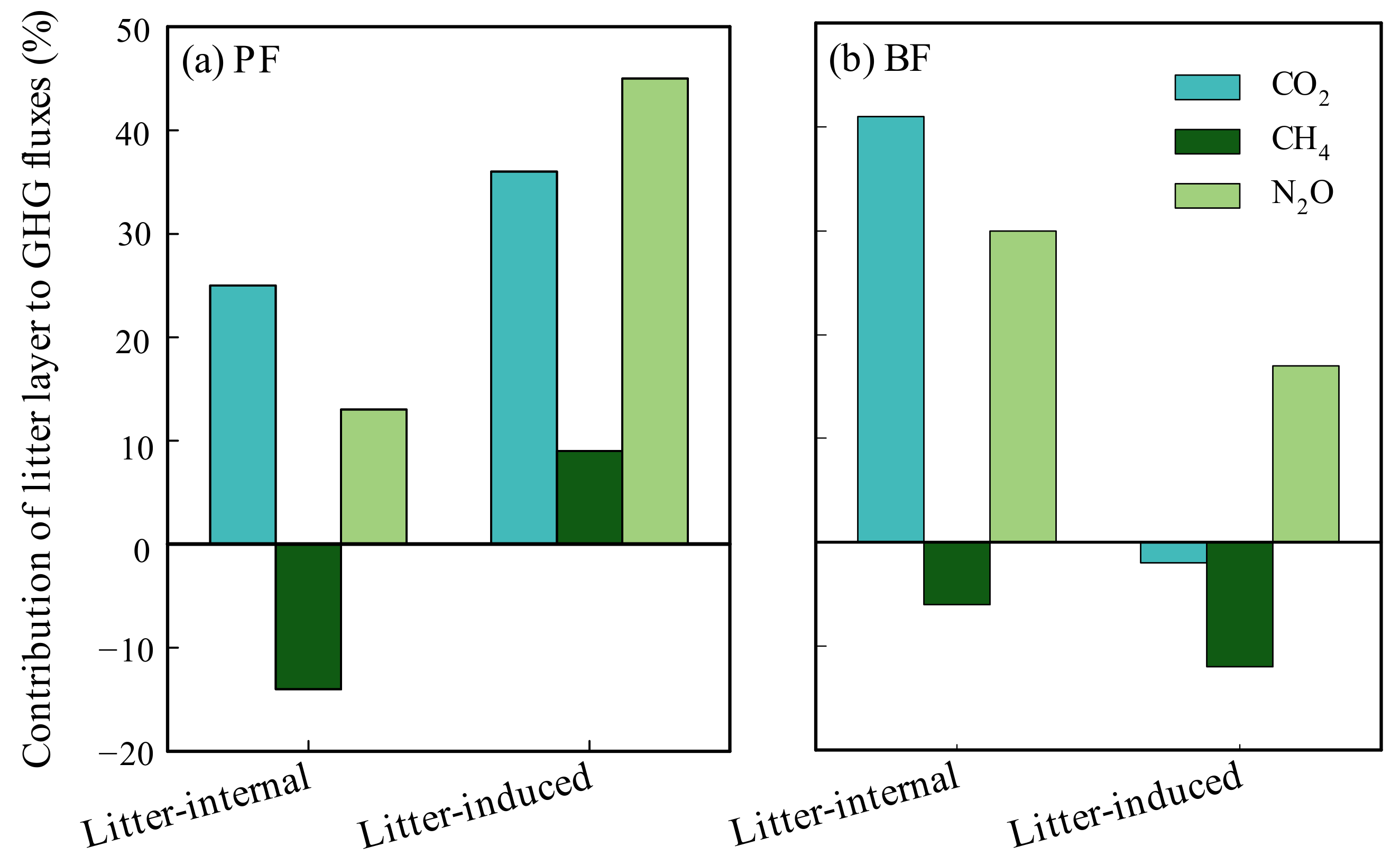
3.4. Contributions of Litter Layer to GHG Fluxes
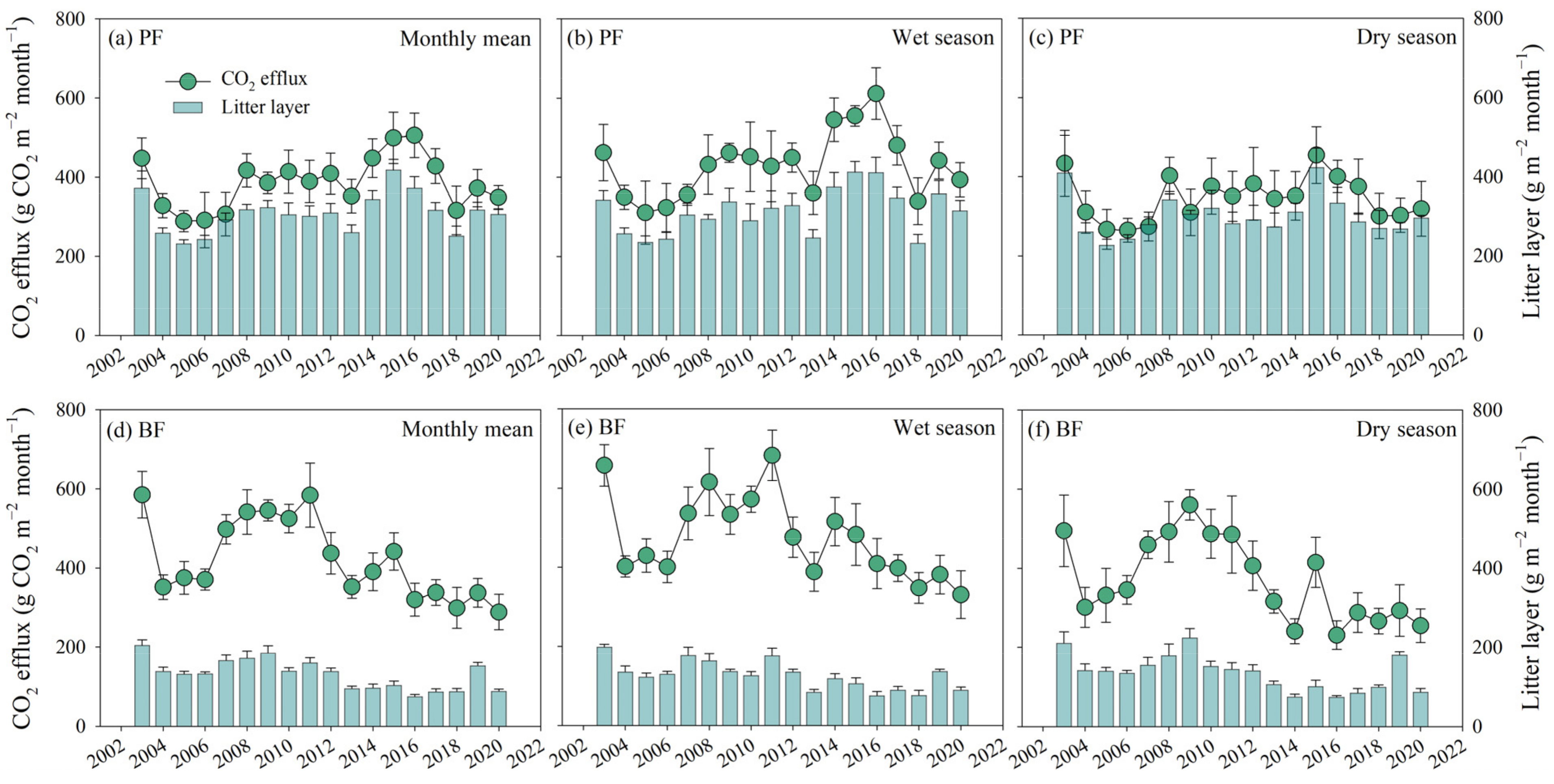
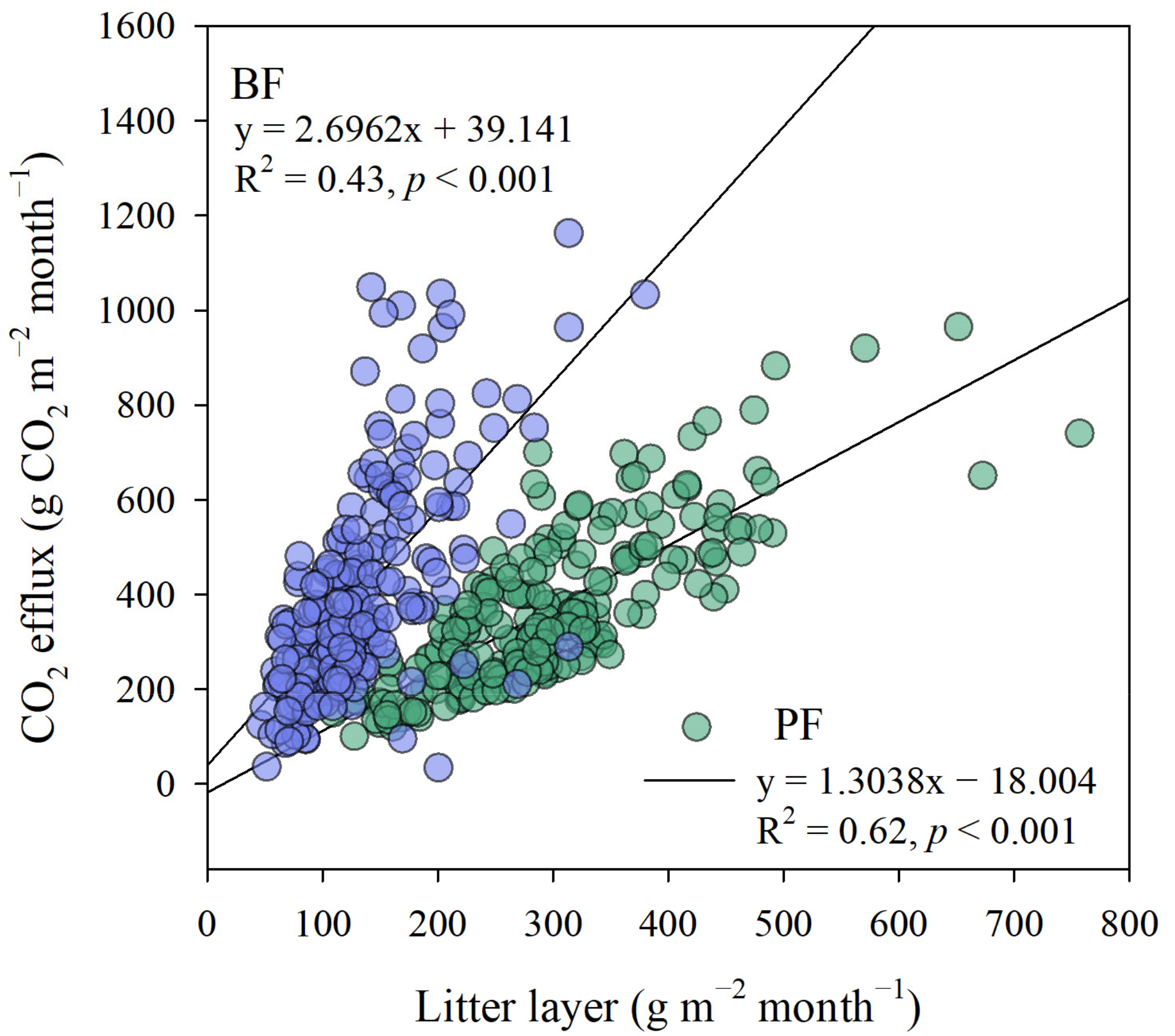
3.5. Interannual Variabilities of the Amount of Litter Layer and CO2 Efflux
4. Discussion
4.1. Effects of Litter Manipulation Treatments on FCO2
4.2. Effects of Litter Manipulation Treatments on FCH4
4.3. Effects of Litter Manipulation Treatments on FN2O
4.4. Forest Succession Regulates the Contributions of Litter Layer to GHG Fluxes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; p. 1535. [Google Scholar]
- Leitner, S.; Sae-Tun, O.; Kranzinger, L.; Zechmeister-Boltenstern, S.; Zimmermann, M. Contribution of litter layer to soil greenhouse gas emissions in a temperate beech forest. Plant Soil 2016, 403, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Peichl, M.; Arain, M.A.; Ullah, S.; Moore, T.R. Carbon dioxide, methane, and nitrous oxide exchanges in an age-sequence of temperate pine forests. Glob. Chang. Biol. 2010, 16, 2198–2212. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, W.; Wang, K.; Qin, F.; Wang, W.; Dai, H.; Li, P. Responses of CO2, N2O and CH4 fluxes between atmosphere and forest soil to changes in multiple environmental conditions. Glob. Chang. Biol. 2014, 20, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Attiwill, P.M.; Adams, M.A. Nutrient cycling in forests. New Phytol. 1993, 124, 561–582. [Google Scholar] [CrossRef]
- Fan, J.; Luo, R.; McConkey, B.G.; Ziadi, N. Effects of nitrogen deposition and litter layer management on soil CO2, N2O, and CH4 emissions in a subtropical pine forestland. Sci. Rep. 2020, 10, 8959. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.H.; Li, Y.L.; Xiao, Y.; Wenigmann, K.O.; Zhou, G.Y.; Zhang, D.Q.; Wenigmann, M.; Tang, X.L.; Liu, J.X. Controls of litter quality on the carbon sink in soils through partitioning the products of decomposing litter in a forest succession series in South China. For. Ecol. Manag. 2011, 261, 1170–1177. [Google Scholar] [CrossRef]
- Leff, J.W.; Wieder, W.R.; Taylor, P.G.; Townsend, A.R.; Nemergut, D.R.; Grandy, A.S.; Cleveland, C.C. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob. Chang. Biol. 2012, 18, 2969–2979. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Zhou, G.; Zhang, D.; Zhou, C. Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Chang. Biol. 2006, 12, 546–560. [Google Scholar] [CrossRef]
- Han, T.; Huang, W.; Liu, J.; Zhou, G.; Xiao, Y. Different soil respiration responses to litter manipulation in three subtropical successional forests. Sci. Rep. 2015, 5, 18166. [Google Scholar] [CrossRef]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E.V.J. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Chang. 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Cepakova, S.; Frouz, J. Changes in chemical composition of litter during decomposition: A review of published C-13 NMR spectra. J. Soil Sci. Plant. Nutr. 2015, 15, 805–815. [Google Scholar]
- Kooch, Y.; Bayranvand, M. Labile soil organic matter changes related to forest floor quality of tree species mixtures in Oriental beech forests. Ecol. Indic. 2019, 107, 105598. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Z.; Pan, X.; Chen, X.; Derrien, D.; Hu, F.; Liu, M.; Hattenschwiler, S. Carbon and nitrogen transfer from litter to soil is higher in slow than rapid decomposing plant litter: A synthesis of stable isotope studies. Soil Biol. Biochem. 2021, 156, 108196. [Google Scholar] [CrossRef]
- Tian, H.; Xu, X.; Lu, C.; Liu, M.; Ren, W.; Chen, G.; Melillo, J.; Liu, J. Net exchanges of CO2, CH4, and N2O between China's terrestrial ecosystems and the atmosphere and their contributions to global climate warming. J. Geophys. Res. Biogeosci. 2011, 116, G02011. [Google Scholar] [CrossRef] [Green Version]
- Del Grosso, S.J.; Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Potter, C.S.; Borken, W.; Brumme, R.; Butterbach-Bahl, K.; Crill, P.M.; Dobbie, K.; et al. General CH4 oxidation model and comparisons of CH4 oxidation in natural and managed systems. Glob. Biogeochem. Cycles 2000, 14, 999–1019. [Google Scholar] [CrossRef]
- Hashimoto, S.; Morishita, T.; Sakata, T.; Ishizuka, S.; Kaneko, S.; Takahashi, M. Simple models for soil CO2, CH4, and N2O fluxes calibrated using a Bayesian approach and multi-site data. Ecol. Model. 2011, 222, 1283–1292. [Google Scholar] [CrossRef]
- Luo, Y.; Gerten, D.; Le Maire, G.; Parton, W.J.; Weng, E.; Zhou, X.; Keough, C.; Beier, C.; Ciais, P.; Cramer, W.; et al. Modeled interactive effects of precipitation, temperature, and CO2 on ecosystem carbon and water dynamics in different climatic zones. Glob. Change Biol. 2008, 14, 1986–1999. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, D.; Zhou, G.; Liu, J. Soil respiration associated with forest succession in subtropical forests in Dinghushan Biosphere Reserve. Soil Biol. Biochem. 2009, 41, 991–999. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Peng, X.; Huang, Z.; Liu, S.; Zhang, Q. The mechanism for exclusion of Pinus massoniana during the succession in subtropical forest ecosystems: Light competition or stoichiometric homoeostasis? Sci. Rep. 2015, 5, 10994. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z.; et al. Litterfall Production Along Successional and Altitudinal Gradients of Subtropical Monsoon Evergreen Broadleaved Forests in Guangdong, China. Plant Ecol. 2006, 188, 77–89. [Google Scholar] [CrossRef]
- Peng, S.L.; Wang, B.S. Forest succession at Dinghushan, Guangdong, China. Chin. J. Bot. 1995, 7, 75–80. [Google Scholar]
- Yan, J.; Li, K.; Wang, W.; Zhang, D.; Zhou, G. Changes in dissolved organic carbon and total dissolved nitrogen fluxes across subtropical forest ecosystems at different successional stages: Carbon and nitrogen fluxes response to forest succession. Water Resour. Res. 2015, 51, 3681–3694. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.P.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhou, G.; Zhang, D. Estimates of soil respiration and net primary production of three forests at different succession stages in South China. Glob. Chang. Biol. 2006, 12, 810–821. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Peng, S.; Kong, G. Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. For. Ecol. Manag. 2003, 175, 573–583. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Li, Z.; Zhang, D.; Tang, X.; Zhou, C.; Yan, J.; Mo, J. Old-growth forests can accumulate carbon in soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Wang, Y.P.; Yu, M.; Li, K.; Shao, Y.; Yan, J. Responses of soil buffering capacity to acid treatment in three typical subtropical forests. Sci. Total Environ. 2016, 563–564, 1068–1077. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wang, Y.H. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv. Atmos. Sci. 2003, 20, 842–844. [Google Scholar]
- Frostegard, A.; Baath, E.; Tunlid, A. Shifts in the structure of soil microbial coumunites in limed forests as revealed by phospholipid fatty-acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Haettenschwiler, S.; Aeschlimann, B.; Couteaux, M.M.; Roy, J.; Bonal, D. High variation in foliage and leaf litter chemistry among 45 tree species of a neotropical rainforest community. New Phytol. 2008, 179, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, T.; Wang, S.; Liu, L. Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric. For. Meteorol. 2013, 178, 152–160. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Ma, Z.; Dai, X.; Wen, X.; Liu, Y.; Wang, Z.L. The litter layer acts as a moisture-induced bidirectional buffer for atmospheric methane uptake by soil of a subtropical pine plantation. Soil Biol. Biochem. 2013, 66, 45–50. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Bowden, R.D.; Yano, Y.; Brant, J.B.; Caldwell, B.A.; Sulzman, E.W. Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. For. Ecol. Manag. 2009, 258, 2224–2232. [Google Scholar] [CrossRef]
- Kalbitz, K.; Meyer, A.; Yang, R.; Gerstberger, P. Response of dissolved organic matter in the forest floor to long-term manipulation of litter and throughfall inputs. Biogeochemistry 2007, 86, 301–318. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Reed, S.C.; Townsend, A.R. Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 2006, 87, 492–503. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barre, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.A.; Cattanio, J.H.; Ackerman, I.L. Land-Use Change and Biogeochemical Controls of Methane Fluxes in Soils of Eastern Amazonia. Ecosystems 2000, 3, 41–56. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Wang, Z.L.; Ma, Z.; Dai, X.; Wen, X.; Liu, Y. Effect of litter layer on soil-atmosphere N2O flux of a subtropical pine plantation in China. Atmos. Environ. 2014, 82, 106–112. [Google Scholar] [CrossRef]
- Ley, M.; Lehmann, M.F.; Niklaus, P.A.; Luster, J. Alteration of nitrous oxide emissions from floodplain soils by aggregate size, litter accumulation and plant-soil interactions. Biogeosciences 2018, 15, 7043–7057. [Google Scholar] [CrossRef] [Green Version]
- Eickenscheidt, N.; Brumme, R. Regulation of N2O and NOx emission patterns in six acid temperate beech forest soils by soil gas diffusivity, N turnover, and atmospheric NOx concentrations. Plant Soil 2013, 369, 515–529. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Mo, J.; Yu, G.; Fang, Y.; Li, D.; Lu, X.; Wang, H. Emissions of nitrous oxide from three tropical forests in Southern China in response to simulated nitrogen deposition. Plant Soil 2008, 306, 221–236. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, W.; Liu, Y.; Zhu, J.; Sha, L.; Song, Q.; Ji, H.; Lin, Y.; Fei, X.; Bai, X.; et al. Effects of Litter Inputs on N2O Emissions from a Tropical Rainforest in Southwest China. Ecosystems 2018, 21, 1013–1026. [Google Scholar] [CrossRef]
- Kooch, Y.; Parsapour, M.K.; Egli, M.; Moghimian, N. Forest floor and soil properties in different development stages of Oriental beech forests. Appl. Soil Ecol. 2021, 161, 103823. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Koegel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.Y.; Chen, H.Y.H. Fine Root Biomass, Production, Turnover Rates, and Nutrient Contents in Boreal Forest Ecosystems in Relation to Species, Climate, Fertility, and Stand Age: Literature Review and Meta-Analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Rowlings, D.W.; Grace, P.R.; Kiese, R.; Weier, K.L. Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Glob. Chang. Biol. 2012, 18, 726–738. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Wei, H.; Man, X. Increased Litter Greatly Enhancing Soil Respiration in Betula platyphylla Forests of Permafrost Region, Northeast China. Forests 2021, 12, 89. [Google Scholar] [CrossRef]
- Wu, X.; Zang, S.; Ma, D.; Ren, J.; Chen, Q.; Dong, X. Emissions of CO2, CH4, and N2O Fluxes from Forest Soil in Permafrost Region of Daxing'an Mountains, Northeast China. Int. J. Environ. Res. Public Health 2019, 16, 2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nottingham, A.T.; Turner, B.L.; Stott, A.W.; Tanner, E.V.J. Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biol. Biochem. 2015, 80, 26–33. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar] [PubMed]
- Fan, J.; Xu, Y.; Chen, Z.; Xiao, J.; Liu, D.; Luo, J.; Bolan, N.; Ding, W. Sulfur deposition suppressed nitrogen-induced soil N2O emission from a subtropical forestland in southeastern China. Agr. For. Meteorol. 2017, 233, 163–170. [Google Scholar] [CrossRef]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Turner, B.L.; Zhou, G.Y.; Mo, J.M. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef]
- Huang, W.; Spohn, M. Effects of long-term litter manipulation on soil carbon, nitrogen, and phosphorus in a temperate deciduous forest. Soil Biol. Biochem. 2015, 83, 12–18. [Google Scholar] [CrossRef]
- Osborne, B.B.; Soper, F.M.; Nasto, M.K.; Bru, D.; Hwang, S.; Machmuller, M.B.; Morales, M.L.; Philippot, L.; Sullivan, B.W.; Asner, G.P.; et al. Litter inputs drive patterns of soil nitrogen heterogeneity in a diverse tropical forest: Results from a litter manipulation experiment. Soil Biol. Biochem. 2021, 158, 108247. [Google Scholar] [CrossRef]
| Parameters | Variables | PF | BF |
|---|---|---|---|
| Site characteristics | Age (year) £ | 80 | >400 |
| Elevation (m) £ | 200–300 | 220–300 | |
| Surface litter | C (mg g−1) & | 523.0 ± 8.0 | 482.0 ± 22.0 |
| N (mg g−1) & | 9.1 ± 0.8 | 17.0 ± 0.9 | |
| P (mg g−1) & | 0.2 ± 0.0 | 0.6 ± 0.1 | |
| C/N & | 57.5 ± 3.8 | 28.3 ± 2.7 | |
| N/P & | 53.5 ± 2.1 | 28.4 ± 3.3 | |
| Mineral soil | Soil pH £ | 4.0 ± 0.1 | 3.8 ± 0.1 |
| Soil moisture content (%) £ | 22.4 ± 2.2 | 33.5 ± 2.6 | |
| Bulk density (g cm−3) § | 1.4 ± 0.1 | 1.0 ± 0.0 | |
| Clay content (%) § | 12.5 ± 0.7 | 19.7 ± 0.8 | |
| Organic C (g kg−1) § | 23.9 ± 1.8 | 34.5 ± 3.1 | |
| Total N (g kg−1) § | 1.6 ± 0.1 | 2.4 ± 0.2 |
| Forest Type | Treatment | Soil Temperature (°C) | Soil Moisture (%) | ||||
|---|---|---|---|---|---|---|---|
| Wet Season | Dry Season | Annual Mean | Wet Season | Dry Season | Annual Mean | ||
| PF | LR | 25.1 ± 0.8 a | 15.3 ± 0.9 a | 20.3 ± 1.2 a | 15.4 ± 0.9 b | 10.1 ± 0.5 b | 13.0 ± 0.7 b |
| CT | 24.0 ± 1.4 a | 15.9 ± 1.6 a | 19.9 ± 1.6 a | 22.2 ± 0.8 a | 14.2 ± 1.1 a | 18.2 ± 1.4 a | |
| LD | 23.8 ± 1.7 a | 15.6 ± 0.7 a | 19.5 ± 1.1 a | 23.4 ± 0.9 a | 15.0 ± 1.3 a | 17.7 ± 1.7 a | |
| LE | 22.7 ± 1.5 a | 16.3 ± 1.4 a | 18.7 ± 0.6 a | 22.6 ± 0.5 a | 14.8 ± 0.8 a | 18.9 ± 0.3 a | |
| BF | LR | 23.3 ± 0.9 a | 18.2 ± 1.2 a | 19.8 ± 0.8 a | 26.9 ± 1.1 a | 21.5 ± 0.7 a | 23.8 ± 0.6 a |
| CT | 23.5 ± 1.1 a | 17.1 ± 1.3 a | 20.3 ± 1.3 a | 27.2 ± 0.8 a | 20.9 ± 0.8 a | 24.1 ± 1.1 a | |
| LD | 23.4 ± 1.5 a | 16.7 ± 0.3 a | 20.9 ± 1.6 a | 27.8 ± 1.2 a | 22.5 ± 1.6 a | 24.3 ± 0.9 a | |
| LE | 24.1 ± 0.7 a | 17.6 ± 0.8 a | 19.5 ± 1.1 a | 26.8 ± 0.5 a | 21.3 ± 1.4 a | 23.7 ± 1.6 a | |
| Forest Type | Treatment | Bacteria | Fungi | G+ | G− | ACT |
|---|---|---|---|---|---|---|
| PF | LR | 825.3 ± 55.2 b | 193.4 ± 27.6 b | 428.1 ± 27.6 b | 383.4 ± 41.4 b | 193.4 ± 27.6 a |
| CT | 1227.2 ± 124.3 a | 287.8 ± 37.8 a | 667.4 ± 78.3 a | 547.2 ± 55.2 a | 196.8 ± 41.4 a | |
| LD | 1266.0 ± 193.4 a | 294.6 ± 56.7 a | 685.5 ± 96.7 a | 593.9 ± 61.3 a | 221.5 ± 23.0 a | |
| LE | 1473.2 ± 188.7 a | 359.1 ± 46.0 a | 779.2 ± 101.3 a | 662.9 ± 82.9 a | 210.8 ± 46.0 a | |
| BF | LR | 1528.7 ± 104.5 b | 321.8 ± 60.3 b | 774.4 ± 70.4 b | 744.3 ± 130.2 a | 297.3 ± 55.3 a |
| CT | 1569.0 ± 141.4 b | 352.0 ± 51.3 b | 824.7 ± 90.5 b | 754.3 ± 171.0 a | 301.2 ± 47.2 a | |
| LD | 2293.1 ± 202.3 a | 593.0 ± 72.4 a | 1377.3 ± 111.1 a | 1035.9 ± 181.0 a | 332.0 ± 69.3 a | |
| LE | 2285.9 ± 231.9 a | 604.0 ± 100.6 a | 1318.7 ± 150.9 a | 1057.2 ± 171.0 a | 346.2 ± 78.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Wang, Y.-P.; Zhang, H.; Yu, M.; Liu, F.; Xia, S.; Yan, J. Contribution of Litter Layer to Greenhouse Gas Fluxes between Atmosphere and Soil Varies with Forest Succession. Forests 2022, 13, 544. https://doi.org/10.3390/f13040544
Jiang J, Wang Y-P, Zhang H, Yu M, Liu F, Xia S, Yan J. Contribution of Litter Layer to Greenhouse Gas Fluxes between Atmosphere and Soil Varies with Forest Succession. Forests. 2022; 13(4):544. https://doi.org/10.3390/f13040544
Chicago/Turabian StyleJiang, Jun, Ying-Ping Wang, Hao Zhang, Mengxiao Yu, Fengcai Liu, Shiting Xia, and Junhua Yan. 2022. "Contribution of Litter Layer to Greenhouse Gas Fluxes between Atmosphere and Soil Varies with Forest Succession" Forests 13, no. 4: 544. https://doi.org/10.3390/f13040544






