Effects of Moisture and Temperature on Soil Organic Carbon Decomposition along a Vegetation Restoration Gradient of Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling and Preparation
2.3. Soil Properties and PLFA Analyses
2.4. Soil Incubation Experiment
2.5. Statistical Analysis
3. Results
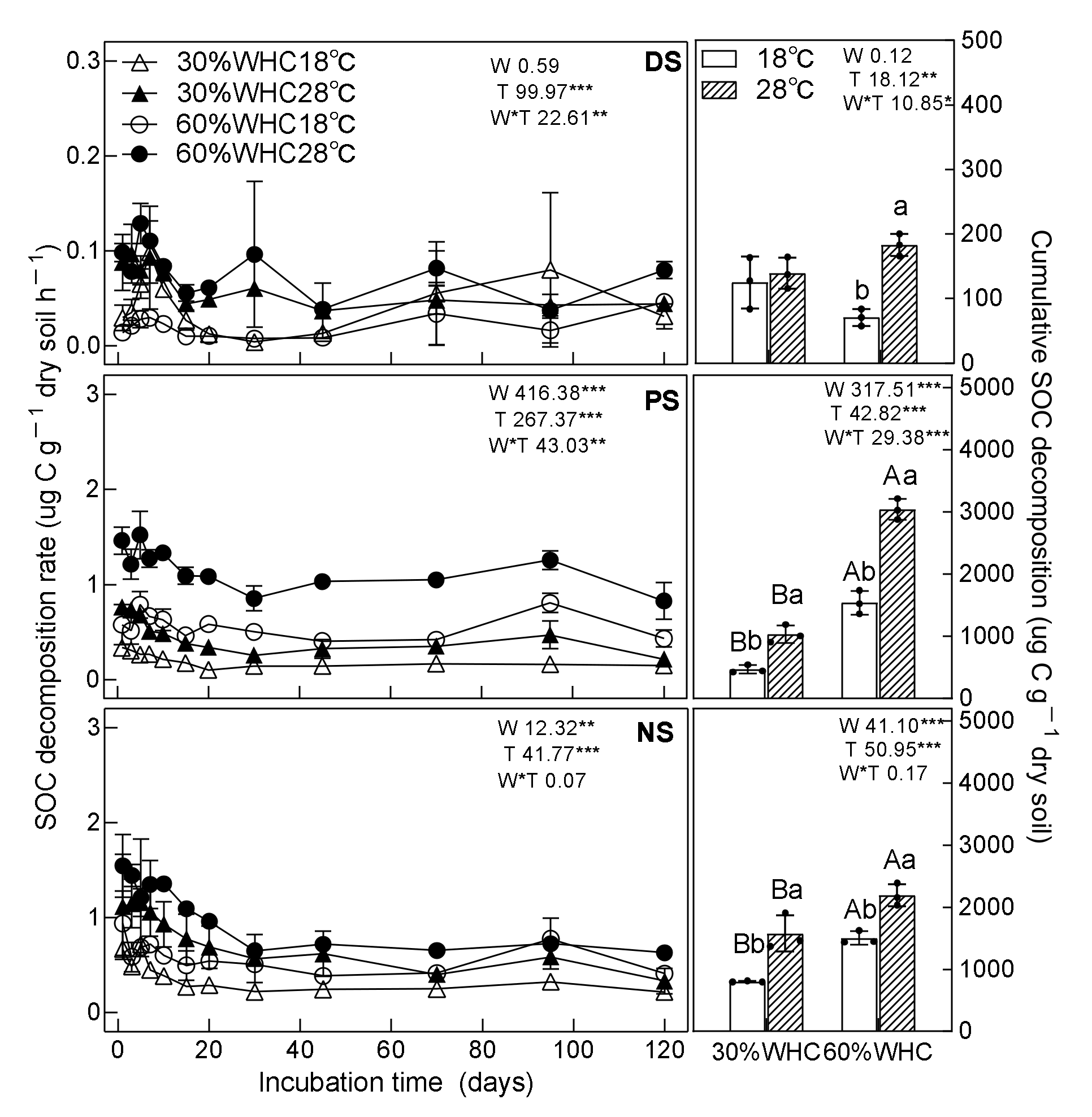
3.1. SOC Decomposition
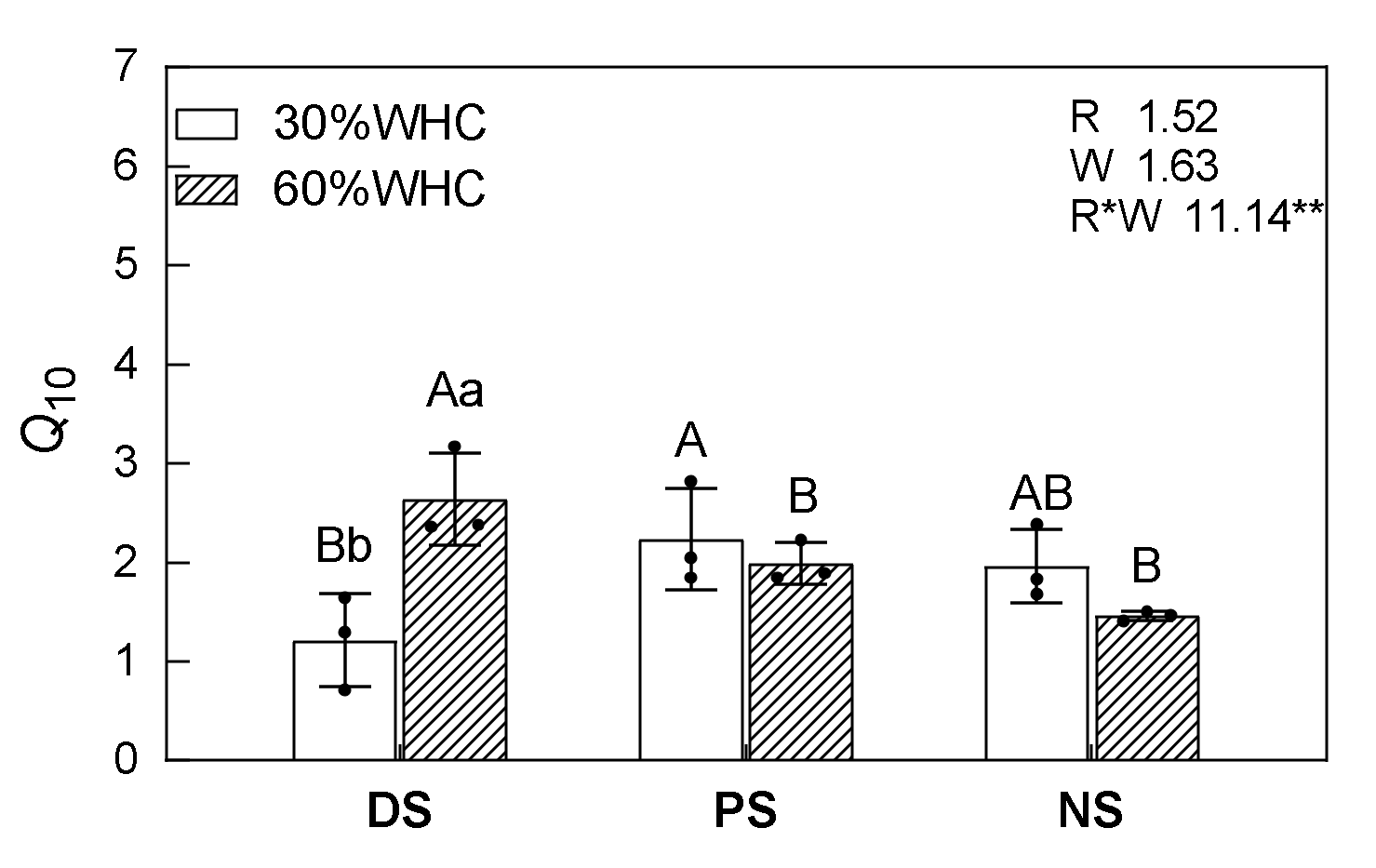
3.2. Temperature Sensitivity
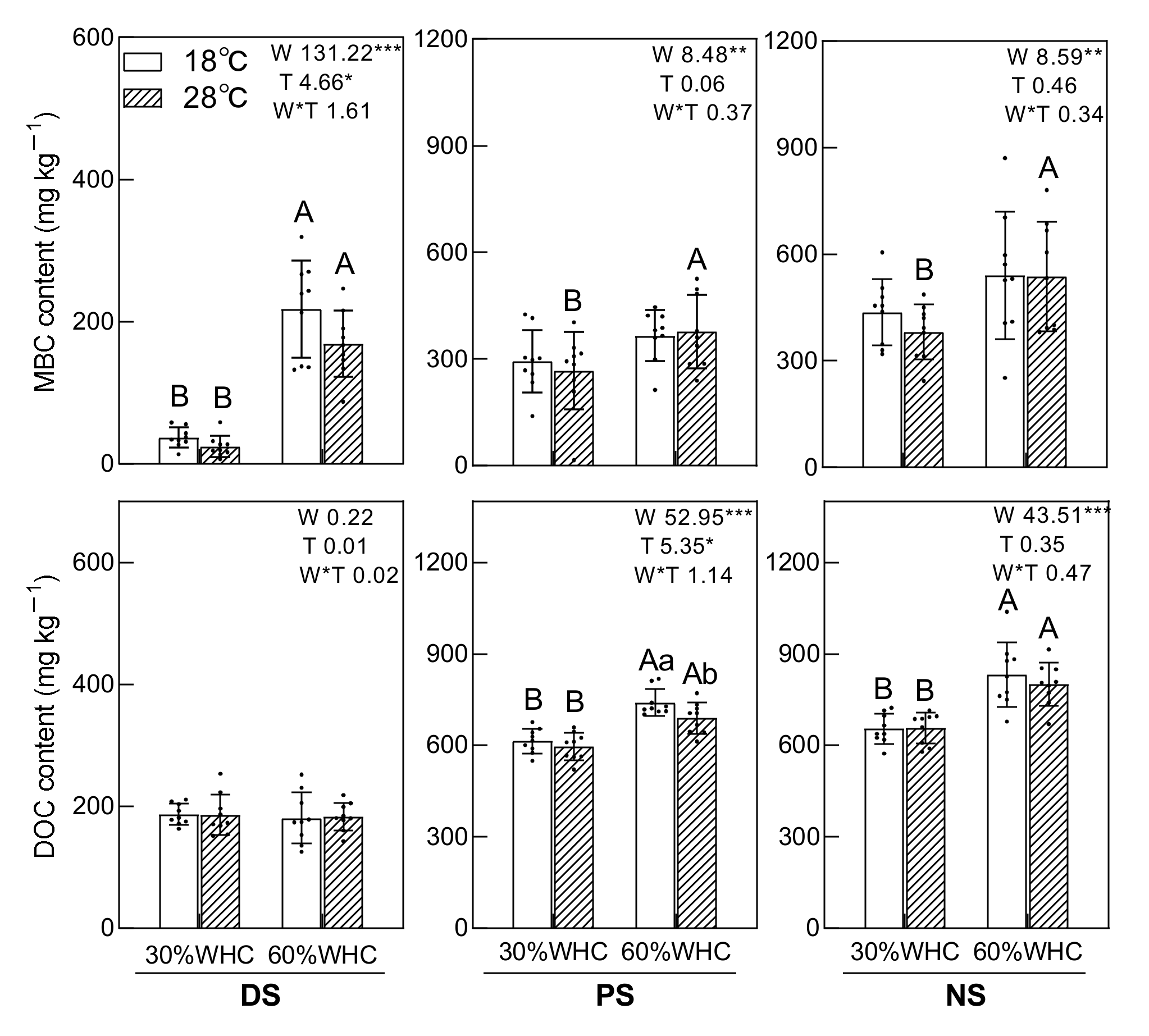
3.3. Soil MBC, DOC, qCO2, Rh: DOC, and Correlations
3.4. Soil Microbial Composition
3.5. Soil Properties among Vegetation Restoration Stages
4. Discussion
4.1. Responses of SOC Decomposition to Vegetation Restoration, Moisture, and Temperature
4.2. Responses of Q10 to Vegetation Restoration and Soil Moisture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.F.; Sun, W.J.; Zhu, J.J.; Liu, G.B.; Zhou, W.M.; Zhang, Q.F.; Shi, P.L.; Liu, X.P.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef] [Green Version]
- Pang, D.B.; Cui, M.; Liu, Y.G.; Wang, G.Z.; Cao, J.H.; Wang, X.R.; Dan, X.Q.; Zhou, J.X. Responses of soil labile organic carbon fractions and stocks to different vegetation restoration strategies in degraded karst ecosystems of southwest China. Ecol. Eng. 2019, 138, 391–402. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Piñeiro, G.; Jobbágy, E.G.; Jackson, R.B. Soil C and N changes with afforestation of grasslands across gradients of precipitation and plantation age. Ecol. Appl. 2012, 22, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.S.; Wang, L.X.; Yang, Z.J.; Xu, C.; Xie, J.S.; Chen, G.S.; Lin, C.F.; Guo, J.F.; Liu, X.F.; Xiong, D.C.; et al. Large ecosystem service benefits of assisted natural regeneration. J. Geophys. Res. Biogeosci. 2018, 123, 676–687. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; DeHollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Yang, H.; Mo, B.; Zhou, M.; Zhu, T.; Cao, J. Effects of plum plantation ages on soil organic carbon mineralization in the karst rocky desertification ecosystem of southwest china. Forests 2019, 10, 1107. [Google Scholar] [CrossRef] [Green Version]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Wei, H.; Guenet, B.; Vicca, S.; Nunan, N.; Asard, H.; AbdElgawad, H.; Shen, W.J.; Janssens, I.A. High clay content accelerates the decomposition of fresh organic matter in artificial soils. Soil Biol. Biochem. 2014, 77, 100–108. [Google Scholar] [CrossRef]
- Zhang, K.R.; Li, X.S.; Cheng, X.L.; Zhang, Z.H.; Zhang, Q.F. Changes in soil properties rather than functional gene abundance control carbon and nitrogen mineralization rates during long-term natural revegetation. Plant Soil 2019, 443, 293–306. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Tuo, D.F.; Wang, C.; Fu, B.J.; Lv, Y.H.; Liu, G.H. Land use change and stand age regulate soil respiration by influencing soil substrate supply and microbial community. Geoderma 2020, 359, 113991. [Google Scholar] [CrossRef]
- Auwal, M.; Singh, B.P.; Chen, Z.Y.; Kumar, A.; Pan, S.T.; Luo, Y.; Xu, J.M. Nutrients addition regulates temperature sensitivity of maize straw mineralization. J. Soils Sediments 2021, 21, 2778–2790. [Google Scholar] [CrossRef]
- Huang, J.X.; Lin, T.C.; Xiong, D.C.; Yang, Z.J.; Liu, X.F.; Chen, G.S.; Xie, J.S.; Li, Y.Q.; Yang, Y.S. Organic carbon mineralization in soils of a natural forest and a forest plantation of southeastern China. Geoderma 2019, 344, 119–126. [Google Scholar] [CrossRef]
- Conant, R.T.; Steinweg, J.M.; Haddix, M.L.; Paul, E.A.; Plante, A.F.; Six, J. Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 2008, 89, 2384–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershenson, A.; Bader, N.E.; Cheng, W.X. Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Glob. Chang. Biol. 2009, 15, 176–183. [Google Scholar] [CrossRef]
- Côté, L.; Brown, S.; Paré, D.; Fyles, J.; Bauhus, J. Dynamics of carbon and nitrogen mineralization in relation to stand type, stand age and soil texture in the boreal mixed wood. Soil Biol. Biochem. 2000, 32, 1079–1090. [Google Scholar] [CrossRef]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.Y.; Qu, C.; Huang, W.J.; Zhang, D.Q.; Li, Y.L.; Liu, J.X. Translocating subtropical forest soils to a warmer region alters microbial communities and increases the decomposition of mineral-associated organic carbon. Soil Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Streit, K.; Hagedorn, F.; Hiltbrunner, D.; Portmann, M.; Saurer, M.; Buchmann, N.; Wild, B.; Richter, A.; Wipf, S.; Siegwolf, R.T.W. Soil warming alters microbial substrate use in alpine soils. Glob. Chang. Biol. 2013, 142, 1327–1338. [Google Scholar] [CrossRef]
- Thiessen, S.; Gleixner, G.; Wutzler, T.; Reichstein, M. Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass-An incubation study. Soil Biol. Biochem. 2013, 57, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.K.; Zhao, X.C.; Chen, L.C.; Yang, Q.P.; Chen, S.; Zhang, W.D. Global synthesis of temperature sensitivity of soil organic carbon decomposition: Latitudinal patterns and mechanisms. Funct. Ecol. 2019, 33, 514–523. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Canarini, A.; Kiaer, L.P.; Dijkstra, F.A. Soil carbon loss regulated by drought intensity and available substrate: A meta-analysis. Soil Biol. Biochem. 2017, 112, 90–99. [Google Scholar] [CrossRef]
- Craine, J.M.; Gelderman, T.M. Soil moisture controls on temperature sensitivity of soil organic carbon decomposition for a mesic grassland. Soil Biol. Biochem. 2011, 43, 455–457. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, Z.; Wan, S.Q. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Chang. Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.H.; Yang, Q.; Li, H.; Luo, Y.Q.; Fang, C.M.; Chen, J.K.; Yang, X.; Li, B. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology 2013, 94, 726–738. [Google Scholar] [CrossRef]
- Moyano, F.E.; Vasilyeva, N.; Bouckaert, L.; Cook, F.; Craine, J.; Curiel Yuste, J.; Don, A.; Epron, D.; Formanek, P.; Franzluebbers, A.; et al. The moisture response of soil heterotrophic respiration: Interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.P.; Hui, D.F.; Shen, W.J. Effects of soil moisture on the temperature sensitivity of soil heterotrophic respiration: A laboratory incubation study. PLoS ONE 2014, 9, e92531. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.A.; Lin, J.J.; Kurganova, I.; Wang, X.G.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.L.; Kuyper, T.W.; Xu, M.; Li, X.L.; Zhang, J.L. High microbial diversity stabilizes the responses of soil organic carbon decomposition to warming in the subsoil on the Tibetan Plateau. Glob. Chang. Biol. 2021, 27, 2061–2075. [Google Scholar] [CrossRef]
- Buol, S.W.; Southard, R.J.; Graham, R.C.; McDaniel, P.A. Soil Genesis and Classification, 5th ed.; Iowa State Press: Ames, IA, USA, 2003. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: Arapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhou, G.Y.; Li, Y.L.; Liu, S.Z.; Chu, G.W.; Xu, Z.H.; Liu, J.X. Warming effects on biomass and composition of microbial communities and enzyme activities within soil aggregates in subtropical forest. Biol. Fertil. Soils. 2016, 52, 353–365. [Google Scholar] [CrossRef]
- Conant, R.T.; Drijber, R.A.; Haddix, M.L.; Parton, W.J.; Paul, E.A.; Plante, A.F.; Steinweg, J.M. Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Chang. Biol. 2008, 14, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, J.; Ostle, N.; McNamara, N.P.; Nottingham, A.T.; Stott, A.W.; Bardgett, R.D.; Salinas, N.; Ccahuana, A.J.Q.; Meir, P. Microbial carbon mineralization intropical lowland and montane forest soils of Peru. Front. Microbiol. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Ruan, H.H.; Luo, Y.Q.; Wang, J.S. Temperature sensitivity increases with soil organic carbon recalcitrance along an elevational gradient in the Wuyi Mountains, China. Soil Biol. Biochem. 2010, 42, 1811–1815. [Google Scholar] [CrossRef]
- Parsapour, M.K.; Kooch, Y.; Hosseini, S.M.; Alavi, S.J. C and N cycle monitoring under Quercus castaneifolia plantation. For. Ecol. Manag. 2018, 427, 26–36. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, G.S.; Robinson, D.; Yang, Z.J.; Guo, J.F.; Xie, J.S.; Fu, S.L.; Zhou, L.X.; Yang, Y.S. Large amounts of easily decomposable carbon stored in subtropical forest subsoil are associated with r-strategy-dominated soil microbes. Soil Biol. Biochem. 2016, 95, 233–242. [Google Scholar] [CrossRef]
- Yang, K.J.; He, R.Y.; Yang, W.Q.; Li, Z.J.; Zhuang, L.Y.; Wu, F.Z.; Tan, B.; Liu, Y.; Zhang, L.; Tu, L.H.; et al. Temperature response of soil carbon decomposition depends strongly on forest management practice and soil layer on the eastern Tibetan Plateau. Sci. Rep. 2017, 7, 4777. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Zhao, L.; Zhou, G.Y.; Huang, W.J.; Liu, J.X. Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests. Plant Soil 2015, 392, 139–153. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Dong, X.J.; Xu, B.X.; Chen, Y.L.; Zhao, Y.; Gao, Y.H.; Hu, Y.G.; Huang, L. Soil respiration sensitivities to water and temperature in a revegetated desert. J. Geophys. Res. Biogeosci. 2015, 120, 773–787. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.; Luo, Y.Q. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Balogh, J.; Pintér, K.; Fóti, S.; Cserhalmi, D.; Papp, M.; Nagy, Z. Dependence of soil respiration on soil moisture, clay content, soil organic matter, and CO2 uptake in dry grasslands. Soil Biol. Biochem. 2011, 43, 1006–1013. [Google Scholar] [CrossRef]
- Schindlbache, A.; Rodler, A.; Kuffner, M.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol. Biochem. 2011, 43, 1417–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Q.; Hui, D.F.; Zhang, D.Q.; Zhou, G.Y.; Liu, J.X.; Liu, S.Z.; Chu, G.W.; Li, J. Effects of precipitation increase on soil respiration: A three-year field experiment in subtropical forests in China. PLoS ONE 2012, 7, e41493. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
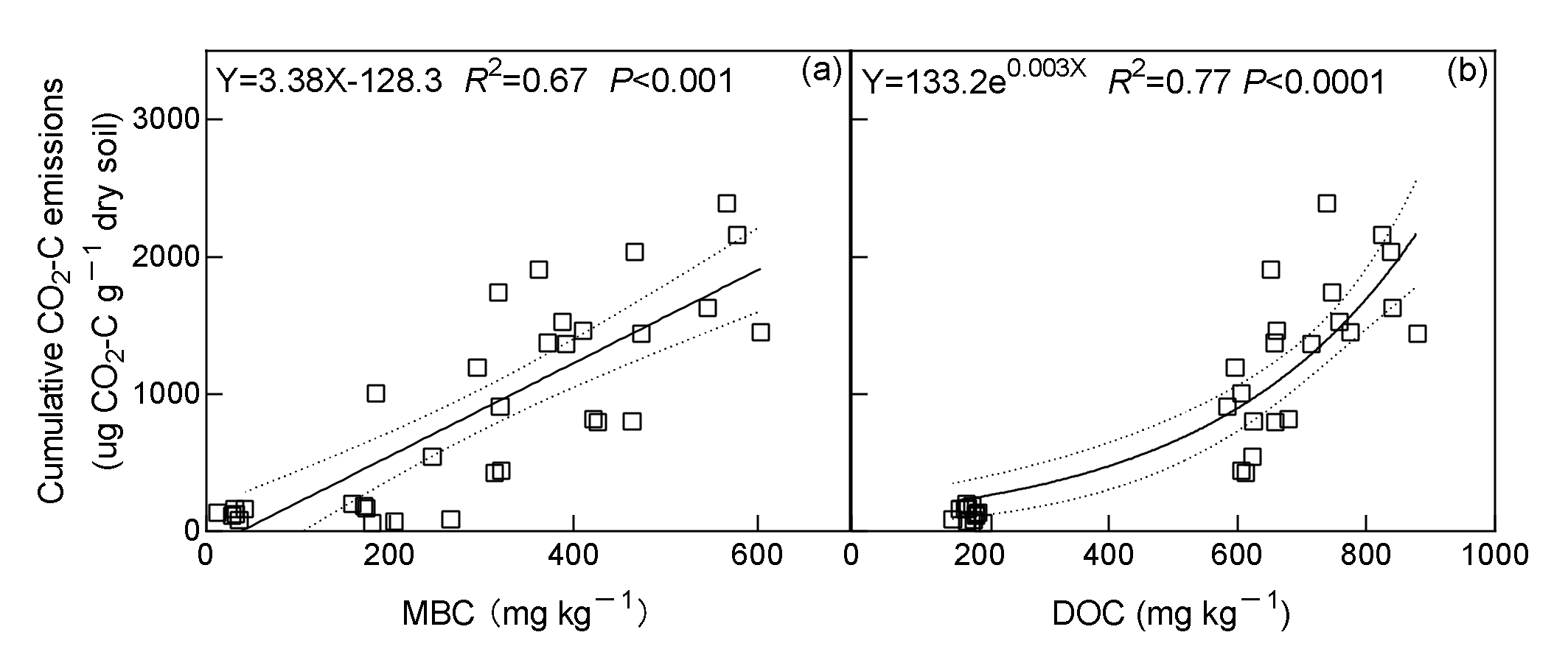
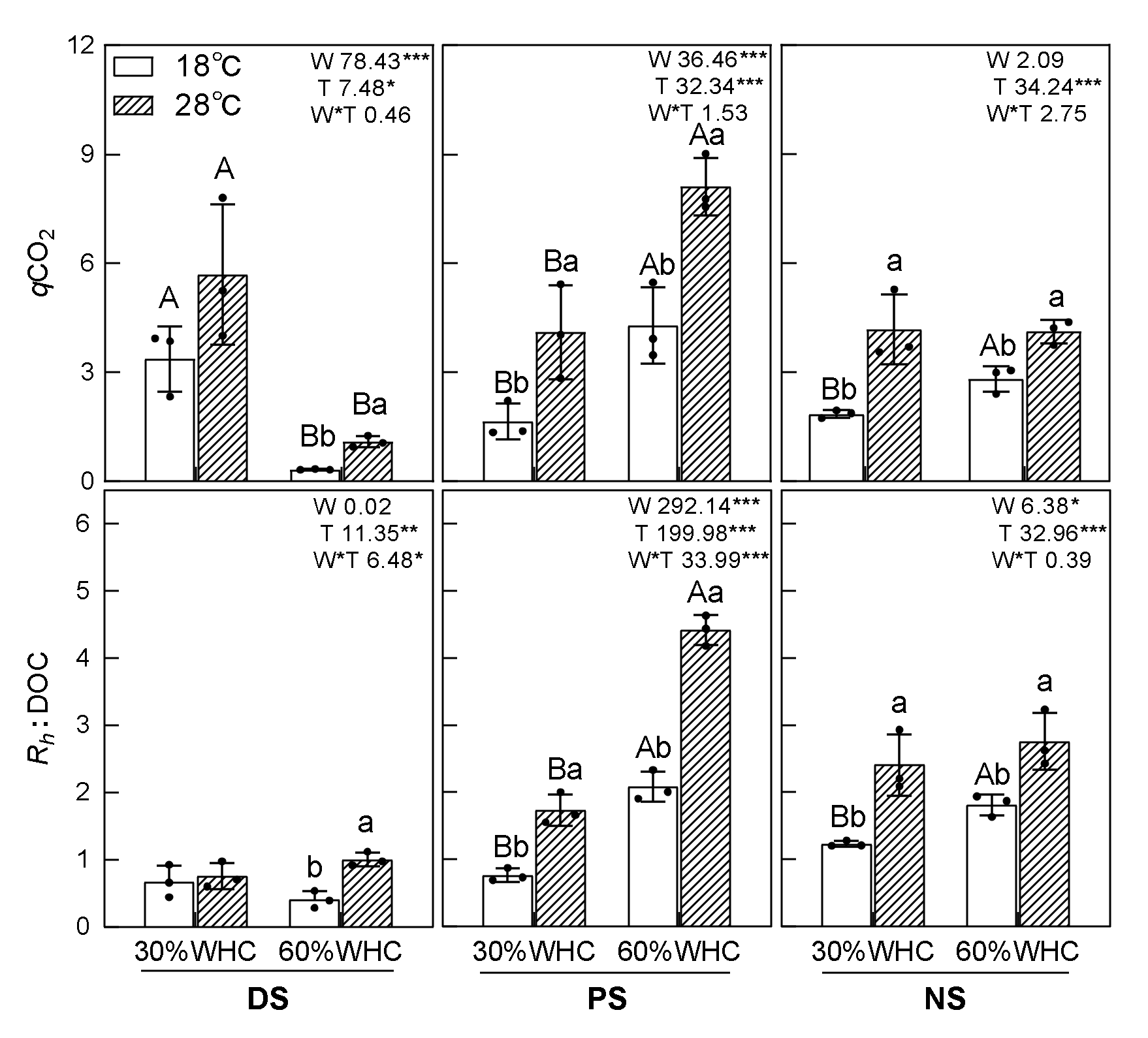

| Main Effect or Interactions | Dependent Variable | |||||||
|---|---|---|---|---|---|---|---|---|
| Cm | MBC | DOC | qCO2 | Rh: DOC | GP | GN | Fungi | |
| Vegetation restoration (R) | 425.98*** | 126.63*** | 1161.2*** | 31.55*** | 145.32*** | 36.64*** | 73.39*** | 59.91*** |
| Soil moisture (W) | 183.54*** | 46.78*** | 76.37*** | 1.59 | 103.39*** | 0.28 | 0.51 | 8.70** |
| Soil temperature (T) | 263.88*** | 1.48 | 2.54*** | 67.25*** | 160.67*** | 3.75 | 4.52* | 12.18*** |
| R×W | 98.92*** | 1.21 | 23.31*** | 57.65*** | 56.95*** | 0.54 | 0.12 | 3.44* |
| R×T | 40.55*** | 0.16 | 1.04 | 6.45** | 22.27*** | 1.71 | 0.57 | 1.73 |
| W×T | 12.37** | 0.23 | 1.07 | 0.01 | 11.68** | 1.64 | 1.80 | 0.23 |
| R×T×W | 12.23** | 0.53 | 0.35 | 2.25 | 8.20** | 0.23 | 0.16 | 0.60 |
| Parameters | Vegetation Restoration Stages | ||
|---|---|---|---|
| DS | PS | NS | |
| pH | 4.56 ± 0.05 a | 4.21 ± 0.15 b | 4.00 ± 0.12 b |
| SOC (g kg−1) | 3.53 ± 0.25 c | 16.03 ± 3.38 b | 34.13 ± 4.56 a |
| TN (g kg−1) | 1.23 ± 0.11 b | 1.78 ± 0.30 b | 2.94 ± 0.37 a |
| TP (g kg−1) | 0.04 ± 0.00 c | 0.08 ± 0.01 b | 0.13 ± 0.01 a |
| MBC (mg kg−1) | 206.6 ± 52.4 b | 931.9 ± 190.2 a | 963.9 ± 45.4 a |
| DOC (mg kg−1) | 154.9 ± 24.5 c | 483.9 ± 160.0 b | 709.3 ± 40.8 a |
| POC (g kg−1) | 1.76 ± 0.06 b | 10.42 ± 4.14 a | 15.44 ± 5.02 a |
| GP (%mol) | 29.11 ± 4.04 a | 19.6 ± 2.51 b | 22.66 ± 0.15 b |
| GN (%mol) | 7.27 ± 3.37 c | 18.44 ± 4.83 b | 25.91 ± 0.09 a |
| Fungi (%mol) | 13.89 ± 3.12 ab | 17.3 ± 0.75 a | 11.58 ± 0.15 b |
| GP:GN | 4.69 ± 2.22 a | 1.13 ± 0.38 b | 0.87 ± 0.00 b |
| F:B | 0.27 ± 0.05 a | 0.28 ± 0.01 a | 0.17 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Zhu, Y.-L.; Liu, J.-D.; Lin, X.-P.; Sun, H.-Z.; Tang, X.-H.; Hu, Y.-L.; Huang, Y.-P.; Yi, Z.-G. Effects of Moisture and Temperature on Soil Organic Carbon Decomposition along a Vegetation Restoration Gradient of Subtropical China. Forests 2022, 13, 578. https://doi.org/10.3390/f13040578
Fang X, Zhu Y-L, Liu J-D, Lin X-P, Sun H-Z, Tang X-H, Hu Y-L, Huang Y-P, Yi Z-G. Effects of Moisture and Temperature on Soil Organic Carbon Decomposition along a Vegetation Restoration Gradient of Subtropical China. Forests. 2022; 13(4):578. https://doi.org/10.3390/f13040578
Chicago/Turabian StyleFang, Xiong, Yu-Lin Zhu, Jun-Di Liu, Xue-Ping Lin, Hao-Zhao Sun, Xing-Hao Tang, Ya-Lin Hu, Yun-Peng Huang, and Zhi-Gang Yi. 2022. "Effects of Moisture and Temperature on Soil Organic Carbon Decomposition along a Vegetation Restoration Gradient of Subtropical China" Forests 13, no. 4: 578. https://doi.org/10.3390/f13040578





