The Shift from Energy to Water Limitation in Local Canopy Height from Temperate to Tropical Forests in China
Abstract
:1. Introduction
2. Materials and Methods
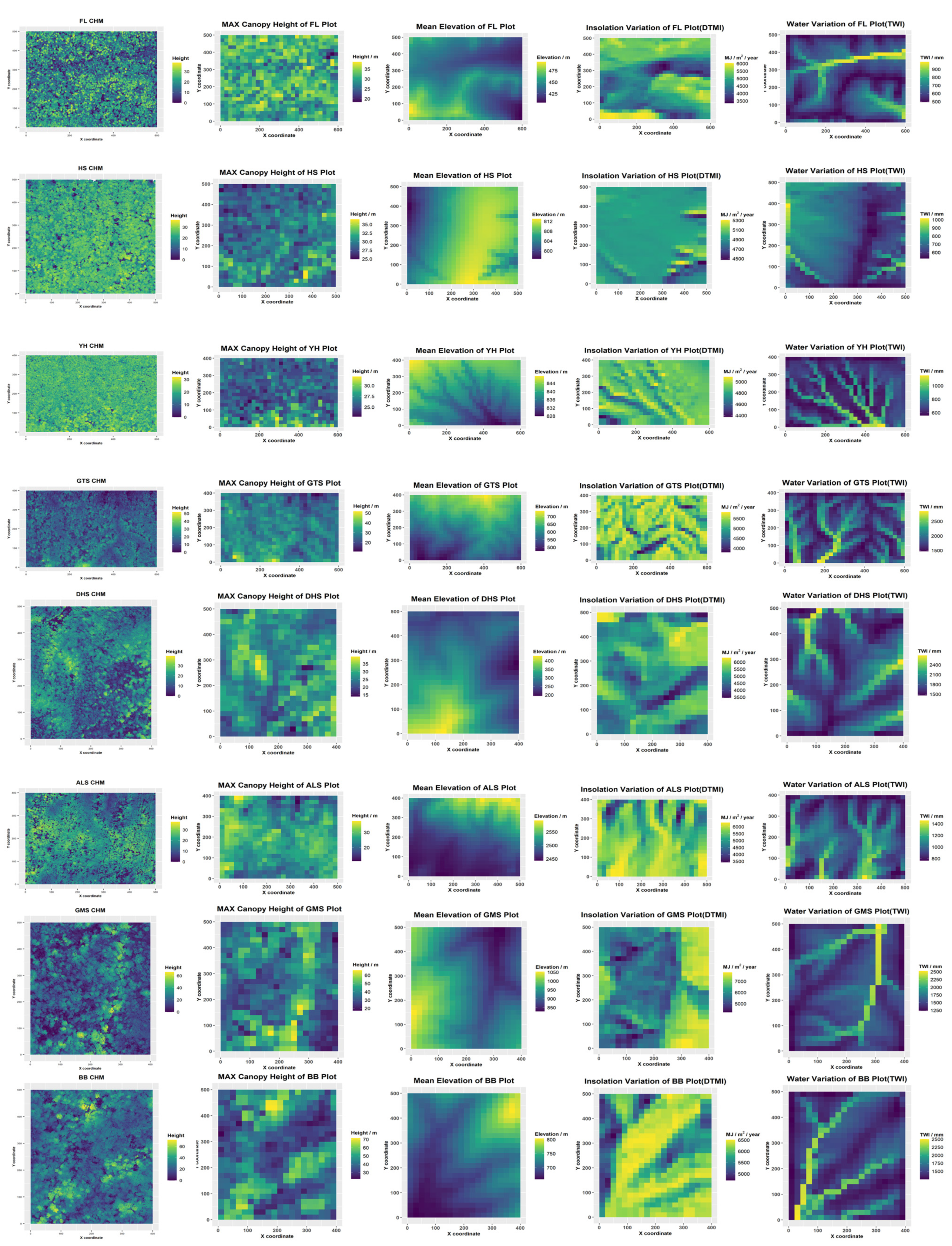
2.1. Study Sites and Forest Plots
2.2. Near-Surface LiDAR Data
2.3. Parameter Extraction
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Plot Name | Soil Type | Point Cloud Density |
|---|---|---|
| FL | mountain brown forest soil | 20 points/m2 |
| HS | mountain dark brown forest soil | 88 points/m2 |
| YH | mountain dark brown forest soil | 124 points/m2 |
| GTS | krasnozem | 20 points/m2 |
| ALS | mountain yellow brown soil | 57 points/m2 |
| DHS | latosolic red soil | 90 points/m2 |
| GMS | Laterite | 30 points/m2 |
| BB | Laterite | 22 points/m2 |
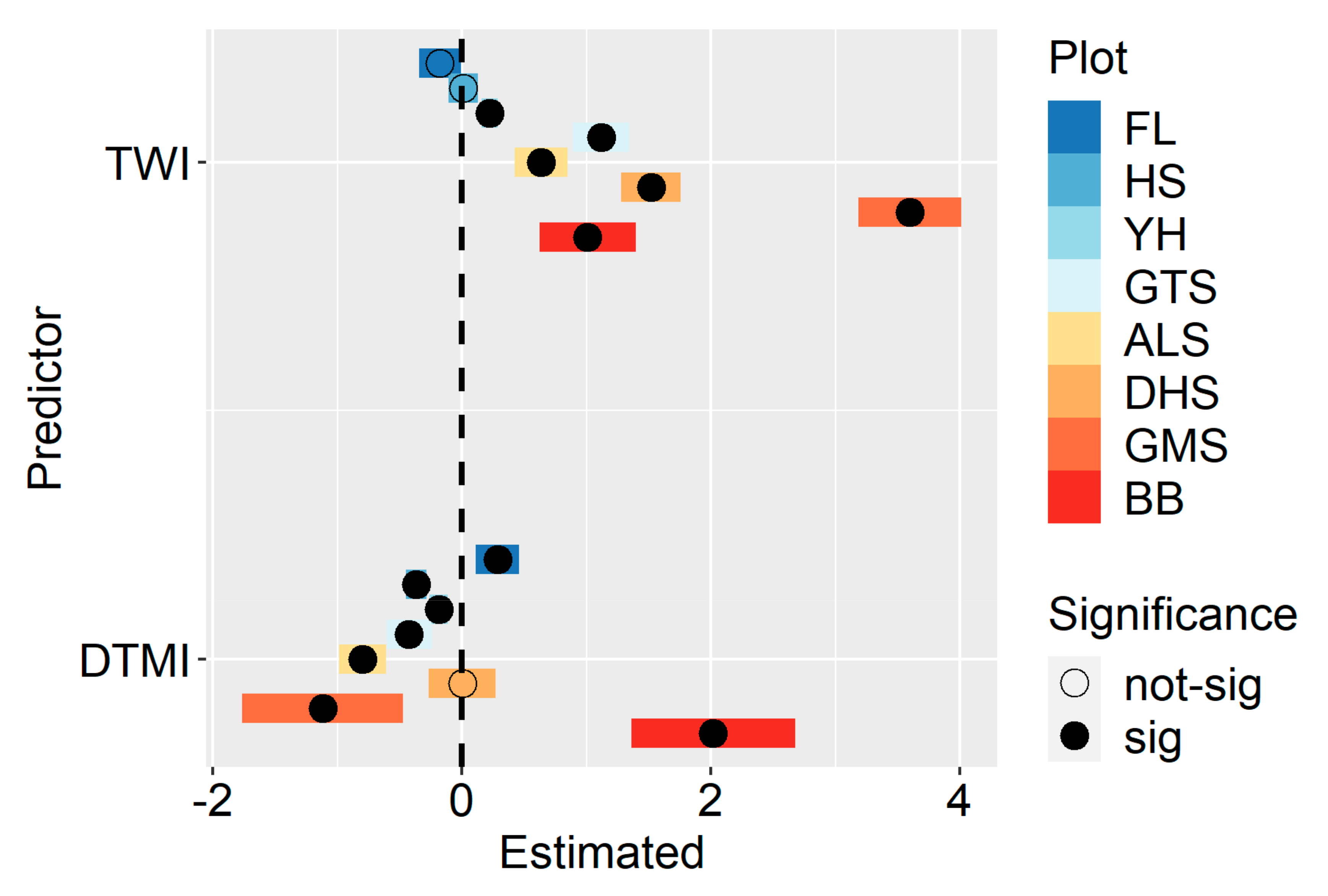
| Plot | Predictor | p Value | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| FL | TWI | 0.29 | −0.18 | 0.17 | −0.35 | −0.008 |
| FL | DEML | . | 0.29 | 0.17 | 0.11 | 0.46 |
| HS | TWI | 0.93 | 0.01 | 0.12 | −0.11 | 0.13 |
| HS | DEML | *** | −0.37 | 0.08 | −0.45 | −0.28 |
| YH | TWI | *** | 0.22 | 0.07 | 0.16 | 0.29 |
| YH | DEML | * | −0.19 | 0.07 | −0.26 | −0.11 |
| GTS | TWI | *** | 1.12 | 0.23 | 0.89 | 1.35 |
| GTS | DEML | * | −0.42 | 0.18 | −0.60 | −0.24 |
| ALS | TWI | ** | 0.64 | 0.21 | 0.43 | 0.85 |
| ALS | DEML | *** | −0.80 | 0.19 | −0.99 | −0.61 |
| DHS | TWI | *** | 1.52 | 0.24 | 1.28 | 1.76 |
| DHS | DEML | 0.99 | 0.001 | 0.27 | −0.26 | 0.27 |
| GMS | TWI | *** | 3.60 | 0.41 | 3.18 | 4.01 |
| GMS | DEML | . | −1.12 | 0.65 | −1.76 | −0.47 |
| BB | TWI | ** | 1.01 | 0.39 | 0.62 | 1.40 |
| BB | DEML | ** | 2.02 | 0.66 | 1.36 | 2.68 |
| Plot Name | TWI (%) | DTMI (%) |
|---|---|---|
| FL | 5.67 | 94.33 |
| HS | 2.21 | 97.79 |
| YH | 59.82 | 40.18 |
| GTS | 83.65 | 16.35 |
| ALS | 53.30 | 46.70 |
| DHS | 97.08 | 2.92 |
| GMS | 95.52 | 4.48 |
| BB | 84.18 | 15.82 |
References
- Ozanne, C.M.P.; Anhuf, D.; Boulter, S.L.; Keller, M.; Kitching, R.L.; Körner, C.; Meinzer, F.C.; Mitchell, A.W.; Nakashizuka, T.; Dias, P.L.S.; et al. Biodiversity Meets the Atmosphere: A Global View of Forest Canopies. Science 2003, 301, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotis, A.T.; Morin, T.H.; Fahey, R.T.; Hardiman, B.S.; Bohrer, G.; Curtis, P.S. Forest Structure in Space and Time: Biotic and Abiotic Determinants of Canopy Complexity and Their Effects on Net Primary Productivity. Agric. For. Meteorol. 2018, 250–251, 181–191. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Harding, D.J.; Keller, M.; Cohen, W.B.; Carabajal, C.C.; Del Bom Espirito-Santo, F.; Hunter, M.O.; de Oliveira, R., Jr. Estimates of Forest Canopy Height and Aboveground Biomass Using ICESat. Geophys. Res. Lett. 2005, 32, L22S02. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Fatoyinbo, L.; Smetanka, C.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Thomas, N.; Van der Stocken, T. Mangrove Canopy Height Globally Related to Precipitation, Temperature and Cyclone Frequency. Nat. Geosci. 2019, 12, 40–45. [Google Scholar] [CrossRef]
- Lefsky, M.A. A Global Forest Canopy Height Map from the Moderate Resolution Imaging Spectroradiometer and the Geoscience Laser Altimeter System. Geophys. Res. Lett. 2010, 37, L15401. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Pinto, N.; Fisher, J.B.; Baccini, A. Mapping Forest Canopy Height Globally with Spaceborne Lidar. J. Geophys. Res. Biogeosci. 2011, 116, G04021. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, U.; Schneider, H.; Wegner, L.H.; Haase, A. Water Ascent in Tall Trees: Does Evolution of Land Plants Rely on a Highly Metastable State? New Phytol. 2004, 162, 575–615. [Google Scholar] [CrossRef]
- Cramer, M. Unravelling the Limits to Tree Height: A Major Role for Water and Nutrient Trade-Offs. Oecologia 2011, 169, 61–72. [Google Scholar] [CrossRef]
- Canny, M.J. A New Theory for the Ascent of Sap—Cohesion Supported by Tissue Pressure. Ann. Bot. 1995, 75, 343–357. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G.; Barnard, H.R.; Fownes, J. Age-Related Decline in Forest Ecosystem Growth: An Individual-Tree, Stand-Structure Hypothesis. Ecosystems 2002, 5, 58–67. [Google Scholar] [CrossRef]
- Rust, S.; Roloff, A. Reduced Photosynthesis in Old Oak (Quercus Robur): The Impact of Crown and Hydraulic Architecture. Tree Physiol. 2002, 22, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N. Actual Evapotranspiration and Deficit: Biologically Meaningful Correlates of Vegetation Distribution across Spatial Scales. J. Biogeogr. 1998, 25, 855–870. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic Limits to Tree Height and Tree Growth. BioScience 1997, 47, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Larjavaara, M. The World’s Tallest Trees Grow in Thermally Similar Climates. New Phytol. 2014, 202, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nielsen, S.E.; Mao, L.; Chen, S.; Svenning, J.-C. Regional and Historical Factors Supplement Current Climate in Shaping Global Forest Canopy Height. J. Ecol. 2016, 104, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The Limits to Tree Height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature Drives Global Patterns in Forest Biomass Distribution in Leaves, Stems, and Roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [Green Version]
- Moles, A.T.; Warton, D.I.; Warman, L.; Swenson, N.G.; Laffan, S.W.; Zanne, A.E.; Pitman, A.; Hemmings, F.A.; Leishman, M.R. Global Patterns in Plant Height. J. Ecol. 2009, 97, 923–932. [Google Scholar] [CrossRef]
- Klein, T.; Randin, C.; Körner, C. Water Availability Predicts Forest Canopy Height at the Global Scale. Ecol. Lett. 2015, 18, 1311–1320. [Google Scholar] [CrossRef]
- Tao, S.; Guo, Q.; Li, C.; Wang, Z.; Fang, J. Global Patterns and Determinants of Forest Canopy Height. Ecology 2016, 97, 3265–3270. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Davies, S.J.; Chuyong, G.B.; Kenfack, D.; et al. Soil Resources and Topography Shape Local Tree Community Structure in Tropical Forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122532. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Bongalov, B.; Burslem, D.F.R.P.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography Shapes the Structure, Composition and Function of Tropical Forest Landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, C.; Lasky, J.R.; Uriarte, M.; Valencia, R.; Wright, S.J.; Garwood, N.C.; Kraft, N.J.B. Topography and Neighborhood Crowding Can Interact to Shape Species Growth and Distribution in a Diverse Amazonian Forest. Ecology 2018, 99, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Mi, X.; Yan, H.; Li, F.Y.; Svenning, J.-C.; Ma, K. CForBio: A Network Monitoring Chinese Forest Biodiversity. Sci. Bull. 2016, 61, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Fricker, G.A.; Synes, N.W.; Serra-Diaz, J.M.; North, M.P.; Davis, F.W.; Franklin, J. More than Climate? Predictors of Tree Canopy Height Vary with Scale in Complex Terrain, Sierra Nevada, CA (USA). For. Ecol. Manag. 2019, 434, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, R.; Zinko, U.; Seibert, J. On the Calculation of the Topographic Wetness Index: Evaluation of Different Methods Based on Field Observations. Hydrol. Earth Syst. Sci. Discuss. 2006, 10, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Grabs, T.; Seibert, J.; Bishop, K.; Laudon, H. Modeling Spatial Patterns of Saturated Areas: A Comparison of the Topographic Wetness Index and a Dynamic Distributed Model. J. Hydrol. 2009, 373, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Skidmore, A.K.; Knowles, E. Modelling Topographic Variation in Solar Radiation in a GIS Environment. Int. J. Geogr. Inf. Sci. 1997, 11, 475–497. [Google Scholar] [CrossRef]
- Fu, P.; Rich, P.M. A Geometric Solar Radiation Model with Applications in Agriculture and Forestry. Comput. Electron. Agric. 2002, 37, 25–35. [Google Scholar] [CrossRef]
- Kissling, W.D.; Carl, G. Spatial Autocorrelation and the Selection of Simultaneous Autoregressive Models. Glob. Ecol Biogeogr. 2007, 17, 59–71. [Google Scholar] [CrossRef]
- Belmaker, J.; Jetz, W. Cross-Scale Variation in Species Richness-Environment Associations: Richness-Environment Scaling. Glob. Ecol. Biogeogr. 2011, 20, 464–474. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef] [Green Version]
- Givnish, T.J.; Wong, S.C.; Stuart-Williams, H.; Holloway-Phillips, M.; Farquhar, G.D. Determinants of Maximum Tree Height in Eucalyptus Species along a Rainfall Gradient in Victoria, Australia. Ecology 2014, 95, 2991–3007. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Cowan, T. Dynamics of Late Autumn Rainfall Reduction over Southeastern Australia. Geophys. Res. Lett. 2008, 35, L09708. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.-I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of Increasing Drought Severity Caused by Temperature Rise in Southern Europe. Environ. Res. Lett. 2014, 9, 044001. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought Sensitivity Shapes Species Distribution Patterns in Tropical Forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Markesteijn, L. Seedling Traits Determine Drought Tolerance of Tropical Tree Species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Liu, H.; Gleason, S.M.; Hao, G.; Hua, L.; He, P.; Goldstein, G.; Ye, Q. Hydraulic Traits Are Coordinated with Maximum Plant Height at the Global Scale. Sci. Adv. 2019, 5, eaav1332. [Google Scholar] [CrossRef] [Green Version]
- Comita, L.S.; Condit, R.; Hubbell, S.P. Developmental Changes in Habitat Associations of Tropical Trees. J. Ecol. 2007, 95, 482–492. [Google Scholar] [CrossRef]
- Webb, C.O.; Peart, D.R. Habitat Associations of Trees and Seedlings in a Bornean Rain Forest. J. Ecol. 2000, 88, 464–478. [Google Scholar] [CrossRef]
- Allié, E.; Pélissier, R.; Engel, J.; Petronelli, P.; Freycon, V.; Deblauwe, V.; Soucémarianadin, L.; Weigel, J.; Baraloto, C. Pervasive Local-Scale Tree-Soil Habitat Association in a Tropical Forest Community. PLoS ONE 2015, 10, e0141488. [Google Scholar] [CrossRef] [PubMed]
- Ameztegui, A.; Rodrigues, M.; Gelabert, P.J.; Lavaquiol, B.; Coll, L. Maximum Height of Mountain Forests Abruptly Decreases above an Elevation Breakpoint. GIScience Remote Sens. 2021, 58, 442–454. [Google Scholar] [CrossRef]


| Plot | Forest Region | Vegetation Type | Size | Location | Elevation | MAP | MAS |
|---|---|---|---|---|---|---|---|
| FL | Temperate | Primary broad-leaved Korean pine forest | 30 ha (600 × 500 m) | 48.133° N 129.200° E | 419 (66) | 629 | 4879.7 |
| HS | Temperate | Primary broad-leaved Korean pine forest | 25 ha (500 × 500 m) | 42.383° N 128.089° E | 801 (18) | 680 | 4929.6 |
| YH | Temperate | Secondary poplar-birch forest | 24 ha (600 × 400 m) | 42.372° N 128.005° E | 832 (22) | 701 | 4865.8 |
| GTS | Subtropical | Evergreen broad-leaved forest | 24 ha (600 × 400 m) | 27.25° N 118.12° E | 618 (277) | 1844 | 5301.6 |
| ALS | Subtropical | Mid-montane moist evergreen broad-leaved forest | 20 ha (500 × 400 m) | 24.53° N 101.03° E | 2509 (157) | 985 | 5515.1 |
| DHS | Subtropical | Evergreen broad-leaved forest | 20 ha (400 × 500 m) | 23.167° N 112.591° E | 350 (240) | 1818 | 5022.9 |
| GMS | Tropical | Tropical mountain rainforest | 20 ha (500 × 400 m) | 22.246° N 100.599° E | 949 (237) | 1574 | 6097.2 |
| BB | Tropical | Tropical seasonal rainforest | 20 ha (400 × 500 m) | 21.613° N 101.580° E | 735.5 (153) | 1698 | 5967.9 |
| Plot | Mean Hmax | Hmax Range | Number of Quadrats |
|---|---|---|---|
| FL | 30.87 | 18.44–38.57 | 750 (613) |
| HS | 29.56 | 24.96–36.49 | 625 (509) |
| YH | 26.14 | 22.81–32.11 | 600 (476) |
| GTS | 28.54 | 11.05–51.69 | 600 (483) |
| ALS | 25.65 | 11.15–37.52 | 500 (394) |
| DHS | 26.73 | 13.97–39.74 | 500 (400) |
| GMS | 37.70 | 17.27–66.03 | 500 (399) |
| BB | 41.9 | 22.24–71.51 | 500 (393) |
| LMM Type | R2 | AIC | TWI | DTMI |
|---|---|---|---|---|
| Random intercept *** | 0.591 | 22,128.89 | 6.19 *** | −0.06 |
| Random slope model *** | 0.611 | 21,968.44 | 3.72 | −0.22 |
| Random intercept and slope *** | 0.633 | 21,787.15 | 4.21 * | −0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Fang, S.; Wang, Y.; Guo, Q.; Hu, T.; Mi, X.; Lin, L.; Jin, G.; Coomes, D.A.; Yuan, Z.; et al. The Shift from Energy to Water Limitation in Local Canopy Height from Temperate to Tropical Forests in China. Forests 2022, 13, 639. https://doi.org/10.3390/f13050639
Wang B, Fang S, Wang Y, Guo Q, Hu T, Mi X, Lin L, Jin G, Coomes DA, Yuan Z, et al. The Shift from Energy to Water Limitation in Local Canopy Height from Temperate to Tropical Forests in China. Forests. 2022; 13(5):639. https://doi.org/10.3390/f13050639
Chicago/Turabian StyleWang, Bojian, Shuai Fang, Yunyun Wang, Qinghua Guo, Tianyu Hu, Xiangcheng Mi, Luxiang Lin, Guangze Jin, David Anthony Coomes, Zuoqiang Yuan, and et al. 2022. "The Shift from Energy to Water Limitation in Local Canopy Height from Temperate to Tropical Forests in China" Forests 13, no. 5: 639. https://doi.org/10.3390/f13050639
APA StyleWang, B., Fang, S., Wang, Y., Guo, Q., Hu, T., Mi, X., Lin, L., Jin, G., Coomes, D. A., Yuan, Z., Ye, J., Wang, X., Lin, F., & Hao, Z. (2022). The Shift from Energy to Water Limitation in Local Canopy Height from Temperate to Tropical Forests in China. Forests, 13(5), 639. https://doi.org/10.3390/f13050639







