Long-Term Maternal Fertilizer Addition Increased Seed Size but Decreased Germination Capacity and Offspring Performance in Taxus baccata L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Seed Size and Morphology
2.3. Metabolite Profile
2.4. Germination Rate and the Ratio of Viable Seeds
2.5. Seedling Growth
2.6. Statistical Analysis
3. Results
3.1. Seed Size and Morphology
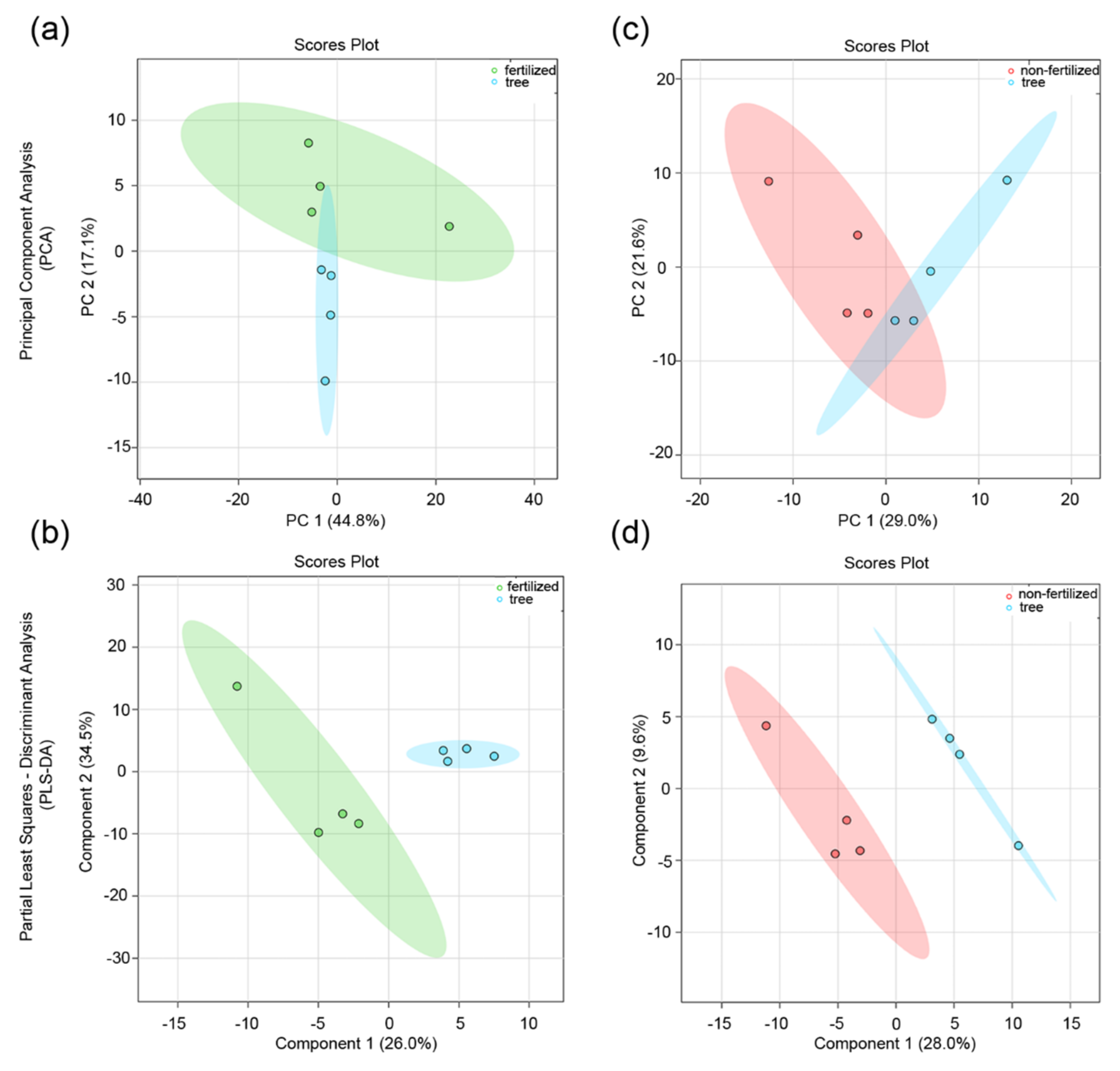
3.2. Effect of the Maternal Environment on the Metabolite Profile of Seeds
3.3. Germination Capacity
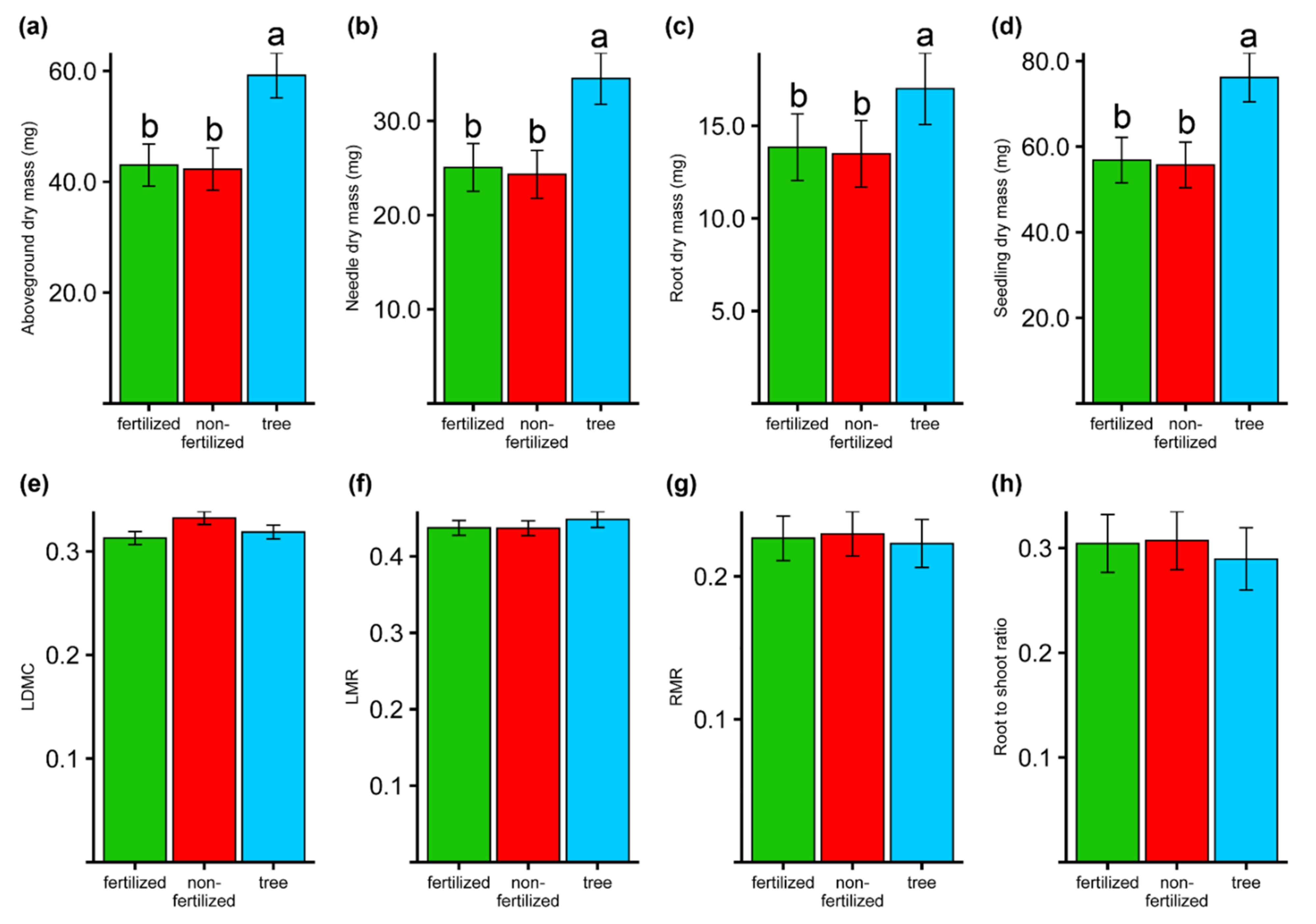
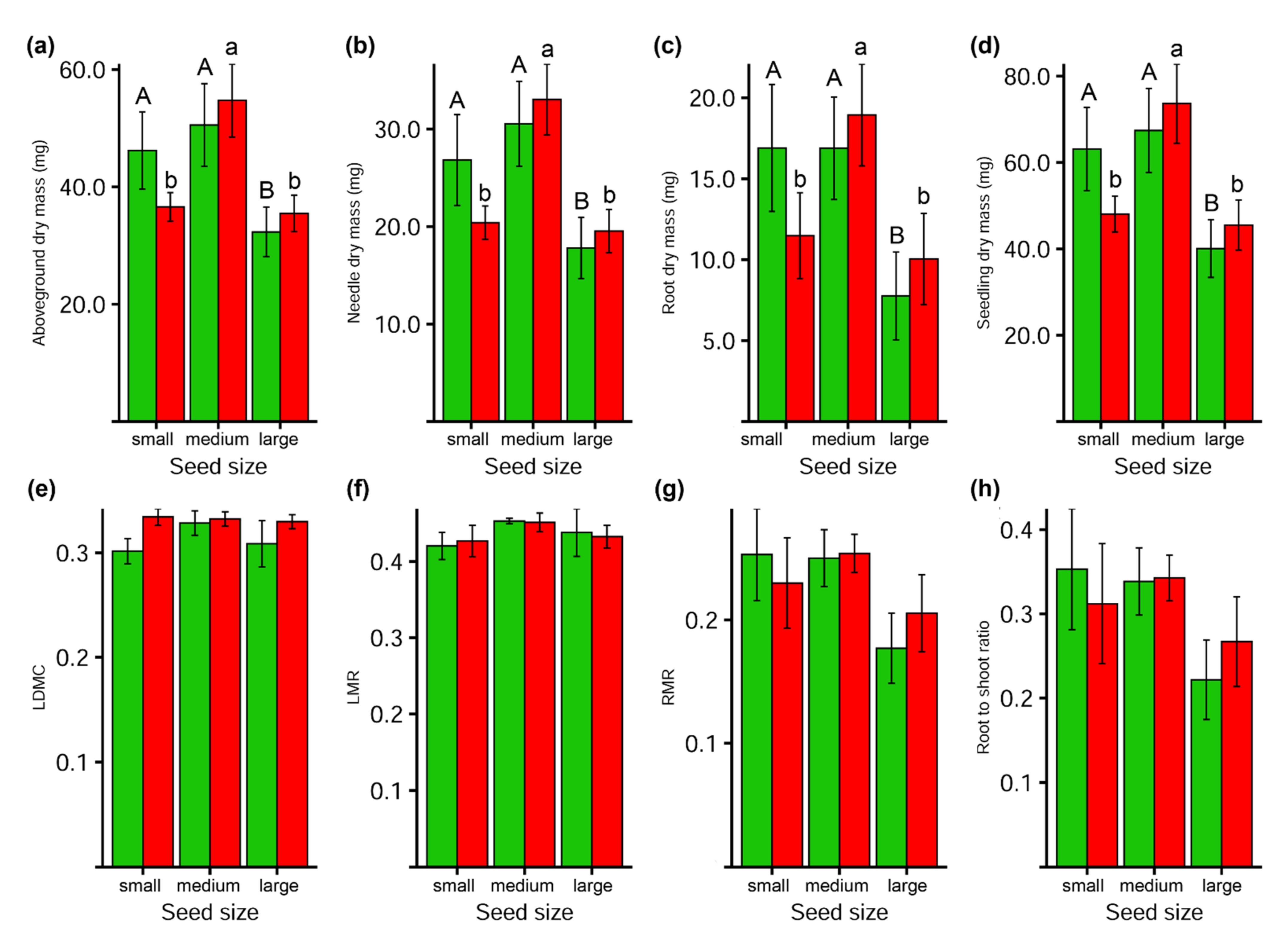
3.4. Seedlings Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Bogdziewicz, M.; Crone, E.E.; Steele, M.A.; Zwolak, R. Effects of nitrogen deposition on reproduction in a masting tree: Benefits of higher seed production are trumped by negative biotic interactions. J. Ecol. 2017, 105, 310–320. [Google Scholar] [CrossRef]
- Pers-Kamczyc, E.; Iszkuło, G.; Rabska, M.; Wrońska-Pilarek, D.; Kamczyc, J. More isn’t always better—The effect of environmental nutritional richness on male reproduction of Taxus baccata L. Environ. Exp. Bot. 2019, 162, 468–478. [Google Scholar] [CrossRef]
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Jeyasingh, P.D.; Neiman, M.; Richardson, A.E.; Soltis, P.S.; et al. Impacts of nitrogen and phosphorus: From genomes to natural ecosystems and agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.; Thomas, V.F.D.; Quiring, R.; Flückiger, W. Does nitrogen deposition increase forest production. The role of phosphorus. Environ. Pollut. 2010, 158, 2043–2052. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Maaroufi, N.I.; De Long, J.R. Global change impacts on forest soils: Linkage between soil biota and carbon-nitrogen-phosphorus stoichiometry. Front. For. Glob. Chang. 2020, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Mao, W.; Felton, A.J.; Zhang, T. Linking changes to intraspecific trait diversity to community functional diversity and biomass in response to snow and nitrogen addition within an inner Mongolian grassland. Front. Plant Sci. 2017, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Etzold, S.; Ferretti, M.; Reinds, G.J.; Solberg, S.; Gessler, A.; Waldner, P.; Schaub, M.; Simpson, D.; Benham, S.; Hansen, K.; et al. Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests. For. Ecol. Manag. 2020, 458, 117762. [Google Scholar] [CrossRef]
- Soons, M.B.; Hefting, M.M.; Dorland, E.; Lamers, L.P.M.; Versteeg, C.; Bobbink, R. Nitrogen effects on plant species richness in herbaceous communities are more widespread and stronger than those of phosphorus. Biol. Conserv. 2017, 212, 390–397. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [Green Version]
- Bobbink, R. Plant Species Richness and the Exceedance of Empirical Nitrogen Critical Loads: An Inventory; Utrecht University, Faculty of Biology: Utrecht, The Netherlands, 2004. [Google Scholar]
- Velthof, G.; Barot, S.; Bloem, J.; Butterbach-Bahl, K.; de Vries, W.; Kros, J.; Lavelle, P.; Olesen, J.E.; Oenema, O. Nitrogen as a threat to European soil quality. In The European Nitrogen Assessment. Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, UK, 2011; pp. 495–510. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U. Effects on beech forest species of experimentally enhanced nitrogen deposition. Flora 1993, 188, 85–91. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Chiariello, N.R.; Coley, P.D.; Pitelka, L.F. Resources to allocating defense and new assessments of the costs and benefits of allocation patterns in plants are relating ecological roles to resource use. Bioscience 1987, 37, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.A.; Polwart, A. Taxus baccata. J. Ecol. 2003, 91, 489–524. [Google Scholar] [CrossRef]
- Nowak, K.; Giertych, M.J.; Pers-Kamczyc, E.; Thomas, P.A.; Iszkuło, G. Rich but not poor conditions determine sex-specific differences in growth rate of juvenile dioecious plants. J. Plant Res. 2021, 134, 947–962. [Google Scholar] [CrossRef]
- Robakowski, P.; Pers-Kamczyc, E.; Ratajczak, E.; Thomas, P.A.P.A.; Ye, Z.-P.P.; Rabska, M.; Iszkuło, G. Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Front. Plant Sci. 2018, 9, 742. [Google Scholar] [CrossRef]
- Sanz, R.; Pulido, F. Pollen limitation and fruit abortion in a declining rare tree, the Eurasian yew (Taxus baccata L.): A reproductive cost of ecological marginality. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2015, 149, 818–826. [Google Scholar] [CrossRef]
- Iszkuło, G.; Pers-Kamczyc, E.; Nalepka, D.; Rabska, M.; Walas, Ł.; Dering, M. Postglacial migration dynamics helps to explain current scattered distribution of Taxus baccata. Dendrobiology 2016, 76, 81–89. [Google Scholar] [CrossRef]
- Vencurik, J.; Bosela, M.; Sedmáková, D.; Pittner, J.; Kucbel, S.; Jaloviar, P.; Parobeková, Z.; Saniga, M. Tree species diversity facilitates conservation efforts of European yew. Biodivers. Conserv. 2019, 28, 791–810. [Google Scholar] [CrossRef]
- Iszkuło, G. Success and failure of endangered tree species: Low temperatures and low light availability affect survival and growth of european yew (Taxus baccata L.) seedlings. Polish J. Ecol. 2010, 58, 259–271. [Google Scholar]
- Pers-Kamczyc, E.; Tyrała-Wierucka, Ż.; Rabska, M.; Wrońska-Pilarek, D.; Kamczyc, J. The higher availability of nutrients increases the production but decreases the quality of pollen grains in Juniperus communis L. J. Plant Physiol. 2020, 248, 153156. [Google Scholar] [CrossRef] [PubMed]
- Gruwez, R.; De Frenne, P.; De Schrijver, A.; Leroux, O.; Vangansbeke, P.; Verheyen, K. Negative effects of temperature and atmospheric depositions on the seed viability of common juniper (Juniperus communis). Ann. Bot. 2014, 113, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Pers-Kamczyc, E.; Mąderek, E.; Kamczyc, J. Seed quantity or quality?—Reproductive responses of female of two dioecious woody species to long term-fertilisation. Int. J. Mol. Sci. 2022, 23, 3187. [Google Scholar] [CrossRef]
- Poorter, L.; Rose, S.A. Light-dependent changes in the relationship between seed mass and seedling traits: A meta-analysis for rain forest tree species. Oecologia 2005, 142, 378–387. [Google Scholar] [CrossRef]
- Domic, A.I.; Capriles, J.M.; Camilo, G.R. Evaluating the fitness effects of seed size and maternal tree size on Polylepis tomentella (Rosaceae) seed germination and seedling performance. J. Trop. Ecol. 2020, 36, 115–122. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R.; Poorter, L. Seed-mass effects in four Mediterranean Quercus species (Fagaceae) growing in contrasting light environments. Am. J. Bot. 2007, 94, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Larios, E.; Venable, D.L. Selection for seed size: The unexpected effects of water availability and density. Funct. Ecol. 2018, 32, 2216–2224. [Google Scholar] [CrossRef]
- Souza, M.L.; Fagundes, M. Seed size as key factor in germination and seedling development of Copaifera langsdorffii (Fabaceae). Am. J. Plant Sci. 2014, 5, 2566–2573. [Google Scholar] [CrossRef] [Green Version]
- Tumpa, K.; Vidaković, A.; Drvodelić, D.; Šango, M.; Idžojtić, M.; Perković, I.; Poljak, I. The effect of seed size on germination and seedling growth in sweet chestnut (Castanea sativa mill.). Forests 2021, 12, 858. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.O.; Cross, A.T.T.; et al. A research agenda for seed-trait functional ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, E.; Roberts, J.A.; Wagstaff, C. Manipulating resource allocation in plants. J. Exp. Bot. 2012, 63, 3391–3400. [Google Scholar] [CrossRef] [Green Version]
- Soper Gorden, N.L.; Winkler, K.J.; Jahnke, M.R.; Marshall, E.; Horky, J.; Huddelson, C.; Etterson, J.R. Geographic patterns of seed mass are associated with climate factors, but relationships vary between species. Am. J. Bot. 2016, 103, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, Y. Signaling pathways of seed size control in plants. Curr. Opin. Plant Biol. 2016, 33, 23–32. [Google Scholar] [CrossRef]
- Wu, H.; Meng, H.; Wang, S.; Wei, X.; Jiang, M. Geographic patterns and environmental drivers of seed traits of a relict tree species. For. Ecol. Manage. 2018, 422, 59–68. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seed size and plant strategy across the whole life cycle. Oikos 2006, 113, 91–105. [Google Scholar] [CrossRef]
- Gómez, J.M. Bigger is not always better: Conflicting selective pressures on seed size in Quercus ilex. Evolution 2004, 58, 71–80. [Google Scholar] [CrossRef]
- Kołodziejek, J. Effect of seed position and soil nutrients on seed mass, germination and seedling growth in Peucedanum oreoselinum (Apiaceae). Sci. Rep. 2017, 7, 1959. [Google Scholar] [CrossRef]
- Michaels, H.J.; Benner, B.; Hartgerink, A.P.; Lee, T.D.; Rice, S.; Willson, M.F.; Bertin, R.I. Seed size variation: Magnitude, distribution, and ecological correlates. Evol. Ecol. 1988, 2, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Westoby, M.; Jurado, E.; Leishman, M. Comparative evolutionary ecology of seed size. Trends Ecol. Evol. 1992, 7, 368–372. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Milberg, P.; Lamont, B.B. Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol. 1997, 137, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Hidawi Publ. Corp. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef] [Green Version]
- Vayda, K.; Donohue, K.; Auge, G.A. Within- and trans-generational plasticity: Seed germination responses to light quantity and quality. AoB Plants 2018, 10, ply023. [Google Scholar] [CrossRef]
- Auge, G.A.; Leverett, L.D.; Edwards, B.R.; Donohue, K. Adjusting phenotypes via within- and across-generational plasticity. New Phytol. 2017, 216, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Suszka, B. Conditions for after-ripening and germination of seeds and for seedling emergence of English yew (Taxus baccata L.). Arbor. Kórnickie 1985, 30, 285–338. [Google Scholar]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [Green Version]
- Green, P.T.; Juniper, P.A. Seed–seedling allometry in tropical rain forest trees: Seed mass-related patterns of resource allocation and the ‘reserve effect’. J. Ecol. 2004, 92, 397–408. [Google Scholar] [CrossRef]
- Nowak, K.; Giertych, M.J.; Pers-Kamczyc, E.; Thomas, P.A.; Iszkuło, G. Defence is a priority in female juveniles and adults of Taxus baccata L. Forests 2021, 12, 844. [Google Scholar] [CrossRef]
- McGinley, M.A.; Charnov, E.L. Multiple resources and the optimal balance between size and number of offspring. Evol. Ecol. 1988, 2, 77–84. [Google Scholar] [CrossRef]
- Khera, N.; Saxena, A.K.; Singh, R.P. Seed size variability and its influence on germination and seedling growth of five multipurpose tree species. Seed Sci. Technol. 2004, 32, 319–330. [Google Scholar] [CrossRef]
- Murali, K.S. Patterns of seed size, germination and seed viability of tropical tree species in Southern India. Biotropica 1997, 29, 271–279. [Google Scholar] [CrossRef]
- Benard, R.B.; Toft, C.A. Effect of seed size on seedling performance in a long-lived desert perennial shrub (Ericameria nauseosa: Asteraceae). Int. J. Plant Sci. 2015, 168, 1027–1033. [Google Scholar] [CrossRef]
- Gruwez, R.; De Frenne, P.; De Schrijver, A.; Vangansbeke, P.; Verheyen, K. Climate warming and atmospheric deposition affect seed viability of common juniper (Juniperus communis) via their impact on the nutrient status of the plant. Ecol. Res. 2017, 32, 135–144. [Google Scholar] [CrossRef]
- Macera, L.G.; Pereira, S.R.; de Souza, A.L.T. Survival and growth of tree seedlings as a function of seed size in a gallery forest under restoration. Acta Bot. Bras. 2017, 31, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The evolutionary ecology of seed size. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 31–57. [Google Scholar] [CrossRef] [Green Version]
- Leishman, M.R. Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 2001, 93, 294–302. [Google Scholar] [CrossRef]
- Mašková, T.; Herben, T. Root:shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef] [Green Version]
- Niemczyk, M.; Chmura, D.J.; Socha, J.; Wojda, T.; Mroczek, P.; Gil, W.; Thomas, B.R. How geographic and climatic factors affect the adaptation of Douglas-fir provenances to the temperate continental climate zone in Europe. Eur. J. For. Res. 2021, 140, 1341–1361. [Google Scholar] [CrossRef]
- Osuna, D.; Prieto, P.; Aguilar, M. Control of seed germination and plant development by carbon and nitrogen availability. Front. Plant Sci. 2015, 6, 1023. [Google Scholar] [CrossRef]
- De Frenne, P.; Kolb, A.; Graae, B.J.; Decocq, G.; Baltora, S.; De Schrijver, A.; Brunet, J.; Chabrerie, O.; Cousins, S.A.O.; Dhondt, R.; et al. A latitudinal gradient in seed nutrients of the forest herb Anemone nemorosa. Plant Biol. 2011, 13, 493–501. [Google Scholar] [CrossRef]
- Abeli, T.; Brancaleoni, L.; Marchesini, R.; Orsenigo, S.; Rossi, G.; Gerdol, R. Fertiliser application positively affects plants performance but reduces seed viability in seashore mallow (Kosteletzkya pentacarpos): Implication for biomass production and species conservation. Ann. Appl. Biol. 2017, 170, 263–272. [Google Scholar] [CrossRef]
- Gao, S.; Wang, J.; Knops, J.M.H.; Wang, J. Nitrogen addition increases sexual reproduction and improves seedling growth in the perennial rhizomatous grass Leymus chinensis. BMC Plant Biol. 2020, 20, 106. [Google Scholar] [CrossRef]
- He, J.S.; Flynn, D.F.B.; Wolfe-Bellin, K.; Fang, J.; Bazzaz, F.A. CO2 and nitrogen, but not population density, alter the size and C/N ratio of Phytolacca americana seeds. Funct. Ecol. 2005, 19, 437–444. [Google Scholar] [CrossRef]
- De Frenne, P.; Blondeel, H.; Brunet, J.; Carón, M.M.; Chabrerie, O.; Cougnon, M.; Cousins, S.A.O.; Decocq, G.; Diekmann, M.; Graae, B.J.; et al. Atmospheric nitrogen deposition on petals enhances seed quality of the forest herb Anemone nemorosa. Plant Biol. 2018, 20, 619–626. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy1[OPEN]. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, K.; Adriaenssens, S.; Gruwez, R.; Michalczyk, I.M.; Ward, L.K.; Rosseel, Y.; Van den Broeck, A.; García, D. Juniperus communis: Victim of the combined action of climate warming and nitrogen deposition? Plant Biol. 2009, 11, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Miao, Y.; Han, S.; Wang, D. Nitrogen addition decreases seed germination in a temperate steppe. Ecol. Evol. 2019, 9, 8441–8449. [Google Scholar] [CrossRef]




| Maternal Treatment | Seed Group | No. of Seed | Seed Mass (mg) | Project Area (mm2) | Curved Length (mm) | Curved Width (mm) | SMA Ratio 1 |
|---|---|---|---|---|---|---|---|
| F | small | 121 | 44.35 ± 0.36 d | 20.07 ± 0.12 e | 6.16 ± 0.03 c | 4.30 ± 0.02 d | 2.24 ± 0.01 e |
| medium | 326 | 53.09 ± 0.22 c | 21.68 ± 0.06 c | 6.44 ± 0.02 b | 4.40 ± 0.01 c | 2.45 ± 0.01 c | |
| large | 193 | 66.11 ± 0.28 a | 24.83 ± 0.12 a | 6.79 ± 0.02 a | 4.73 ± 0.01 a | 2.66 ± 0.01 a | |
| NF | small | 200 | 41.45 ± 0.28 e | 17.63 ± 0.13 f | 5.72 ± 0.02 d | 4.14 ± 0.01 e | 2.35 ± 0.01 d |
| medium | 309 | 53.39 ± 0.22 c | 21.33 ± 0.07 d | 6.44 ± 0.02 b | 4.38 ± 0.01 c | 2.51 ± 0.01 b | |
| large | 123 | 63.62 ± 0.36 b | 24.09 ± 0.13 b | 6.80 ± 0.03 a | 4.63 ± 0.02 b | 2.64 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pers-Kamczyc, E.; Suszka, J. Long-Term Maternal Fertilizer Addition Increased Seed Size but Decreased Germination Capacity and Offspring Performance in Taxus baccata L. Forests 2022, 13, 670. https://doi.org/10.3390/f13050670
Pers-Kamczyc E, Suszka J. Long-Term Maternal Fertilizer Addition Increased Seed Size but Decreased Germination Capacity and Offspring Performance in Taxus baccata L. Forests. 2022; 13(5):670. https://doi.org/10.3390/f13050670
Chicago/Turabian StylePers-Kamczyc, Emilia, and Jan Suszka. 2022. "Long-Term Maternal Fertilizer Addition Increased Seed Size but Decreased Germination Capacity and Offspring Performance in Taxus baccata L." Forests 13, no. 5: 670. https://doi.org/10.3390/f13050670
APA StylePers-Kamczyc, E., & Suszka, J. (2022). Long-Term Maternal Fertilizer Addition Increased Seed Size but Decreased Germination Capacity and Offspring Performance in Taxus baccata L. Forests, 13(5), 670. https://doi.org/10.3390/f13050670






