Beta Diversity Patterns Unlock the Community Assembly of Woody Plant Communities in the Riparian Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.2.1. Field Survey
2.2.2. Functional Traits Measurement
2.2.3. Environmental Variables Measurement
2.3. Statistical Analyses
2.3.1. Spatial Variables
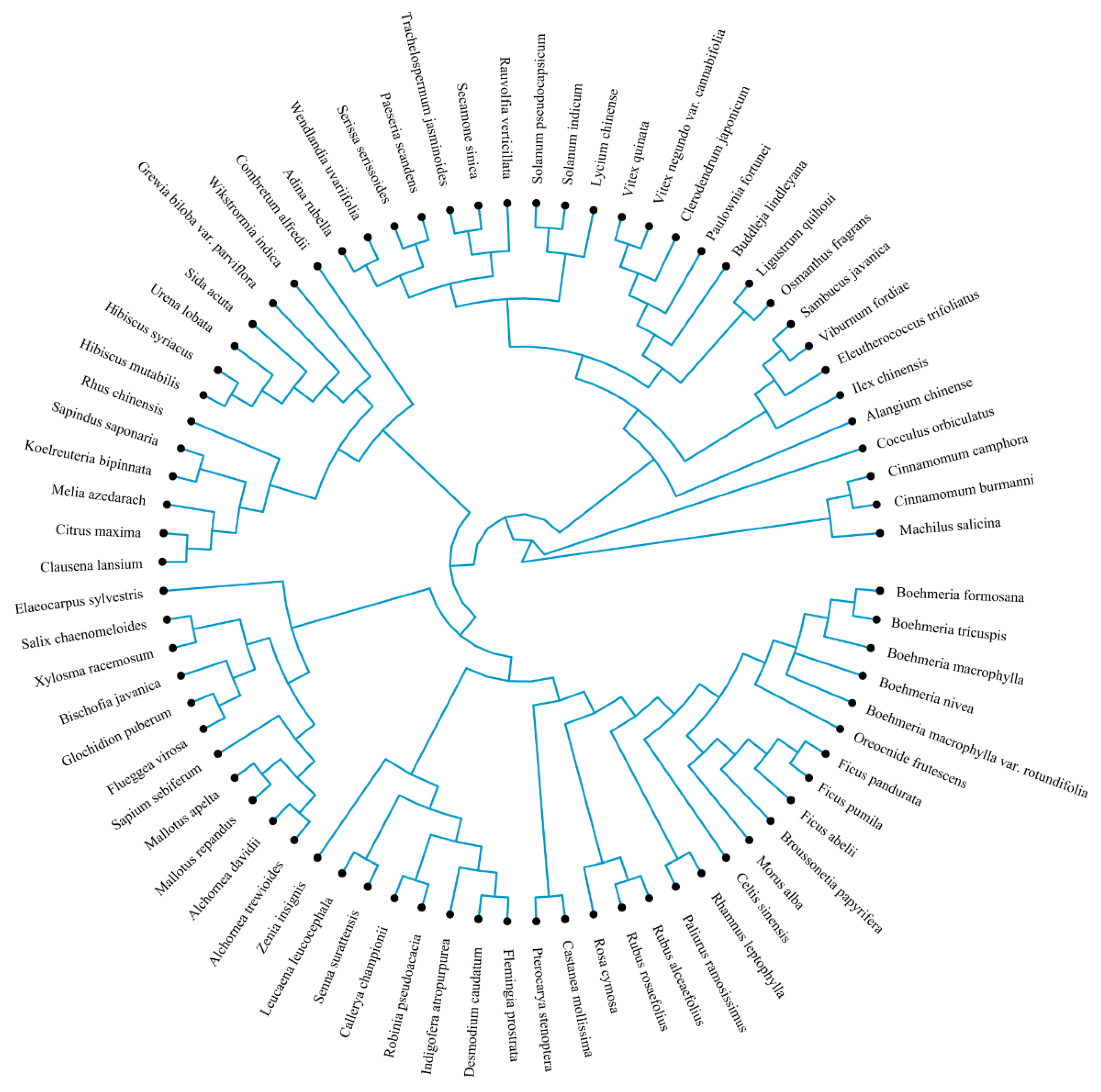
2.3.2. Phylogenetic Tree Construction
2.3.3. Beta Diversity Calculation
3. Results
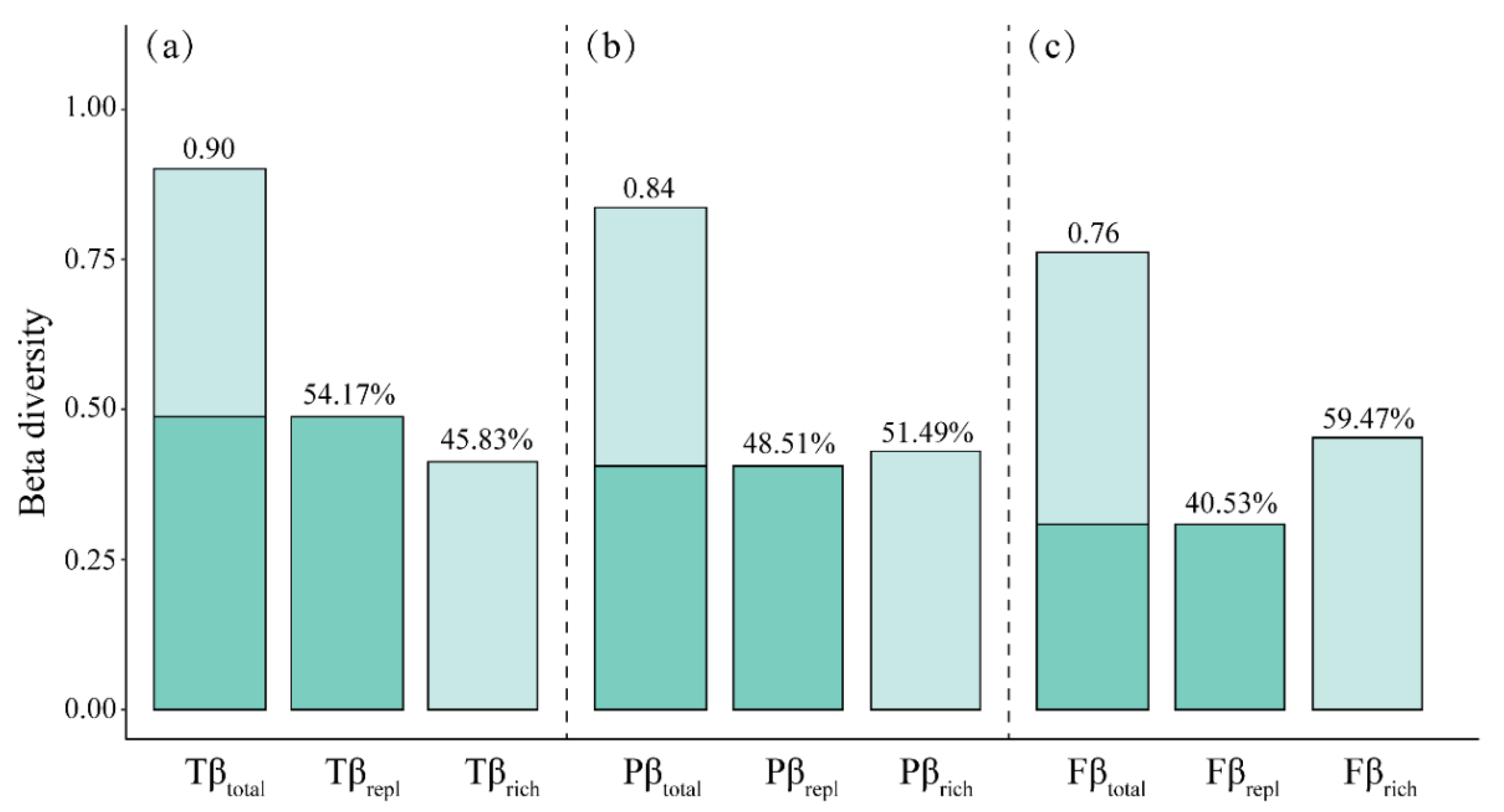
3.1. Patterns of Taxonomic, Functional and Phylogenetic Beta Diversity with Their Two Components
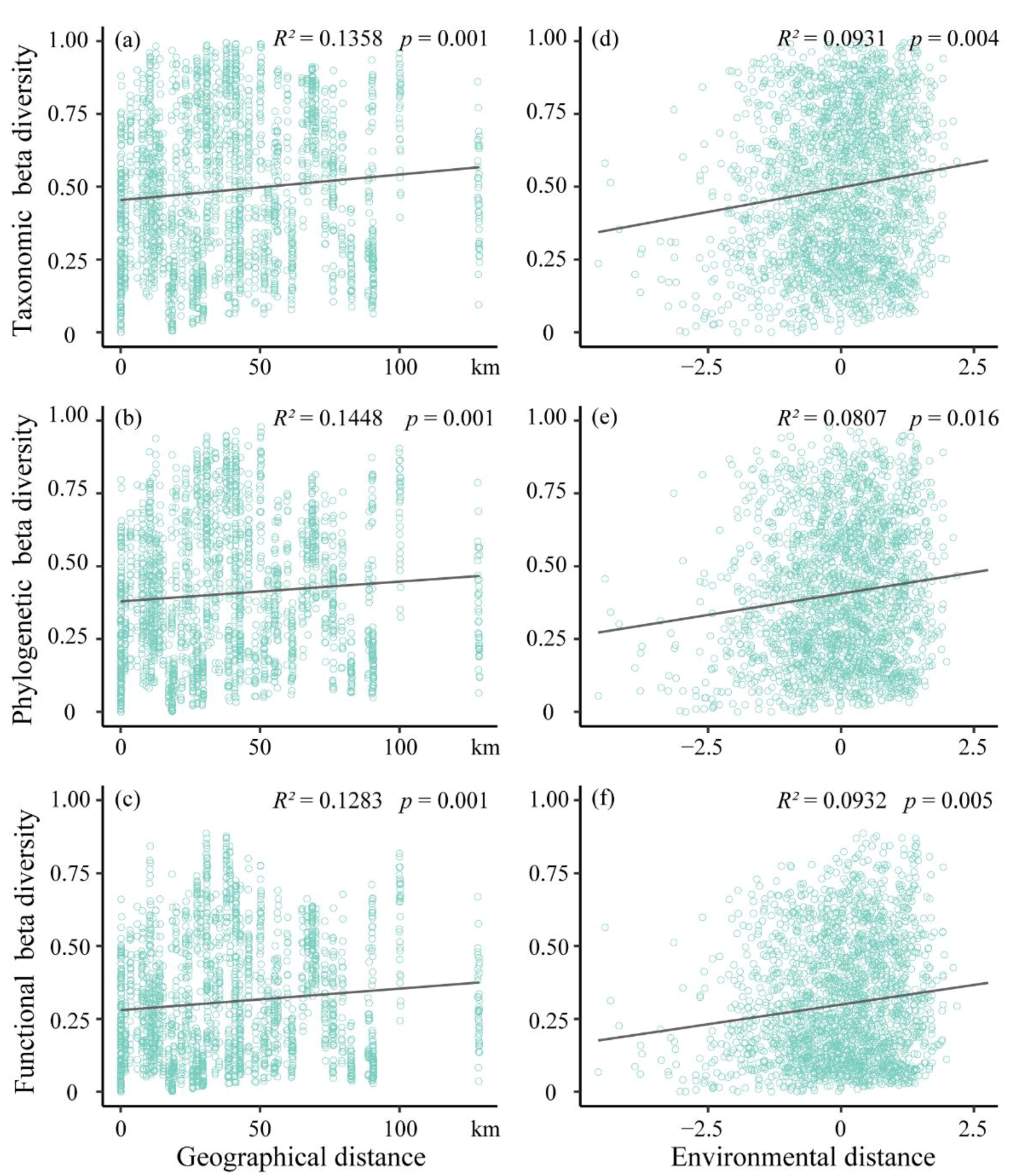
3.2. The Influence of Environmental Filtering and Dispersal Limitation on Beta Diversity Patterns
3.3. The Influence of Environmental Factors on Beta Diversity
4. Discussion
4.1. The Composition of Taxonomic, Functional and Phylogenetic Beta Diversity with Their Two Components
4.2. The Relative Importance of Dispersal Limitation and Environmental Filtering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Site | Plot | Longitude | Latitude | Community Type |
|---|---|---|---|---|
| Caiyuan | 1–7 | 110°27′41.00″ E | 25°33′41.37″ N | ASS. P. stenoptera-F. abelii |
| Zhuzhi | 8–12 | 110°24′20.68″ E | 25°28′47.21″ N | ASS. C. sinensis-B. formosana + F. abelii |
| Yangjia | 13–17 | 110°22′30.08″ E | 25°24′48.87″ N | ASS. P. stenoptera-F. abelii |
| Nanchang | 18–22 | 110°22′33.14″ E | 25°24′46.64″ N | ASS. P. stenoptera-A. chinense |
| Nanzhou | 23–26 | 110°19′49.48″ E | 25°20′23.83″ N | ASS. C. sinensis-F. abelii |
| Dahe | 27–32 | 110°19′22.99″ E | 25°19′31.87″ N | ASS. C. sinensis-F. abelii |
| Biyan | 33–37 | 110°25′09.45″ E | 25°06′30.65″ N | ASS. C. burmannii-R. verticillate |
| Duchuan | 38–41 | 110°25′29.78″ E | 25°05′47.63″ N | ASS. R. verticillate |
| Longmen | 42–46 | 110°20′58.79″ E | 25°12′12.54″ N | ASS. T. sebiferum-A. rubella |
| Yueguang | 47–52 | 110°27′17.94″ E | 25°00′12.81″ N | ASS. C. sinensis-V. negundo var. cannabifolia |
| Luoshi | 53–57 | 110°30′05.50″ E | 24°54′22.97″ N | ASS. T. sebiferum-B. formosana |
| Sanhe | 58–65 | 110°34′49.15″ E | 24°44′58.25″ N | ASS. T. sebiferum-B. formosana |
Appendix B
Phylogenetic Tree Construction
References
- Münkemüller, T.; De, B.F.; Meynard, C.N.; Gravel, D.; Lavergne, S.; Mouillot, D.; Mouquet, N.; Thuiller, W. From diversity indices to community assembly processes: A test with simulated data. Ecography 2012, 35, 468–480. [Google Scholar] [CrossRef]
- Jiang, L.M.; Lv, G.H.; Gong, Y.M.; Li, Y.; Wang, H.F.; Wu, D.Y. Characteristics and driving mechanisms of species beta diversity in desert plant communities. PLoS ONE 2021, 16, e0245249. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, Z.J.J.; Kunin, W.E.; Edwards, D.P. How Should Beta-diversity Inform Biodiversity Conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Jiang, L.M.; Hou, Z.F.; Zhang, J.; Wang, H.F.; Lv, G.H. Environmental filtration and dispersal limitation explain different aspects of beta diversity in desert plant communities. Glob. Ecol. Conserv. 2022, 33, e01956. [Google Scholar] [CrossRef]
- García-Giróna, J.; Fernández-Aláeza, C.; Fernández-Aláeza, M.; Lahuhta, J.A. Untangling the assembly of macrophyte metacommunities by means of taxonomic, functional and phylogenetic beta diversity patterns. Sci. Total Environ. 2019, 693, 133616. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Angeler, D.G. Revealing a conservation challenge through partitioned long-term beta diversity: Increasing turnover and decreasing nestedness of boreal lake metacommunities. Divers. Distrib. 2013, 19, 772–781. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Gutiérrez-Cánovas, C.; Millán, A.; Velasco, J.; Vaughan, I.P.; Ormerod, S.J. Contrasting Effects of Natural and Anthropogenic Stressors on Beta Diversity in River Organisms. Glob. Ecol. Biogeogr. 2013, 22, 796–805. [Google Scholar] [CrossRef]
- Leprieur, F.; Tedesco, P.A.; Hugueny, B.; Beauchard, O.; Dürr, H.H.; Brosse, S.; Oberdorff, T. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecol. Lett. 2011, 14, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, R.S.; Bastazini, V.A.G.; Velez-Martin, E.; Debastiani, V.; Zanini, K.J.; Loyola, R.; Muller, S.C. Linking beta diversity patterns to protected areas: Lessons from the Brazilian Atlantic Rainforest. Biodivers. Conserv. 2017, 26, 1557–1568. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Jeppesen, E.; Ge, D.; Li, W.; Zou, D.; Huang, Z.; Wu, A.; Liu, Q. Local and regional drivers of turnover and nestedness components of species and functional beta diversity in lake macrophyte communities in China. Sci. Total Environ. 2019, 687, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Giron, J.; Heino, J.; Baastrup-Spohr, L.; Bove, C.P.; Clayton, J.; de Winton, M.; Feldmann, T.; Fernandez-Alaez, M.; Ecke, F.; Grillas, P.; et al. Global patterns and determinants of lake macrophyte taxonomic, functional and phylogenetic beta diversity. Sci. Total Environ. 2020, 723, 138021. [Google Scholar] [CrossRef]
- Li, F.S.; Yan, Y.Z.; Zhang, J.N.; Zhang, Q.; Niu, J.M. Taxonomic, functional, and phylogenetic beta diversity in the Inner Mongolia grassland. Glob. Ecol. Conserv. 2021, 28, e01634. [Google Scholar] [CrossRef]
- Cardoso, P.; Rigal, F.; Carvalho, J.C.; Fortelius, M.; Borges, P.A.V.; Podani, J.; Schmera, D. Partitioning taxon, phylogenetic and functional beta diversity into replacement and richness difference components. J. Biogeogr. 2014, 41, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Siefert, A.; Ravenscroft, C.; Weiser, M.D.; Swenson, N.G. Functional beta-diversity patterns reveal deterministic community assembly processes in eastern North American trees. Glob. Ecol. Biogeogr. 2012, 22, 682–691. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Liu, X.J.; Cai, X.F.; Xing, L.X. Quantitative Remote Sensing Analysis of the Geomorphological Development of the Lijiang River Basin, Southern China. J. Indian Soc. Remote Sens. 2019, 47, 737–747. [Google Scholar] [CrossRef]
- Zhang, Q.; Buyantuev, A.; Fang, X.N.; Han, P.; Li, A.; Li, F.Y.; Liang, C.Z.; Liu, Q.F.; Ma, Q.; Niu, J.M.; et al. Ecology and sustainability of the Inner Mongolian Grassland: Looking back and moving forward. Landsc. Ecol. 2020, 35, 2413–2432. [Google Scholar] [CrossRef]
- Liu, G.; Jin, Q.W.; Li, J.Y.; Li, L.; He, C.X.; Huang, Y.Q.; Yao, Y.F. Policy factors impact analysis based on remote sensing data and the CLUE-S model in the Lijiang River Basin, China. CATENA 2017, 158, 286–297. [Google Scholar] [CrossRef]
- Huang, W.; Chak, H.; Peng, Y.; Li, L. Qualitative risk assessment of soil erosion for karst landforms in Chahe town, Southwest China: A hazard index approach. CATENA 2016, 144, 184–193. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Wright, I.; Reich, P.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Kang, M.; Chang, S.C.; Yan, E.R.; Wang, X.H. Trait variability differs between leaf and wood tissues across ecological scales in subtropical forests. J. Veg. Sci. 2014, 25, 703–714. [Google Scholar] [CrossRef]
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, E.; Renaud, A.; Richardson, A.D.; Roggy, J.C.; Schimann, H.; Uddling, J.; Hérault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: China, Beijing, 2000. (In Chinese) [Google Scholar]
- Hijmans, R.J. Geosphere: Spherical Trigonometry. R Package Version 1.5-14. 2021. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 12 October 2021).
- Jin, Y.; Qian, H.V. PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef] [Green Version]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Talbot, J.M.; Bruns, T.D.; Taylor, J.W.; Smith, D.P.; Branco, S.; Glassman, S.E.S.; Vilgalys, R.; Liao, H.L.; Smith, M.E.; Peay, K.G. Endemism and functional convergence across the North American soil mycobiome. Proc. Natl. Acad. Sci. USA 2014, 111, 6341–6346. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, X.; Yao, J.; Wang, Z.W.; Deng, Y.; Cheng, W.X.; Zhou, J.Z.; Han, X.G. Habitat-specific patterns and drivers of bacterial β-diversity in China’s drylands. ISME J. 2017, 11, 1345–1358. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 October 2021).
- Cardoso, P.; Mammola, S.; Rigal, F.; Carvalho, J. BAT: Biodiversity Assessment Tools. R Package Version 2.7.0. 2021. Available online: https://CRAN.R-project.org/package=BAT (accessed on 12 October 2021).
- Goslee, S.C.; Urban, D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 October 2021).
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Gianuca, A.T.; Engelen, J.; Brans, K.I.; Hanashiro, F.T.T.; Vanhamel, M.; van den Berg, E.M.; Caroline, S.; De Meester, L. Taxonomic, functional and phylogenetic metacommunity ecology of cladoceran zooplankton along urbanization gradients. Ecography 2018, 41, 183–194. [Google Scholar] [CrossRef] [Green Version]
- De Paula, L.F.A.; Colmenares-Trejos, S.L.; Negreiros, D.; Rosado, B.H.P.; De Mattos, E.A.; De Bello, F.; Porembski, S.; Silveira, F.A.O. High plant taxonomic beta diversity and functional and phylogenetic convergence between two Neotropical inselbergs. Plant Ecol. Divers. 2020, 13, 61–73. [Google Scholar] [CrossRef]
- González-Trujillo, J.D.; Saito, V.S.; Petsch, D.K.; Muñoz, I.; Sabater, S. Historical legacies and contemporary processes shape beta diversity in Neotropical montane streams. J. Biogeogr. 2021, 48, 101–177. [Google Scholar] [CrossRef]
- Soininen, J.; Heino, J.; Wang, J. A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob. Ecol. Biogeogr. 2018, 27, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Viana, D.S.; Figuerola, J.; Schwenk, K.; Manca, M.; Hobaek, A.; Mjelde, M.; Preston, C.D.; Gornall, R.J.; Croft, J.M.; King, R.A.; et al. Assembly mechanisms determining high species turnover in aquatic communities over regional and continental scales. Ecography 2016, 39, 281–288. [Google Scholar] [CrossRef]
- Wang, X.; Wiegand, T.; Anderson-Teixeira, K.J.; Bourg, N.A.; Hao, Z.; Howe, R.; Jin, G.; Orwig, D.A.; Spasojevic, M.J.; Wang, S.; et al. Ecological drivers of spatial community dissimilarity, species replacement and species nestedness across temperate forests. Glob. Ecol. Biogeogr. 2018, 27, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Batista, B.C.; De Lima, I.P.; Lima, M.R. Beta diversity patterns of bats in the Atlantic Forest: How does the scale of analysis affect the importance of spatial and environmental factors? J. Biogeogr. 2020, 48, 1–10. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Li, Y.S.; Wu, L.J.; Yu, S.; Xin, C.L.; Sun, P.G.; Xiao, Q.; Zhao, H.J.; Zhang, Y.; Qin, T. Impact of the atmospheric deposition of major acid rain components, especially NH4, on carbonate weathering during recharge in typical karst areas of the Lijiang River basin, southwest China. Appl. Geochem. 2020, 114, 104518. [Google Scholar] [CrossRef]
- Zhang, M.M.; Qin, H.; Wang, Y.; Zhang, F. Beta diversity of wetland vegetation in the middle and upper reaches of the Fenhe River watershed. Acta Ecol. Sin. 2016, 36, 3292–3299. [Google Scholar] [CrossRef]
- Dobrovolski, R.R.; Melo, A.S.; Cassemiro, F.A.S.; Diniz-Filho, J.A.F. Climatic history and dispersal ability explain the relative importance of turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2012, 21, 191–197. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.Q.; Xiang, W.S.; Li, X.K.; Cao, K.F. Environmental filtering and dispersal limitation jointly shaped the taxonomic and phylogenetic beta diversity of natural forests in southern China. Ecol. Evol. 2021, 11, 8783–8794. [Google Scholar] [CrossRef]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness, and growth form of naturalized alien plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Kong, J.J.; Yang, J.; Bai, E. Long-term effects of wildfire on available soil nutrient composition and stoichiometry in a Chinese boreal forest. Sci. Total Environ. 2018, 642, 1353–1361. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Foster, R.B. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Dong, L.; Li, Z.; Zhang, J.; Li, Z.; Miao, B.; Jia, C.; Liang, C.; Wang, L.; Li, F.Y. Phylogenetic structure and formation mechanism of shrub communities in arid and semiarid areas of the Mongolian Plateau. Ecol. Evol. 2019, 9, 13320–13331. [Google Scholar] [CrossRef]
- Sobral, F.L.; Lees, A.C.; Cianciaruso, M.V. Introductions do not compensate for functional and phylogenetic losses following extinctions in insular bird assemblages. Ecol. Lett. 2016, 19, 1091–1100. [Google Scholar] [CrossRef]
- Jiang, X.M.; Pan, B.Z.; Jiang, W.X.; Hou, Y.M.; Yang, H.Q.; Zhu, P.H.; Heino, J. The role of environmental conditions, climatic factors and spatial processes in driving multiple facets of stream macroinvertebrate beta diversity in a climatically heterogeneous mountain region. Ecol. Indic. 2021, 124, 107407. [Google Scholar] [CrossRef]
- Fournier, B.; Mouly, A.; Moretti, M.; Gillet, F. Contrasting processes drive alpha and beta taxonomic, functional and phylogenetic diversity of orthopteran communities in grasslands. Agric. Ecosyst. Environ. 2017, 242, 43–52. [Google Scholar] [CrossRef]

| Explanatory Variables | Taxonomic Beta Diversity | Phylogenetic Beta Diversity | Functional Beta Diversity | |||
|---|---|---|---|---|---|---|
| R2 | Pr (>F) | R2 | Pr (>F) | R2 | Pr (>F) | |
| Soil pH | 0.075 | *** | 0.063 | *** | 0.070 | *** |
| Soil water content | 0.001 | ns | 0.001 | ns | 0.001 | ns |
| Soil organic matter | 0.028 | * | 0.027 | * | 0.015 | ns |
| Total nitrogen | 0.057 | *** | 0.057 | *** | 0.047 | ** |
| Available nitrogen | 0.040 | ** | 0.030 | * | 0.064 | *** |
| Total phosphorus | 0.082 | *** | 0.082 | *** | 0.074 | *** |
| Available phosphorus | 0.056 | *** | 0.062 | ** | 0.083 | *** |
| Total potassium | 0.091 | *** | 0.134 | *** | 0.120 | *** |
| Available potassium | 0.073 | *** | 0.091 | *** | 0.120 | *** |
| Residuals | 0.497 | - | 0.453 | - | 0.406 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liang, S.; Liu, R.; Jiang, Y. Beta Diversity Patterns Unlock the Community Assembly of Woody Plant Communities in the Riparian Zone. Forests 2022, 13, 673. https://doi.org/10.3390/f13050673
He Y, Liang S, Liu R, Jiang Y. Beta Diversity Patterns Unlock the Community Assembly of Woody Plant Communities in the Riparian Zone. Forests. 2022; 13(5):673. https://doi.org/10.3390/f13050673
Chicago/Turabian StyleHe, Yan, Shichu Liang, Runhong Liu, and Yong Jiang. 2022. "Beta Diversity Patterns Unlock the Community Assembly of Woody Plant Communities in the Riparian Zone" Forests 13, no. 5: 673. https://doi.org/10.3390/f13050673
APA StyleHe, Y., Liang, S., Liu, R., & Jiang, Y. (2022). Beta Diversity Patterns Unlock the Community Assembly of Woody Plant Communities in the Riparian Zone. Forests, 13(5), 673. https://doi.org/10.3390/f13050673






