Subfossil Scots Pine (Pinus sylvestris L.) Wood from Northern Finland—Physical, Mechanical, and Chemical Properties and Suitability for Specialty Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subfossil Material
2.2. Physical and Mechanical Analyses
2.3. Chemical Analyses
2.3.1. Specimen Preparation
2.3.2. Structural Compounds
2.3.3. Extractives
2.4. SWOT Analysis
2.5. Statistical Analyses and Comparison to the Recently Grown Scots Pines
3. Results
3.1. Moisture Content, Basic Density, and Ash Content
3.2. Modulus of Elasticity, Modulus of Rupture, and Brinell Hardness
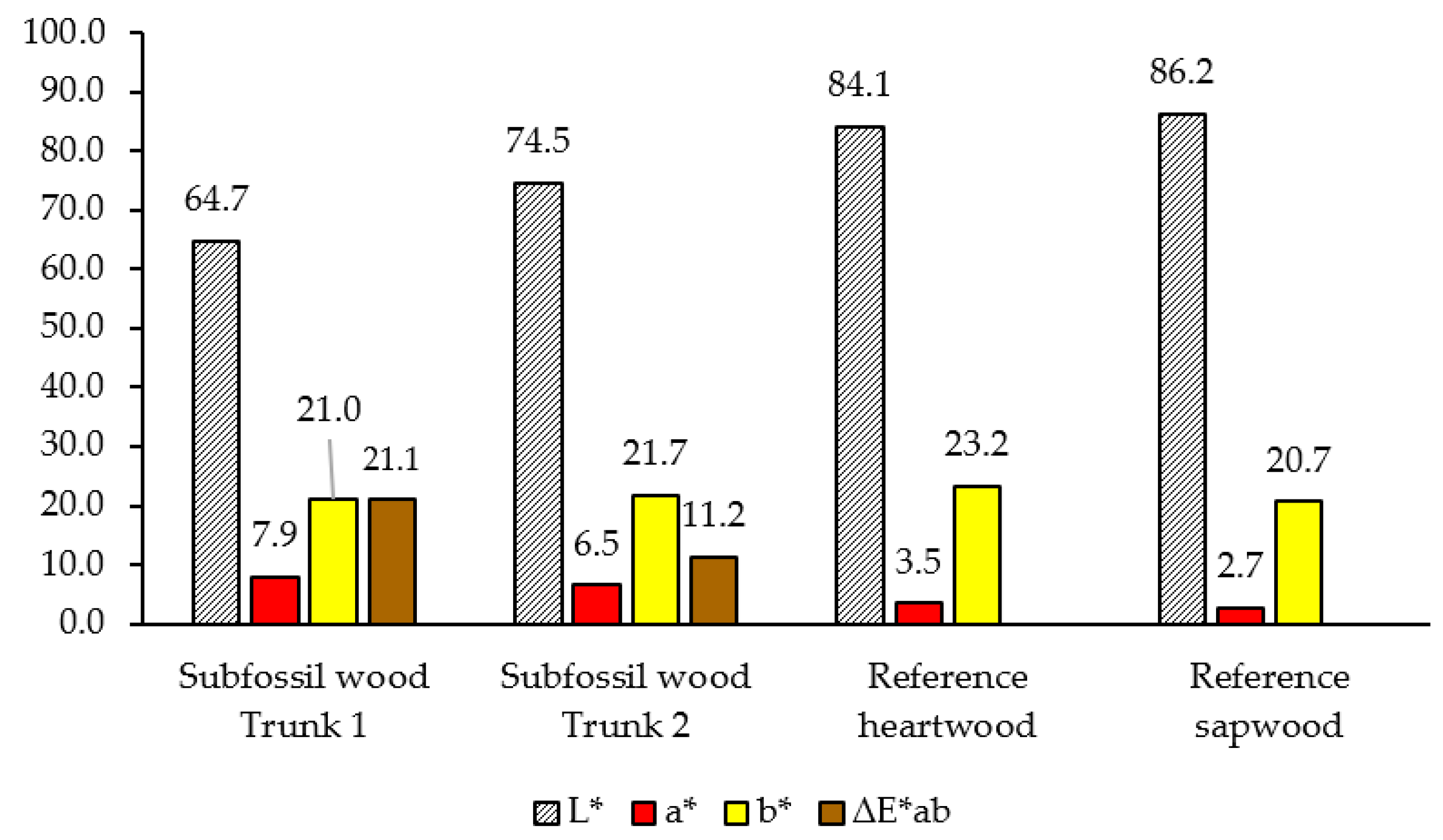
3.3. Colour
3.4. Chemical Properties
3.4.1. Structural Compounds
3.4.2. Extractives
3.5. SWOT Analysis
4. Discussion
4.1. Physical and Mechanical Properties
4.2. Chemical Properties
4.3. SWOT Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Older Stem Wood | Younger Stem Wood | Older Knot Wood | Younger Knot Wood | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | |
| Carboxylyc acids | ||||||||||||||||
| Succinic acid | 0.3 | 0.0 | 0.7 | 0.3 | 0.1 | 0.0 | 0.2 | 0.1 | 0.1 | 0.0 | 0.6 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fatty acids | ||||||||||||||||
| Acid 14:0 | 91.9 | 4.8 | 432.1 | 190.2 | 500.0 | 7.5 | 1330.1 | 617.3 | 43.2 | 4.0 | 131.7 | 51.1 | 91.8 | 5.3 | 136.1 | 50.2 |
| Acid 15:0 | 202.7 | 10.2 | 425.2 | 159.0 | 14.7 | 3.7 | 29.4 | 13.2 | 222.3 | 8.4 | 662.3 | 285.6 | 23.6 | 0.3 | 53.2 | 21.2 |
| Acid 16:0 | 1200.7 | 118.3 | 4845.0 | 2041.2 | 2917.8 | 64.6 | 6969.3 | 3297.5 | 466.3 | 274.6 | 858.0 | 235.0 | 465.1 | 185.1 | 616.3 | 163.5 |
| Acid 17:0 | 26.0 | 0.3 | 110.0 | 47.1 | 76.4 | 1.1 | 180.1 | 89.4 | 18.3 | 0.0 | 34.9 | 15.5 | 37.7 | 26.9 | 52.6 | 9.8 |
| Acid 18:0 | 763.5 | 113.6 | 1590.3 | 632.8 | 59.4 | 11.0 | 126.7 | 51.3 | 176.7 | 1.6 | 385.6 | 187.4 | 42.0 | 33.6 | 52.0 | 7.2 |
| Acid 18:2 | 60.2 | 3.6 | 170.0 | 77.4 | 489.3 | 1.1 | 1485.0 | 666.3 | 399.4 | 43.5 | 679.1 | 314.7 | 792.2 | 150.2 | 1204.6 | 388.9 |
| Acid 20:0 | 29.3 | 12.0 | 79.6 | 28.3 | 40.8 | 12.5 | 77.9 | 32.6 | 28.4 | 5.5 | 62.4 | 22.9 | 355.3 | 131.5 | 578.3 | 182.0 |
| Acid 22:0 | 61.2 | 43.8 | 95.8 | 21.0 | 66.1 | 42.4 | 102.3 | 28.3 | 92.2 | 48.5 | 148.6 | 42.9 | 127.1 | 108.5 | 147.5 | 17.3 |
| Acid 24:0 | 51.3 | 10.1 | 129.9 | 48.2 | 19.8 | 11.7 | 34.9 | 9.6 | 38.6 | 23.7 | 68.6 | 18.3 | 28.6 | 18.4 | 40.3 | 9.2 |
| 13-Hydroxystearic acid | 15.8 | 1.5 | 55.5 | 22.7 | 50.4 | 0.7 | 121.0 | 61.3 | 57.4 | 5.8 | 126.8 | 43.9 | 91.7 | 31.7 | 132.1 | 37.6 |
| Sum | 2502.7 | 940.1 | 5933.2 | 1982.4 | 4234.6 | 156.8 | 10,412.6 | 4843.3 | 1542.8 | 659.1 | 2499.3 | 788.6 | 2055.1 | 1050.5 | 2594.8 | 617.3 |
| Resin acids | ||||||||||||||||
| Secodehydroabietic acid | 452.3 | 99.9 | 617.0 | 206.6 | 80.8 | 13.4 | 152.8 | 58.3 | 768.9 | 634.4 | 867.5 | 87.5 | 1000.0 | 749.9 | 1292.3 | 193.6 |
| 9,10-epoxy-18:0 acid | 13.4 | 0.0 | 64.2 | 28.4 | 142.0 | 0.4 | 386.9 | 185.0 | 118.1 | 0.0 | 376.2 | 160.0 | 123.0 | 24.3 | 204.9 | 87.1 |
| 8,15-pimaradien-18-oic acid | 18.3 | 0.6 | 72.4 | 31.0 | 10.2 | 0.1 | 39.3 | 16.4 | 116.5 | 71.3 | 220.7 | 63.0 | 137.3 | 104.8 | 162.1 | 22.9 |
| Pimaric acid | 547.2 | 43.3 | 2284.9 | 973.5 | 31.0 | 6.8 | 71.6 | 27.8 | 7008.8 | 759.2 | 14,801.9 | 6435.9 | 6722.2 | 5920.4 | 7957.6 | 749.5 |
| Sandaracopimaric acid | 137.4 | 11.6 | 587.3 | 251.8 | 3.9 | 0.0 | 12.4 | 5.0 | 1034.0 | 113.7 | 2402.5 | 982.2 | 1065.7 | 923.8 | 1275.1 | 136.8 |
| Iso-pimaric acid | 153.6 | 0.2 | 665.0 | 286.8 | 3.6 | 2.1 | 5.1 | 1.3 | 2188.4 | 106.8 | 5214.0 | 2092.4 | 2589.6 | 2161.2 | 2965.5 | 326.5 |
| Dihydroabietic acid | 665.8 | 41.1 | 2817.9 | 1205.5 | 25.9 | 7.8 | 48.6 | 20.8 | 11,909.9 | 210.0 | 36,513.1 | 15,352.7 | 11,045.9 | 9212.5 | 14,022.1 | 2106.8 |
| Palustric acid | 67.2 | 1.5 | 293.0 | 126.6 | 1.2 | 0.2 | 3.0 | 1.2 | 2282.2 | 175.7 | 5911.5 | 2351.4 | 4784.9 | 2212.4 | 8300.0 | 2377.5 |
| Levopimaric acid | 18.6 | 0.8 | 48.7 | 19.7 | 4.4 | 0.9 | 10.4 | 3.9 | 625.6 | 59.9 | 1546.5 | 706.6 | 411.6 | 78.4 | 1075.5 | 401.1 |
| Dehydroabietic acid | 5637.3 | 1012.3 | 21,692.8 | 9003.8 | 547.8 | 151.1 | 1162.6 | 438.2 | 10,974.3 | 3892.2 | 26,901.4 | 9272.9 | 14,706.8 | 10,927.7 | 18,906.0 | 3153.9 |
| Tetrahydroabietic acid | 11,163.6 | 1961.1 | 44,227.0 | 18,508.1 | 1368.9 | 275.2 | 3057.3 | 1024.4 | 15,413.4 | 9042.8 | 24,413.2 | 6392.6 | 8322.5 | 2128.7 | 15,979.0 | 6011.1 |
| Abietic acid | 56.4 | 1.7 | 227.4 | 96.1 | 6.4 | 4.0 | 10.9 | 2.8 | 5384.0 | 356.6 | 13,668.8 | 5584.3 | 16,235.9 | 4602.1 | 23,536.6 | 7295.1 |
| Neoabietic acid | 25.0 | 0.9 | 115.6 | 50.7 | 0.7 | 0.1 | 1.8 | 0.7 | 1394.4 | 67.2 | 3393.8 | 1364.1 | 2527.7 | 1267.9 | 5014.8 | 1510.5 |
| x-Hydroxy-tetrahydroabietic acid | 12.0 | 0.7 | 45.5 | 18.9 | 4.9 | 0.8 | 12.7 | 4.6 | 195.5 | 2.9 | 453.3 | 193.3 | 467.8 | 341.8 | 620.2 | 116.8 |
| x-Hydroxy-dehydroabietic acid | 7.6 | 3.5 | 12.0 | 3.6 | 4.7 | 2.9 | 6.1 | 1.3 | 72.1 | 16.4 | 208.6 | 80.0 | 319.2 | 128.6 | 650.9 | 203.9 |
| Sum | 18,975.6 | 3275.0 | 73,700.4 | 30,652.6 | 2236.5 | 468.3 | 4877.9 | 1693.0 | 59,486.1 | 17077.2 | 124,450.5 | 44,730.6 | 70,459.9 | 59,815.3 | 83,903.8 | 8615.3 |
| Sterols | ||||||||||||||||
| Campesterol | 36.6 | 7.0 | 52.2 | 17.3 | 15.9 | 3.7 | 30.9 | 11.6 | 32.8 | 25.1 | 45.5 | 7.7 | 33.5 | 27.5 | 40.9 | 6.7 |
| Campestanol | 39.4 | 11.2 | 75.3 | 23.9 | 6.9 | 3.9 | 9.9 | 2.8 | 41.6 | 24.7 | 72.2 | 17.9 | 33.9 | 21.4 | 43.2 | 8.3 |
| Sitosterol | 586.6 | 81.7 | 809.4 | 299.0 | 270.7 | 54.8 | 521.9 | 199.5 | 556.7 | 451.9 | 684.2 | 94.3 | 564.8 | 509.9 | 619.6 | 51.4 |
| Sitostanol | 96.6 | 25.0 | 139.0 | 44.2 | 55.1 | 9.0 | 117.9 | 44.8 | 60.1 | 40.3 | 74.3 | 13.9 | 72.8 | 55.9 | 110.2 | 21.5 |
| Citrostadienol | 10.7 | 3.3 | 17.0 | 5.5 | 5.6 | 1.5 | 11.1 | 3.8 | 20.8 | 15.8 | 26.1 | 3.8 | 17.1 | 12.7 | 19.2 | 2.6 |
| Stigmasta-3,5-diene | 23.5 | 3.7 | 59.9 | 21.9 | 6.8 | 4.3 | 8.7 | 1.6 | 37.6 | 28.4 | 42.1 | 5.5 | 31.0 | 23.9 | 39.9 | 6.3 |
| Sitosteryl formiate | 17.9 | 4.4 | 37.2 | 13.0 | 4.4 | 3.1 | 5.2 | 0.9 | 11.2 | 5.7 | 18.7 | 5.3 | 14.9 | 7.3 | 25.4 | 6.7 |
| Sum | 811.4 | 136.3 | 1079.0 | 387.8 | 365.3 | 84.9 | 701.2 | 259.8 | 760.8 | 622.3 | 909.7 | 110.1 | 768.0 | 682.4 | 867.6 | 76.2 |
| Terpenes | ||||||||||||||||
| Pimarol | 11.5 | 0.1 | 49.3 | 21.2 | 37.4 | 5.0 | 108.3 | 44.0 | 344.1 | 0.0 | 1340.3 | 560.2 | 270.9 | 151.3 | 374.3 | 100.1 |
| 3-hydroxylambda-8(17),13(16),14-trienoic | 0.5 | 0.1 | 1.3 | 0.5 | 3.0 | 0.0 | 10.3 | 4.3 | 49.9 | 0.9 | 137.7 | 54.6 | 258.6 | 106.4 | 550.3 | 170.9 |
| 18-norabietane+ | 15.8 | 0.2 | 40.0 | 16.2 | 0.7 | 0.2 | 1.5 | 0.5 | 17.3 | 4.2 | 35.2 | 12.2 | 4.5 | 0.0 | 20.3 | 8.8 |
| 18-norabietene | 826.8 | 10.7 | 1455.2 | 597.7 | 10.6 | 7.5 | 14.5 | 2.7 | 2042.7 | 195.8 | 5164.5 | 1873.2 | 44.9 | 6.9 | 134.2 | 51.8 |
| 18-norabietane | 17,579.4 | 161.8 | 36,389.6 | 13,458.5 | 180.8 | 105.1 | 295.6 | 74.0 | 12,760.3 | 1197.3 | 22,942.3 | 9403.9 | 206.9 | 66.3 | 534.1 | 186.8 |
| 18-norabieta-8,11,13-triene | 4433.0 | 238.1 | 6733.9 | 2630.4 | 264.4 | 61.8 | 636.9 | 219.3 | 9527.9 | 4157.4 | 15,646.3 | 5361.0 | 1748.9 | 586.7 | 4621.3 | 1649.1 |
| Cycloartenol | 52.8 | 24.2 | 68.7 | 17.3 | 27.3 | 8.9 | 41.9 | 13.8 | 255.1 | 190.9 | 378.4 | 76.4 | 173.5 | 164.8 | 178.0 | 5.5 |
| Methylene cycloartanol | 6.5 | 1.5 | 9.2 | 3.2 | 6.5 | 1.7 | 10.0 | 3.3 | 15.6 | 2.7 | 24.6 | 9.1 | 20.6 | 16.0 | 25.6 | 3.5 |
| Sum | 22926.4 | 489.2 | 43016.8 | 15,366.3 | 530.8 | 215.1 | 1035.2 | 303.4 | 25,013.0 | 7699.5 | 44,064.1 | 15,795.3 | 2728.9 | 1544.6 | 5775.9 | 1731.4 |
| Free sugars | ||||||||||||||||
| Sugar pentose_1 | 7.9 | 0.6 | 24.5 | 9.4 | 0.3 | 0.1 | 0.5 | 0.2 | 2.4 | 0.2 | 8.1 | 3.3 | 1.8 | 0.5 | 3.3 | 1.2 |
| Sugar hexose_1 | 226.1 | 15.6 | 644.2 | 249.7 | 11.1 | 0.4 | 29.6 | 12.6 | 1365.2 | 310.5 | 3533.9 | 1261.1 | 63.8 | 0.8 | 269.1 | 116.0 |
| Sum | 234.1 | 16.2 | 649.3 | 251.0 | 11.4 | 0.5 | 29.7 | 12.6 | 1367.6 | 310.7 | 3534.3 | 1260.7 | 65.6 | 2.1 | 271.9 | 116.5 |
| Glycerides | ||||||||||||||||
| Diglycerides | 54.2 | 12.2 | 212.5 | 88.6 | 271.6 | 64.0 | 636.1 | 220.4 | 24.3 | 6.4 | 41.5 | 12.9 | 27.1 | 1.6 | 45.9 | 18.4 |
| Triglycerides | 85.4 | 25.8 | 117.5 | 34.8 | 52.5 | 16.3 | 75.5 | 25.9 | 178.4 | 88.4 | 283.1 | 70.8 | 139.6 | 109.8 | 167.6 | 20.5 |
| Sum | 139.6 | 106.4 | 238.3 | 55.9 | 324.1 | 124.5 | 710.7 | 226.2 | 202.8 | 129.9 | 309.8 | 66.6 | 166.8 | 142.5 | 183.6 | 17.3 |
| Steryl esters | 435.8 | 128.8 | 1483.7 | 587.1 | 1061.5 | 741.6 | 1584.1 | 394.4 | 465.3 | 227.4 | 825.6 | 250.9 | 820.8 | 677.9 | 1219.6 | 225.7 |
| Most abundant non-identified | ||||||||||||||||
| RT9.83 | 113.9 | 8.5 | 485.0 | 207.8 | 232.6 | 2.0 | 721.7 | 321.3 | 325.0 | 39.9 | 691.5 | 273.1 | 195.0 | 20.3 | 285.9 | 106.5 |
| RT10.64 | 515.1 | 12.6 | 1067.1 | 423.9 | 10.8 | 8.3 | 16.3 | 3.5 | 882.1 | 62.6 | 1377.8 | 526.8 | 17.4 | 3.4 | 42.7 | 15.3 |
| RT11.86 | 415.1 | 4.6 | 608.2 | 252.6 | 4.0 | 3.3 | 4.4 | 0.4 | 1255.5 | 221.9 | 2192.5 | 779.5 | 51.8 | 3.4 | 186.0 | 76.6 |
| RT12.48 | 124.6 | 9.0 | 221.9 | 90.6 | 6.0 | 3.1 | 14.7 | 4.9 | 1338.3 | 447.4 | 2641.4 | 800.6 | 85.6 | 1.5 | 308.3 | 127.0 |
| RT15.25 | 249.5 | 58.2 | 409.1 | 171.8 | 42.5 | 1.1 | 132.7 | 56.0 | 1091.5 | 52.5 | 2458.3 | 950.2 | 14.7 | 6.9 | 17.6 | 4.6 |
| RT16.62 | 531.3 | 33.0 | 2263.7 | 970.1 | 17.7 | 6.7 | 40.6 | 14.2 | 4425.7 | 283.9 | 11,347.6 | 4314.9 | 3993.7 | 2104.1 | 6860.2 | 1767.1 |
| Sum | 1949.5 | 125.9 | 5055.0 | 2116.8 | 313.6 | 24.5 | 930.4 | 400.3 | 9318.1 | 1108.2 | 20,709.1 | 7645.1 | 4538.2 | 2139.6 | 7700.7 | 2097.1 |
| Total sum, μg/g dry wood | 47975.3 | 11,728.3 | 101,130.0 | 33,787.1 | 9077.9 | 2181.7 | 17,018.2 | 7058.1 | 98,156.6 | 66,787.2 | 155,927.1 | 37,597.0 | 81,423.2 | 75,058.5 | 93,625.9 | 7202.9 |
References
- Fengel, D. Aging and fossilization of wood and its components. Wood Sci. Technol. 1991, 25, 153–177. [Google Scholar] [CrossRef]
- Kullman, L.; Engelmark, O. A high Late Holocene tree-limit and the establishment of the spruce forest-limit—A case study in northern Sweden. Boreas 1990, 19, 323–331. [Google Scholar] [CrossRef]
- Helama, S.; Kuoppamaa, M.; Sutinen, R. Subaerially preserved remains of pine stemwood as indicators of late Holocene timberline fluctuations in Fennoscandia, with comparisons of tree-ring and 14C dated depositional histories of subfossil trees from dry and wet sites. Rev. Palaeobot. Palyno. 2020, 278, 104223. [Google Scholar] [CrossRef]
- Martin, R.E. Taphonomy: A Process Approach; Cambridge Paleobiology Series; Cambridge University Press: Cambridge, UK, 1999; Volume 4, p. 508. ISBN 0 521 59171 6. [Google Scholar]
- Greenwood, D.R. The taphonomy of plant macrofossils. In The Processes of Fossilization; Donovan, S.K., Ed.; Belhaven: London, UK, 1991; pp. 141–169. [Google Scholar]
- Wing, S.L. Depositional environments of plant bearing sediments. Paleontol. Soc. Spec. Publ. 1988, 3, 1–13. [Google Scholar] [CrossRef]
- Rich, F.J. A review of the taphonomy of plant remains in lacustrine sediments. Rev. Palaeobot. Palyno. 1989, 58, 33–46. [Google Scholar] [CrossRef]
- Vogel, J.C.; Casparie, W.A.; Munaut, A.V. Carbon-14 trends in subfossil pine stubs. Science 1969, 166, 1143–1145. [Google Scholar] [CrossRef]
- Kullman, L. Radiocarbon dating of subfossil Scots pine (Pinus sylvestris L.) in the southern Swedish Scandes. Boreas 1980, 9, 101–106. [Google Scholar] [CrossRef]
- Kullman, L. Ecological tree line history and palaeoclimate—Review of megafossil evidence from the Swedish Scandes. Boreas 2013, 42, 555–567. [Google Scholar] [CrossRef]
- Bridge, M.C.; Haggart, B.A.; Lowe, J.J. The history and palaeoclimatic significance of subfossil remains of Pinus sylvestris in blanket peats from Scotland. J. Ecol. 1990, 78, 77–99. [Google Scholar] [CrossRef]
- Linderholm, H.W.; Gunnarson, B.E. Summer temperature variability in central Scandinavia during the last 3600 years. Geogr. Ann. 2005, 87A, 231–241. [Google Scholar] [CrossRef]
- Moir, A.K.; Leroy, S.A.G.; Brown, D.; Collins, P.E.F. Dendrochronological evidence for a lower water-table on peatland around 3200–3000 BC from subfossil pine in northern Scotland. Holocene 2010, 20, 931–942. [Google Scholar] [CrossRef]
- Edvardsson, J.G.; Stoffel, M.; Corona, C.; Bragazza, L.; Leuschner, H.H.; Charman, D.J.; Helama, S. Subfossil peatland trees as proxies for Holocene palaeohydrology and palaeoclimate. Earth-Sci. Rev. 2016, 163, 118–140. [Google Scholar] [CrossRef] [Green Version]
- Achterberg, I.; Frechen, M.; Bauerochse, A.; Eckstein, J.; Leuschner, H.H. The Göttingen tree-ring chronologies of peat-preserved oaks and pines from Northwest Germany. Z. Dtsch. Ges. Geowiss. 2017, 168, 9–19. [Google Scholar] [CrossRef]
- Walker, M.; Johnsen, S.; Rasmussen, S.O.; Popp, T.; Steffensen, J.-P.; Gibbard, P.; Hoek, W.; Lowe, J.; Andrews, J.; Bj€orck, S.; et al. Formal definition and dating of the GSSP (Global Stratotype Section and Point) for the base of the Holocene using the Greenland NGRIP ice core, and selected auxiliary records. J. Quat. Sci. 2009, 24, 3–17. [Google Scholar] [CrossRef]
- Rubiales, J.; Génova, M. Late Holocene pinewoods persistence in the Gredos Mountains (central Spain) inferred from extensive megafossil evidence. Quat. Res. 2015, 84, 12–20. [Google Scholar] [CrossRef]
- Eronen, M.; Hyvärinen, H.; Zetterberg, P. Holocene humidity changes in northern Finnish Lapland inferred from lake sediments and submerged Scots pines dated by tree rings. Holocene 1999, 9, 569–580. [Google Scholar] [CrossRef]
- Eronen, M.; Zetterberg, P.; Briffa, K.R.; Lindholm, M.; Meriläinen, J.; Timonen, M. The supra-long Scots pine tree-ring record for Finnish Lapland: Part 1, chronology construction and initial references. Holocene 2002, 12, 673–680. [Google Scholar] [CrossRef]
- Helama, S.; Arppe, L.; Timonen, M.; Mielikäinen, K.; Oinonen, M. A 7.5 ka chronology of stable carbon isotopes from tree rings with implications for their use in palaeo-cloud reconstruction. Glob. Planet. Chang. 2018, 170, 20–33. [Google Scholar] [CrossRef]
- Eronen, M. The retreat of pine forest in Finnish Lapland since the Holocene climatic optimum: A general discussion with radiocarbon evidence from subfossil pines. Fennia 1979, 157, 93–114. Available online: https://fennia.journal.fi/article/view/9166 (accessed on 12 November 2020).
- Helama, S.; Lindholm, M.; Timonen, M.; Eronen, M. Mid- and late Holocene tree population density changes in northern Fennoscandia derived by a new method using megafossil pines and their tree-ring series. J. Quat. Sci. 2005, 20, 567–575. [Google Scholar] [CrossRef]
- Helama, S.; Vartiainen, M.; Kolström, T.; Meriläinen, J. Dendrochronological investigation of wood extractives. Wood Sci. Technol. 2010, 44, 335–351. [Google Scholar] [CrossRef]
- Hedges, J.I. The chemistry of archaeological wood. Adv. Chem. 1990, 225, 111–140. [Google Scholar] [CrossRef]
- Hoffmann, P.; Jones, M.A. Structure and degradation process for waterlogged archaeological wood. Adv. Chem. 1990, 225, 35–65. [Google Scholar] [CrossRef]
- Schniewind, A.P. Physical and mechanical properties of archaeological wood. Adv. Chem. 1990, 225, 87–109. [Google Scholar] [CrossRef]
- Baar, J.; Paschová, Z.; Hofmann, T.; Kolář, T.; Koch, G.; Saake, B.; Rademacher, P. Natural durability of subfossil oak: Wood chemical composition changes through the ages. Holzforschung 2020, 74, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Guyette, R.; Stambaugh, M. The age and density of ancient and modern oak wood in streams and sediments. IAWA J. 2003, 24, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Xiao, L.; Han, L.; Wu, H.; Yang, T.; Wu, S.; Yin, Y.; Donaldson, L.A. Deterioration of the cell wall in waterlogged wooden archeological artifacts, 2400 years old. IAWA J. 2019, 40, 820–844. [Google Scholar] [CrossRef]
- Čufar, K.; Gričar, J.; Zupančič, M.; Koch, G.; Schmitt, U. Anatomy, cell wall structure and topochemistry of water-logged archaeological wood aged 5200 and 4500 years. IAWA J. 2008, 29, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.; Frosch, M.; Jensen, P.; Schnell, U.; Shashoua, Y.; Nielsen, O.F. Waterlogged archaeological wood—Chemical changes by conservation and degradation. J. Raman Spectrosc. 2006, 37, 1171–1178. [Google Scholar] [CrossRef]
- Christiernin, M.; Notley, S.M.; Zhang, L.; Nilsson, T.; Henriksson, G. Comparison between 10,000-year old and contemporary spruce lignin. Wood Sci. Technol. 2009, 43, 23–41. [Google Scholar] [CrossRef]
- Kolář, T.; Rybníček, M. Physical and mechanical properties of subfossil oak (Quercus, sp.) wood. Acta Univ. Agric. Silvic. Mendel. Brun. 2010, 58, 123–134. Available online: https://acta.mendelu.cz/pdfs/acu/2010/04/12.pdf (accessed on 18 August 2021). [CrossRef] [Green Version]
- Kolář, T.; Rybníček, M.; Střelcová, M.; Hedbávný, J.; Vít, J. The changes in chemical composition and properties of subfossil oak deposited in Holocene sediments. Wood Res. 2014, 59, 149–166. [Google Scholar]
- Rede, V.; Essert, S.; Kodvanj, J. Annual ring orientation effect on bending strength of subfossil elm wood. J. Wood Sci. 2017, 63, 31–36. [Google Scholar] [CrossRef]
- Han, L.; Tian, X.; Keplinger, T.; Zhou, H.; Li, R.; Svedström, K.; Burgert, I.; Yin, Y.; Guo, J. Even visually intact cell walls in waterlogged archaeological wood are chemically deteriorated and mechanically fragile: A case of a 170 year-old shipwreck. Molecules 2020, 25, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björdal, C.G.; Nilsson, T.; Daniel, G. Microbial decay of waterlogged archaeological wood found in Sweden. Applicable to archaeology and conservation. Int. Biodeter. Biodegr. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, Q.; Feng, X.; Chen, D.; Sun, J.; Cui, Y.; Xu, R. Microbial erosion assessment on waterlogged archaeological woods (WAWs) from a Chinese ancient shipwreck, Nanhai No. 1. Herit. Sci. 2018, 6, 53. [Google Scholar] [CrossRef]
- Broda, M.; Frankowski, M. Determination of the content of selected elements in medieval waterlogged oak wood from the Lednica Lake—A case study. Environ. Sci. Pollut. Res. 2017, 24, 23401–23410. [Google Scholar] [CrossRef] [Green Version]
- Borgin, K.; Parameswaran, N.; Liese, W. The effect of aging on the ultrastructure of wood. Wood. Sci Technol. 1975, 9, 87–98. [Google Scholar] [CrossRef]
- Sen, J.; Basak, R.K. The nature of ancient wood. II. The structure and properties of well preserved tracheids and fibres. B. Torrey Bot. Club 1955, 82, 183–195. [Google Scholar] [CrossRef]
- Erhardt, D.; Mecklenburg, M.F.; Tumosa, C.S.; Olstad, T.M. New vs old wood: Differences and similarities in physical, mechanical, and chemical properties. In Proceedings of the ICOM Committee for Conservation, 11th Triennial Meeting in Edinburgh, Edinburgh, Scotland, 1–6 September 1996; Volume II, pp. 903–910. [Google Scholar]
- Tintner, J.; Spangl, B.; Grabner, M.; Helama, S.; Timonen, M.; Kirchhefer, A.J.; Reinig, F.; Nievergelt, D.; Krąpiec, M.; Smidt, E. MD dating: Molecular decay (MD) in pinewood as a dating method. Sci. Rep. 2020, 10, 11255. [Google Scholar] [CrossRef]
- Dinwoodie, J.M. Timber: Its Nature and Behaviour, 2nd ed.; E & FN Spon: London, UK; New York, NY, USA, 2000; p. 272. ISBN 0419235809. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Grekin, M. Color and color uniformity variation of Scots pine wood in the air-dry condition. Wood Fiber Sci. 2007, 39, 279–290. [Google Scholar]
- Sundberg, A.; Sundberg, K.; Lillandt, C.; Holmbom, B.R. Determination of Hemicelluloses and Pectins in Wood and Pulp Fibres by Acid Methanolysis and Gas Chromatography. Nord. Pulp Pap. Res. J. 1996, 11, 216–219. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Klason Lignin: Modifications to Improve the Precision of the Standardized Determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- Grekin, M. Nordic Scots Pine vs. Selected Competing Species and Non-Wood Substitute Materials in Mechanical Wood Products; Literature Survey; Finnish Forest Research Institute: Helsinki, Finland, 2006; Volume 82, p. 66. ISBN 978-951-40-2019-3. [Google Scholar]
- Hakkila, P. Utilization of Residual Forest Biomass; Springer Series in Wood Science; Springer: Berlin, Germany, 1989; p. 568. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Tymendorf, Ł.; Trzciński, G. Importance of the moisture content of large-sized Scots pine (Pinus sylvestris L.) roundwood in its road transport. Forests 2021, 12, 879. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective hemicellulose hydrolysis of Scots pine sawdust. Biomass Conv. Bioref. 2019, 9, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.M.; Pettersen, R.; Han, J.S.; Rowell, J.S.; Tshabalala, M.A. Cell Wall Chemistry: Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; pp. 35–74. [Google Scholar]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989; p. 613. [Google Scholar]
- Tintner, J. Recent developments in using the molecular decay dating method: A review. Ann. NY. Acad. Sci. 2021, 1493, 29–40. [Google Scholar] [CrossRef]
- Babiński, L.; Fabisiak, E.; Dąbrowski, H.P.; Kittel, P. Study on dimensional stabilization of 12,500-year-old waterlogged subfossil Scots pine wood from the Koźmin Las site, Poland. J. Cult. Herit. 2017, 23, 119–127. [Google Scholar] [CrossRef]
- Hakkila, P. Investigations on the basic density of Finnish pine, spruce and birch wood. Commun. Inst. For. Fenn. 1966, 61, 1–98. [Google Scholar]
- Werkelin, J.; Skrifvars, B.-J.; Hupa, M. Ash-forming elements in four Scandinavian wood species. Part 1: Summer harvest. Biomass Bioenerg. 2005, 29, 451–466. [Google Scholar] [CrossRef]
- Paukkunen, S.; Sikanen, L.; Ikonen, R. Ash content of wood pellets made from small Scots pine (Pinus sylvestris) trees with bark. Forest Prod. J. 2015, 65, 337–345. [Google Scholar] [CrossRef]
- Jalava, M. Suomalaisen männyn, kuusen, koivun ja haavan lujuusominaisuuksista. Summary: Strength properties of Finnish pine, spruce, birch and aspen. Commun. Inst. For. Fenn. 1945, 33, 1–66, (In Finnish with English Summary). [Google Scholar]
- Verkasalo, E.; Leban, J.-M. MOE and MOR in static bending of small clear specimens of Scots pine, Norway spruce and European fir from Finland and France and their prediction for the comparison of wood quality. Pap. Tim. 2002, 84, 332–340. [Google Scholar]
- Grekin, M.; Verkasalo, E. Variations in and models for Brinell hardness of Scots pine wood from Finland and Sweden. Baltic For. 2013, 19, 128–136. [Google Scholar]
- Endo, R.; Kamei, K.; Iida, I.; Yokoyama, M.; Kawahara, Y. Physical and mechanical properties of waterlogged wood treated with hydrolyzed feather keratin. J. Archaeol. Sci. 2010, 37, 1311–1316. [Google Scholar] [CrossRef]
- Simpson, W.T. Specific Gravity, Moisture Content, and Density Relationship for Wood; Gen. Tech. Rep. FPL-GTR-76; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1993; p. 13.
- Barcík, Š.; Gašparík, M.; Razumov, E.Y. Effect of thermal modification on the colour changes of oak wood. Wood Res. 2015, 60, 385–396. [Google Scholar]
- Veizović, M.; Popović, Z.; Todorović, N.; Goran, M. Drying quality and colour of subfossil oak from central Serbia. Glas. Sumar. Fak. 2018, 117, 157–172. [Google Scholar] [CrossRef] [Green Version]
- van Bürck, U.; Wagner, F.E.; Lerf, A. Mössbauer studies of subfossil oak. Hyperfine Interact. 2012, 208, 105–110. [Google Scholar] [CrossRef]
- Kránitz, K.; Sonderegger, W.; Bues, C.T.; Niemz, P. Effects of aging on wood: A literature review. Wood Sci. Technol. 2016, 50, 7–22. [Google Scholar] [CrossRef]
- Obst, J.R.; McMillan, N.J.; Blanchette, R.A.; Christensen, D.J.; Faix, O.; Han, J.S.; Kuster, T.A.; Landucci, L.L.; Newman, R.H.; Pettersen, R.C.; et al. Characterization of Canadian Arctic Fossil Woods. In Tertiary Fossil Forests of the Geodetic Hills, Axel Heiberg Island, Arctic Archipelago; Christie, R.L., McMillan, N.J., Eds.; Geological Survey of Canada: Ottawa, ON, Canada, 1991; Volume 403, pp. 123–146. [Google Scholar]
- Mansikkala, T.; Patanen, M.; Kärkönen, A.; Korpinen, R.; Pranovich, A.; Ohigashi, T.; Swaraj, S.; Seitsonen, J.; Ruokolainen, J.; Huttula, M.; et al. Lignans in knotwood of Norway spruce: Localisation with soft X-ray microscopy and scanning transmission electron microscopy with energy dispersive X-ray spectroscopy. Molecules 2020, 25, 2997. [Google Scholar] [CrossRef]
- Timell, T.E. Compression Wood in Gymnosperms; Springer: Berlin/Heidelberg, Germany, 1986; p. 625. ISBN 978-3-642-64887-8. [Google Scholar]
- Nisula, L. Wood Extractives in Conifers. Ph.D. Thesis, Åbo Akademi University Press, Turku/Åbo, Finland, 2018; p. 253. [Google Scholar]
- Willför, S.; Hemming, J.; Reunanen, R.; Eckerman, C.; Holmbom, B. Lignans and lipophilic extractives in Norway spruce knots and stemwood. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Yildirim, H.; Holmbom, B. Investigations on the Wood Extractives of Pine Species from Turkey; Acta Academiae Aboensis. Series B, Mathematica et Physica; Åbo Academy: Turku, Finland, 1977. [Google Scholar]
- Reunanen, M.; Ekman, R.; Heinonen, M. Analysis of Finnish pine tar and tar from the wreck of frigate St. Nikolai. Holzforschung 1989, 43, 33–39. [Google Scholar] [CrossRef]
- Reunanen, M.; Ekman, R.; Heinonen, M. Long-term alteration of pine tar in a marine environment. Holzforschung 1990, 44, 277–278. [Google Scholar] [CrossRef]
- Staccioli, G.; Meli, A.; Menchi, G.; Matteoli, U.; Ricottini, G. Role of a labile terpene compound in the assessment of the age of a fossil wood from Siena (Tuscany, Italy). Holzforschung 2000, 54, 591–596. [Google Scholar] [CrossRef]
- Staccioli, G.; Sturaro, A.; Parvoli, G.; Menchi, G.; Matteoli, U. The lipophilic extractives of an interglacial fossil Picea abies from Zeifen (Germany). Holzforschung 1999, 53, 391–396. [Google Scholar] [CrossRef]


| Wood Property | Standard |
|---|---|
| Moisture content (MC) | ISO 13061-1:2014 |
| Basic density (ρy) | ISO 13061-2:2014 |
| Ash content (AC) | TAPPI T 211:2007 |
| Modulus of elasticity (Ew) | ISO 13061-3:2014 |
| Modulus of rupture (σb) | ISO 13061-4:2014 |
| Colour | ISO 11664-2: 2007. Colorimetry. Part 2: CIE standard illuminants. ISO 11664-4: 2008: Colorimetry. Part 4: CIE 1976 L*a*b* colour space. ISO 11664-6: 2013. Colorimetry. Part 6: CIEDE2000 Colour-difference formula. |
| Brinell hardness (HB) | EN 1534:2010 |
| Variable | Sample | N | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|---|
| ρy | Older stem wood | 21 | 361.54 | 324.72 | 426.30 | 28.81 |
| (kg/m3) | Younger stem wood | 10 | 330.70 | 310.72 | 374.42 | 16.95 |
| Older knot wood | 6 | 632.21 | 596.68 | 683.68 | 32.43 | |
| Younger knot wood | 2 | 546.69 | 525.69 | 567.69 | - | |
| Reference stem wood [49] | 381–427 | |||||
| HB | Older stem wood | 26 | 7.36 | 5.13 | 11.23 | 1.51 |
| (MPa) | Younger stem wood | 24 | 7.14 | 6.04 | 8.45 | 0.76 |
| Older knot wood | 10 | 21.15 | 10.47 | 48.64 | 11.29 | |
| Younger knot wood | 10 | 19.03 | 7.96 | 35.90 | 9.18 | |
| Reference stem wood [49] | 13–24 | |||||
| Ew | Older stem wood | 42 | 5.43 | 4.37 | 6.59 | 0.65 |
| (GPa) | Younger stem wood | 14 | 5.36 | 4.12 | 6.42 | 0.58 |
| Reference stem wood [49] | 10.6–12.7 | |||||
| σb | Older stem wood | 21 | 50.0 | 38.7 | 56.7 | 5.1 |
| (MPa) | Younger stem wood | 7 | 42.6 | 28.9 | 56.6 | 10.3 |
| Reference stem wood [49] | 51–98 | |||||
| AC | Older stem wood | 9 | 0.43 | 0.38 | 0.54 | 0.05 |
| (%) | Younger stem wood | 6 | 0.39 | 0.34 | 0.44 | 0.04 |
| Older knot wood | 9 | 0.42 | 0.35 | 0.52 | 0.06 | |
| Younger knot wood | 6 | 0.47 | 0.40 | 0.57 | 0.07 | |
| Reference stem wood [49] | 0.30–0.40 | |||||
| MC | Older stem wood | 5 | 206.8 | 168.2 | 230.9 | 23.3 |
| (%) | Younger stem wood | 5 | 234.9 | 208.5 | 274.7 | 27.5 |
| Older knot wood | 5 | 90.8 | 62.5 | 110.7 | 18.6 | |
| Younger knot wood | 5 | 102.3 | 73.7 | 173.8 | 41.2 | |
| Reference stem wood [50,51] | 50–95 |
| Compound | Sample | N | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|---|
| Klason lignin | Older stem wood | 5 | 28.87 | 27.21 | 30.95 | 1.46 |
| Younger stem wood | 5 | 28.99 | 27.16 | 32.04 | 1.84 | |
| Older knot wood | 5 | 31.27 | 29.22 | 32.70 | 1.47 | |
| Younger knot wood | 5 | 31.24 | 30.20 | 32.73 | 0.94 | |
| Reference | 26 | |||||
| AS lignin | Older stem wood | 5 | 0.18 | 0.16 | 0.21 | 0.02 |
| Younger stem wood | 5 | 0.20 | 0.16 | 0.23 | 0.03 | |
| Older knot wood | 5 | 0.23 | 0.20 | 0.26 | 0.02 | |
| Younger knot wood | 5 | 0.22 | 0.20 | 0.24 | 0.02 | |
| Reference | - | |||||
| Hemicelluloses | Older stem wood | 5 | 23.70 | 22.55 | 24.41 | 0.94 |
| Younger stem wood | 5 | 24.15 | 23.83 | 24.94 | 0.45 | |
| Older knot wood | 5 | 26.49 | 25.73 | 27.22 | 0.57 | |
| Younger knot wood | 5 | 26.56 | 24.58 | 27.63 | 1.16 | |
| Reference | 26 | |||||
| Cellulose | Older stem wood | 5 | 39.75 | 37.23 | 41.57 | 1.59 |
| Younger stem wood | 5 | 40.99 | 38.30 | 42.85 | 1.65 | |
| Older knot wood | 5 | 36.00 | 34.19 | 37.09 | 1.21 | |
| Younger knot wood | 5 | 36.39 | 35.15 | 37.41 | 0.92 | |
| Reference | 44 | |||||
| Total | Older stem wood | 5 | 92.51 | 90.83 | 94.55 | 1.57 |
| Younger stem wood | 5 | 94.33 | 93.50 | 95.02 | 0.56 | |
| Older knot wood | 5 | 93.99 | 93.06 | 95.76 | 1.14 | |
| Younger knot wood | 5 | 94.40 | 93.22 | 95.76 | 0.96 | |
| Reference | 96 |
| Compound | Sample | N | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|---|
| Arabinose | Older stem wood | 5 | 0.934 | 0.774 | 1.194 | 0.172 |
| Younger stem wood | 5 | 1.223 | 1.091 | 1.320 | 0.086 | |
| Older knot wood | 5 | 1.158 | 0.964 | 1.567 | 0.240 | |
| Younger knot wood | 5 | 1.394 | 1.285 | 1.518 | 0.097 | |
| Rhamnose | Older stem wood | 5 | 0.220 | 0.198 | 0.249 | 0.019 |
| Younger stem wood | 5 | 0.227 | 0.216 | 0.235 | 0.008 | |
| Older knot wood | 5 | 0.244 | 0.228 | 0.263 | 0.013 | |
| Younger knot wood | 5 | 0.259 | 0.240 | 0.284 | 0.019 | |
| Xylose | Older stem wood | 5 | 5.122 | 4.708 | 5.558 | 0.388 |
| Younger stem wood | 5 | 4.950 | 4.390 | 5.235 | 0.342 | |
| Older knot wood | 5 | 6.029 | 5.450 | 6.390 | 0.385 | |
| Younger knot wood | 5 | 5.805 | 5.565 | 6.180 | 0.235 | |
| Mannose | Older stem wood | 5 | 8.706 | 7.243 | 9.847 | 1.011 |
| Younger stem wood | 5 | 9.845 | 9.172 | 10.377 | 0.432 | |
| Older knot wood | 5 | 9.132 | 7.565 | 10.437 | 1.085 | |
| Younger knot wood | 5 | 9.577 | 8.334 | 10.357 | 0.795 | |
| Galactose | Older stem wood | 5 | 3.414 | 1.790 | 6.235 | 2.161 |
| Younger stem wood | 5 | 2.236 | 1.883 | 3.292 | 0.595 | |
| Older knot wood | 5 | 4.225 | 2.963 | 5.375 | 0.877 | |
| Younger knot wood | 5 | 3.538 | 3.022 | 4.238 | 0.461 | |
| Glucose | Older stem wood | 5 | 3.030 | 2.779 | 3.396 | 0.235 |
| Younger stem wood | 5 | 3.350 | 3.316 | 3.394 | 0.032 | |
| Older knot wood | 5 | 3.283 | 2.799 | 3.679 | 0.353 | |
| Younger knot wood | 5 | 3.455 | 3.109 | 3.845 | 0.352 | |
| Glucuronic | Older stem wood | 5 | 0.026 | 0.022 | 0.032 | 0.004 |
| acid | Younger stem wood | 5 | 0.035 | 0.033 | 0.038 | 0.002 |
| Older knot wood | 5 | 0.030 | 0.027 | 0.035 | 0.003 | |
| Younger knot wood | 5 | 0.039 | 0.036 | 0.044 | 0.004 | |
| 4-O-methyl- | Older stem wood | 5 | 0.999 | 0.891 | 1.119 | 0.104 |
| glucuronic | Younger stem wood | 5 | 0.921 | 0.780 | 1.008 | 0.087 |
| acid | Older knot wood | 5 | 1.115 | 0.959 | 1.257 | 0.123 |
| Younger knot wood | 5 | 1.068 | 1.029 | 1.158 | 0.054 | |
| Galacturonic | Older stem wood | 5 | 1.254 | 1.194 | 1.332 | 0.054 |
| acid | Younger stem wood | 5 | 1.363 | 1.309 | 1.420 | 0.045 |
| Older knot wood | 5 | 1.273 | 1.166 | 1.439 | 0.111 | |
| Younger knot wood | 5 | 1.421 | 1.332 | 1.498 | 0.075 | |
| Total | Older stem wood | 45 | 23.705 | 22.550 | 24.405 | 0.941 |
| Younger stem wood | 45 | 24.152 | 23.831 | 24.944 | 0.451 | |
| Older knot wood | 45 | 26.490 | 25.731 | 27.218 | 0.573 | |
| Younger knot wood | 45 | 26.557 | 24.581 | 27.628 | 1.158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möttönen, V.; Helama, S.; Pranovich, A.; Korotkova, E.; Xu, C.; Herva, H.; Heräjärvi, H.; Mäkinen, H.; Nöjd, P.; Jyske, T. Subfossil Scots Pine (Pinus sylvestris L.) Wood from Northern Finland—Physical, Mechanical, and Chemical Properties and Suitability for Specialty Products. Forests 2022, 13, 704. https://doi.org/10.3390/f13050704
Möttönen V, Helama S, Pranovich A, Korotkova E, Xu C, Herva H, Heräjärvi H, Mäkinen H, Nöjd P, Jyske T. Subfossil Scots Pine (Pinus sylvestris L.) Wood from Northern Finland—Physical, Mechanical, and Chemical Properties and Suitability for Specialty Products. Forests. 2022; 13(5):704. https://doi.org/10.3390/f13050704
Chicago/Turabian StyleMöttönen, Veikko, Samuli Helama, Andrey Pranovich, Ekaterina Korotkova, Chunlin Xu, Hannu Herva, Henrik Heräjärvi, Harri Mäkinen, Pekka Nöjd, and Tuula Jyske. 2022. "Subfossil Scots Pine (Pinus sylvestris L.) Wood from Northern Finland—Physical, Mechanical, and Chemical Properties and Suitability for Specialty Products" Forests 13, no. 5: 704. https://doi.org/10.3390/f13050704
APA StyleMöttönen, V., Helama, S., Pranovich, A., Korotkova, E., Xu, C., Herva, H., Heräjärvi, H., Mäkinen, H., Nöjd, P., & Jyske, T. (2022). Subfossil Scots Pine (Pinus sylvestris L.) Wood from Northern Finland—Physical, Mechanical, and Chemical Properties and Suitability for Specialty Products. Forests, 13(5), 704. https://doi.org/10.3390/f13050704







