Water Uptake Pattern by Coniferous Forests in Two Habitats Linked to Precipitation Changes in Subtropical Monsoon Climate Region, China
Abstract
:1. Introduction
2. Materials and Methods
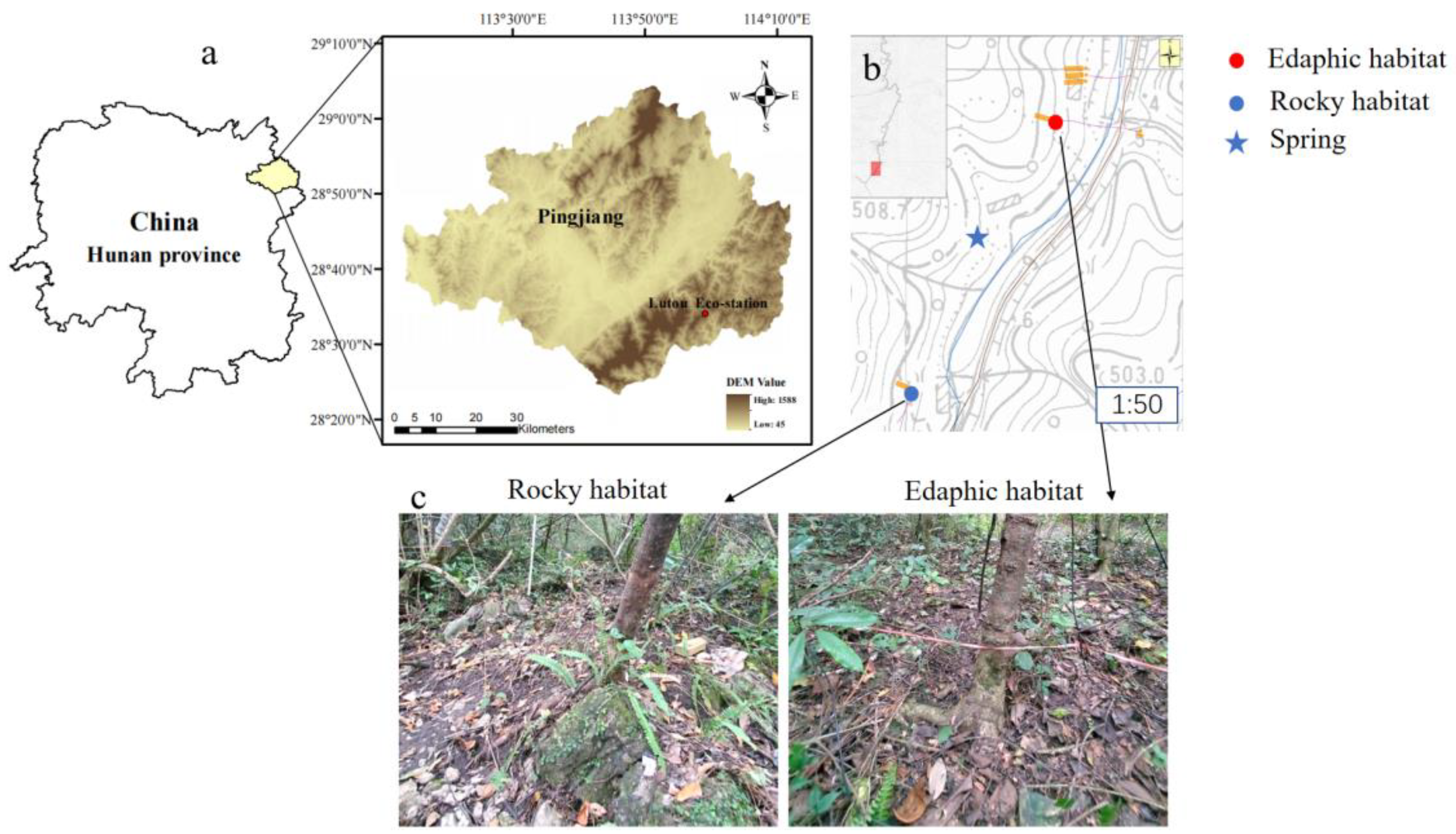
2.1. Study Sites
2.2. Plant and Soil Sampling
2.3. Precipitation and Spring Sampling
2.4. Isotopic Analyses
2.5. Leaf Water Potential
2.6. Data Analysis
3. Results
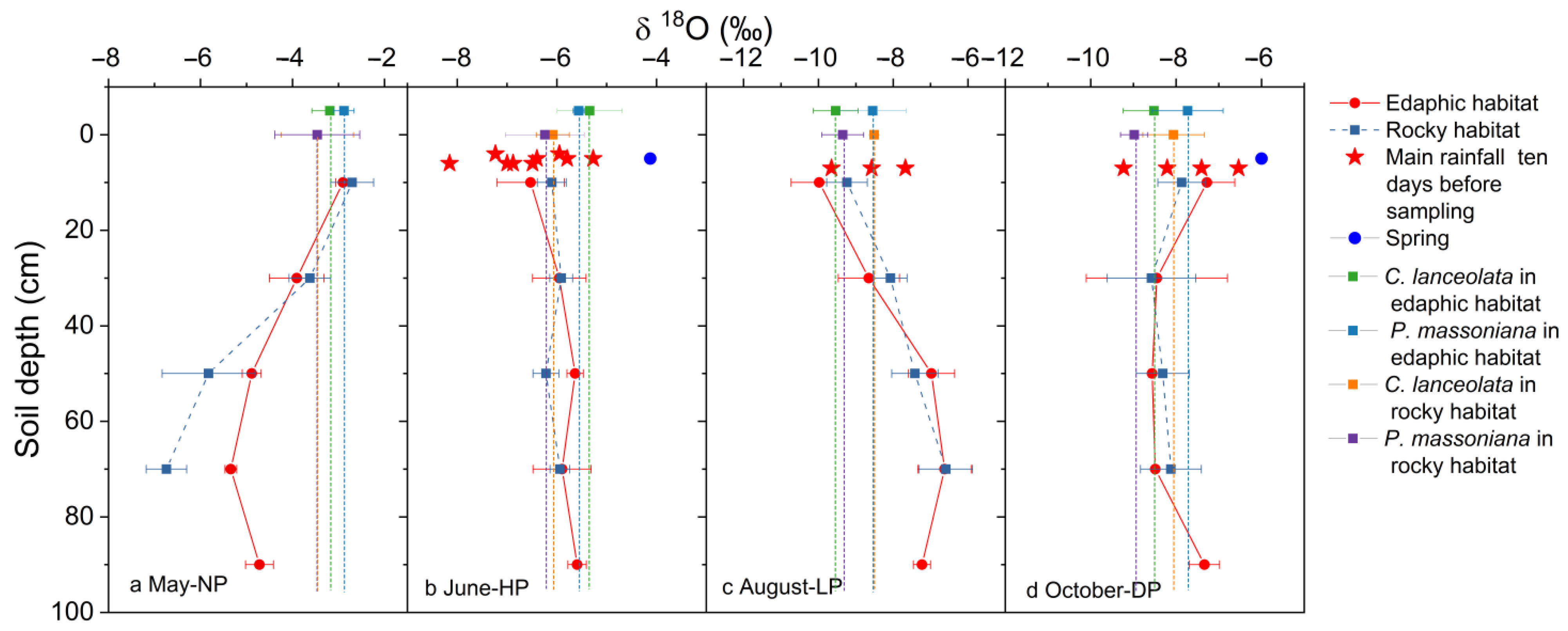
3.1. Isotopic Compositions of Precipitation, Soil Water, and Springs
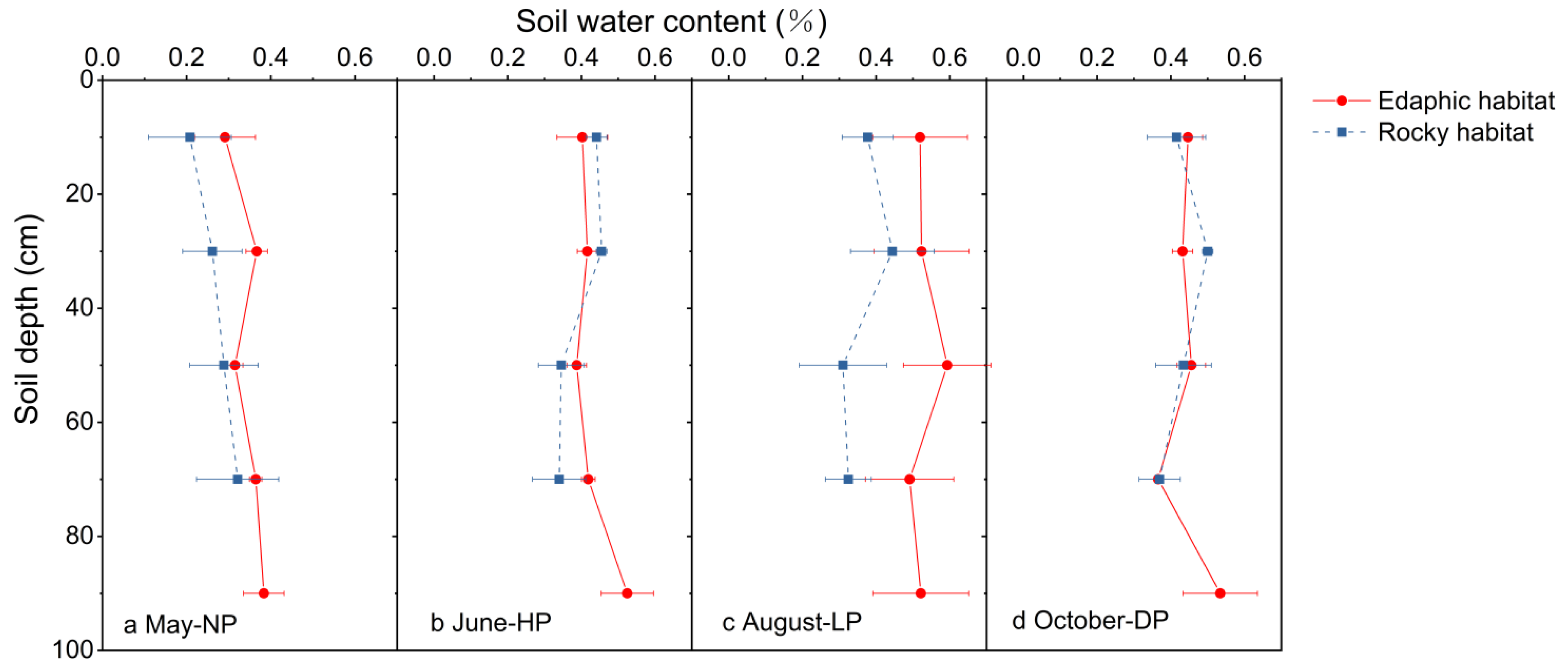
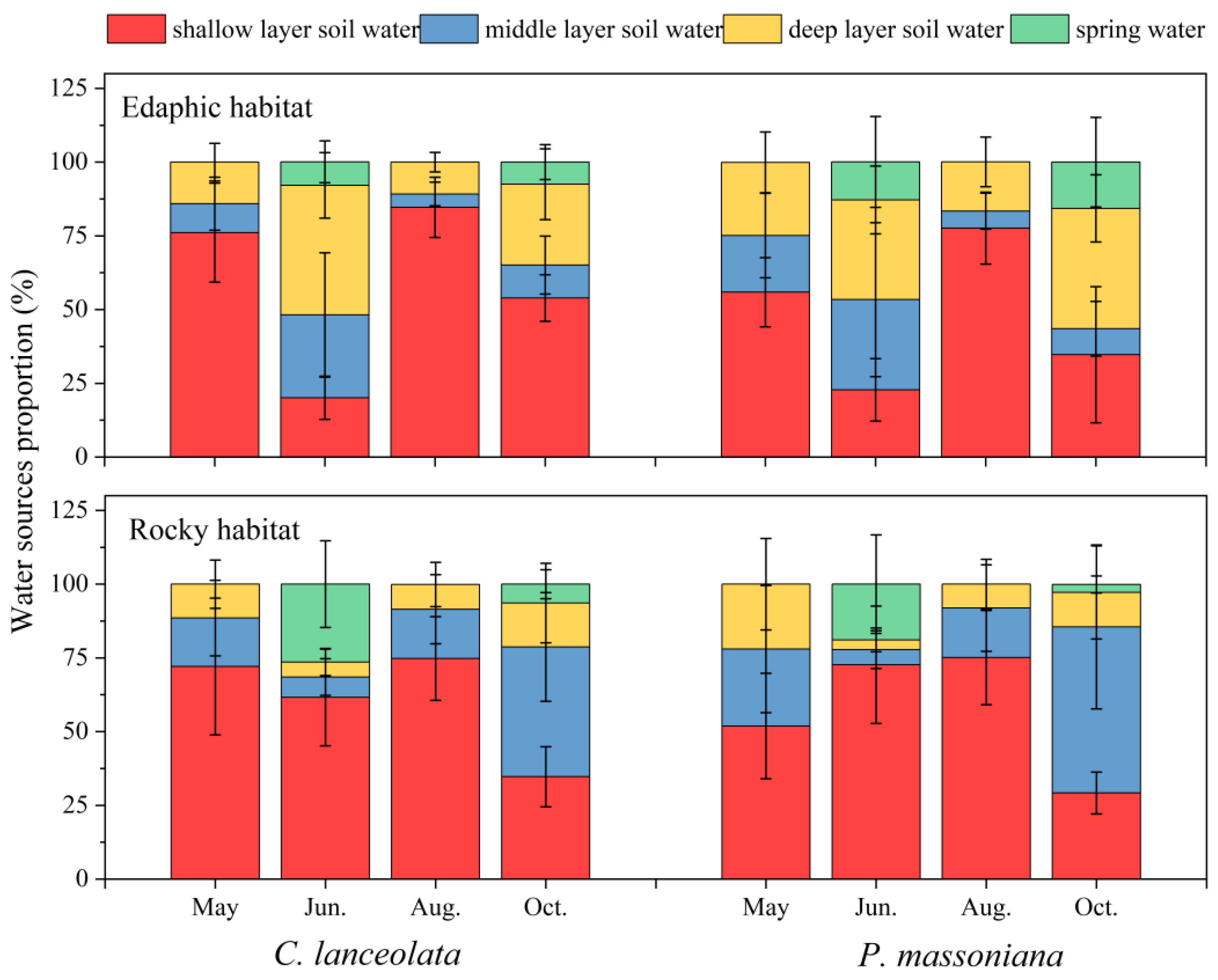
3.2. Variations in Soil Water Content, Water Uptake Patterns, and Their Linkage with Precipitation
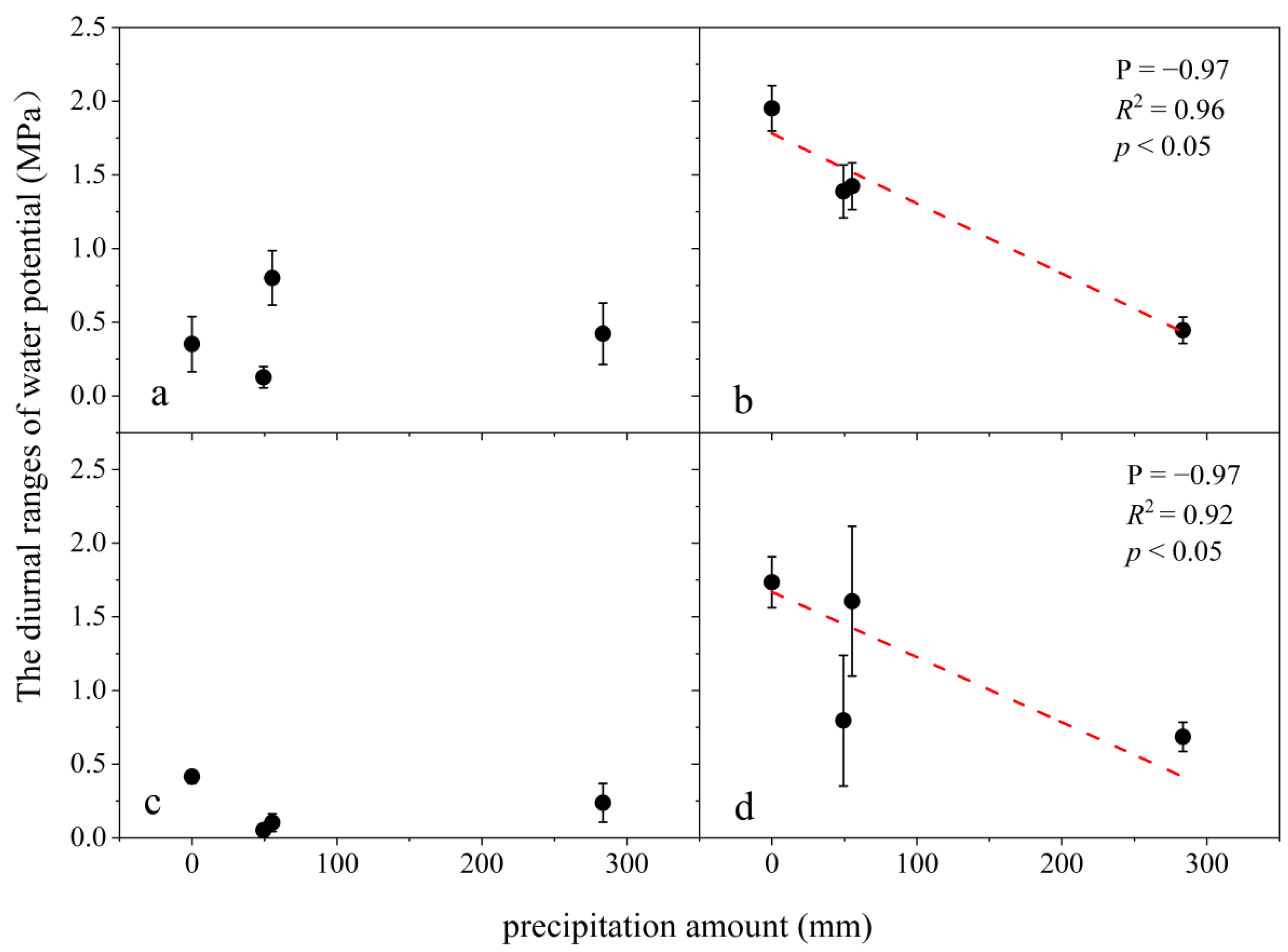
3.3. Variation in Leaf Water Potential and Its Linkage with Precipitation
3.4. Variation in Leaf Water Use Efficiency
4. Discussion
4.1. Water Uptake of Tree Species in Two Habitats
4.2. Water Uptake Pattern and Physiological Factors Change in the Different Tree Species
4.3. Implications for Plant Water Adaptation under Precipitation Changes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forzieri, G.; Miralles, D.G.; Ciais, P.; Alkama, R.; Ryu, Y.; Duveiller, G.; Zhang, K.; Robertson, E.; Kautz, M.; Martens, B. Increased control of vegetation on global terrestrial energy fluxes. Nat. Clim. Chang. 2020, 10, 356–362. [Google Scholar] [CrossRef]
- Piao, S.; Yin, G.; Tan, J.; Cheng, L.; Huang, M.; Li, Y.; Liu, R.; Mao, J.; Myneni, R.B.; Peng, S. Detection and attribution of vegetation greening trend in China over the last 30 years. Glob. Chang. Biol. 2015, 21, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.C.; Piao, S.L.; Myneni, R.B.; Huang, M.; Ning, Z. Greening of the Earth and its drivers. Nat. Clim. Chang. 2016, 6, 182. [Google Scholar] [CrossRef]
- Aguirre, G.J. Drier tropical forests are susceptible to functional changes in response to a long-term drought. Ecol. Lett. 2019, 22, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.H.; Pallardy, S.G.; Hosman, K.P.; Sun, Y. Impacts of precipitation variability on plant species and community water stress in a temperate deciduous forest in the central US. Agr. For. Meteorol. 2016, 217, 120–136. [Google Scholar] [CrossRef] [Green Version]
- Malhi, Y.; Roberts, T.J.; Betts, R.A.; Killeen, T.J.; Li, W.; Nobre, C.A. Climate change, deforestation, and the fate of the Amazon. Science 2008, 319, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, L.; Kahmen, A. Water relations of drought-stressed temperate trees benefit from short drought-intermitting rainfall events. Agric. For. Meteorol. 2019, 265, 70–77. [Google Scholar] [CrossRef]
- Konapala, G.; Mishra, A.K.; Wada, Y.; Mann, M.E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 2020, 11, 3044. [Google Scholar] [CrossRef]
- Anderegg, W.; Konings, A.G.; Trugman, A.T.; Yu, K.; Bowling, D.R.; Gabbitas, R.; Karp, D.S.; Pacala, S.; Sperry, J.S.; Sulman, B.N. Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 2018, 561, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar]
- Macias, F.M. Satellite images show China going green. Nature 2018, 553, 411–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; He, P.; Liu, H.; Zhu, S.; Uyehara, I.K.; Hou, H.; Wu, G.L.; Zhang, H.; You, Z.T.; Xiao, Y.Y. Precipitation has dominant influences on the variation of plant hydraulics of the native Castanopsis fargesii (Fagaceae) in subtropical China. Agric. For. Meteorol. 2019, 271, 83–91. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L. Plant water use strategy in response to spatial and temporal variation in precipitation patterns in China: A stable isotope analysis. Forests 2018, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Kühnhammer, K.; Kübert, A.; Brüggemann, N.; Diaz, P.D.; Dusschoten, D.V.; Javaux, M.; Merz, S.; Vereecken, H.; Dubbert, M.; Rothfuss, Y. Investigating the root plasticity response of Centaurea jacea to soil water availability changes from isotopic analysis. New Phytol. 2020, 226, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, B.J.; Wang, L.X.; Lu, N.; Li, J.Y. Water use characteristics of the common tree species in different plantation types in the Loess Plateau of China. Agric. For. Meteorol. 2020, 288–289, 108020. [Google Scholar] [CrossRef]
- Deng, Y.; Ke, J.; Wu, S.; Jiang, G.; Zhu, A. Responses of plant water uptake to groundwater depth in limestone outcrops. J. Hydrol. 2020, 590, 125377. [Google Scholar] [CrossRef]
- Ceacero, C.J.; Díaz-Hernández, J.L.; Campo, A.D.; Navarro-Cerrillo, R.M. Soil rock fragment is stronger driver of spatio-temporal soil water dynamics and efficiency of water use than cultural management in holm oak plantations. Soil Till Res. 2020, 197, 104495. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Yang, J. Water source utilization by woody plants growing on dolomite outcrops and nearby soils during dry seasons in karst region of Southwest China. J. Hydrol. 2012, 420, 264–274. [Google Scholar] [CrossRef]
- Drake, P.L.; Franks, P.J. Water resource partitioning, stem xylem hydraulic properties, and plant water use strategies in a seasonally dry riparian tropical rainforest. Oecologia 2013, 137, 321–329. [Google Scholar] [CrossRef]
- Yang, B.; Wen, X.F.; Sun, X.M. Seasonal variations in depth of water uptake for a subtropical coniferous plantation subjected to drought in an East Asian monsoon region. Agric. For. Meteorol. 2015, 201, 218–228. [Google Scholar] [CrossRef]
- Yang, F.T.; Feng, Z.M.; Wang, H.; Dai, X.Q.; Fu, X.L. Deep soil water extraction helps to drought avoidance but shallow soil water uptake during dry season controls the inter-annual variation in tree growth in four subtropical plantations. Agric. For. Meteorol. 2017, 234–235, 106–114. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Yu, X.X.; Jia, G.D. Water uptake by coniferous and broad-leaved forest in a rocky mountainous area of northern China. Agric. For. Meteorol. 2019, 265, 381–389. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Boutaleb, S.; Díaz Barradas, M.C.; Esquivias, M.P.; Valera, J.; Jáuregui, J.; Tagma, T.; Ain-Lhout, F. Reliance on deep soil water in the tree species Argania spinosa. Tree Physiol. 2018, 38, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Gao, J.G.; Zhao, P.; Rao, X.Q. Species-specific transpiration and water use patterns of two pioneer dominant tree species under manipulated rainfall in a low-subtropical secondary evergreen forest. Ecohydrology 2020, 13, e2234. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant-Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant. Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Ding, Y.L.; Nie, Y.P.; Chen, H.S.; Wang, K.L.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2020, 229, 1339–1353. [Google Scholar] [CrossRef]
- Eggemeyer, K.D.; Tala, A.; Edwin, H.F.; David, A.W.; Zhou, X.H.; William, Z.C. Seasonal changes in depth of water uptake for encroaching trees Juniperus virginiana and Pinus ponderosa and two dominant C4 grasses in a semiarid grassland. Tree Physiol. 2009, 29, 157–169. [Google Scholar] [CrossRef]
- Liu, W.N.; Chen, H.S.; Zou, Q.Y.; Nie, Y.P. Divergent root water uptake depth and coordinated hydraulic traits among typical karst plantations of subtropical China: Implication for plant water adaptation under precipitation changes. Agric. Water Manag. 2021, 249, 106798. [Google Scholar] [CrossRef]
- Craven, D.; Hall, J.S.; Ashton, M.S.; Berlyn, G.P. Water-use efficiency and whole-plant performance of nine tropical tree species at two sites with contrasting water availability in Panama. Trees 2013, 27, 639–653. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Allen, M.F.; Santiago, L.S. Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 2010, 164, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Ding, Y.L. Seasonal variations in leaf δ13C values: Implications for different water-use strategies among species growing on continuous dolomite outcrops in subtropical China. Acta Physiolo Plant. 2014, 36, 2571–2579. [Google Scholar] [CrossRef]
- Volkmann, T.; Haberer, K.; Gessler, A.; Weiler, M. High-resolution isotope measurements resolve rapid ecohydrological dynamics at the soil–plant interface. New Phytol. 2016, 210, 839–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruelheide, H.; Nadrowski, K.; Assmann, T.; Bauhus, J.; Both, S.; Buscot, F.; Chen, X.Y.; Ding, B.Y.; Durka, W.; Erfmeier, A. Designing forest biodiversity experiments: General considerations illustrated by a new large experiment in subtropical china. Meth. Ecol Evol. 2014, 5, 74–89. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.Y.; Jiang, Z.H.; Dai, J.Y.; Wu, X.C.; Peng, J. Rock crevices determine woody and herbaceous plant cover in the karst critical zone. Sci. China Earth Sci. 2019, 62, 1756–1763. [Google Scholar] [CrossRef]
- Ding, Y.L.; Nie, Y.P.; Susanne, S.; Chen, H.S.; Yang, J.; Zhang, W.; Wang, K.L. A novel approach for estimating groundwater use by plants in rock-dominated habitats. J. Hydrol. 2018, 565, 760–769. [Google Scholar] [CrossRef]
- Agency, I.A.E. A new device for monthly rainfall sampling for GNIP. Water Environ. News 2002, 16, 5. [Google Scholar]
- Ehleringer, J.R.; Roden, J.; Dawson, T.E. Assessing Ecosystem-Level Water Relations Through Stable Isotope Ratio Analyses. In Methods in Ecosystem Science; Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W., Eds.; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Li, S.G.; Romero-Saltos, H.; Tsujimura, M.; Sugimoto, A.; Sasaki, L.; Davaa, G.; Oyunbaatar, D. Plant water sources in the cold semiarid ecosystem of the upper Kherlen River catchment in Mongolia: A stable isotope approach. J. Hydrol. 2007, 333, 109–117. [Google Scholar] [CrossRef]
- Liu, W.R.; Peng, X.H.; Shen, Y.J.; Chen, X.M. Measurements of hydrogen and oxygen isotopes in liquid water by isotope ratio infrared spectroscopy (IRIS) and their spectral contamination corrections. Chin. J. Ecol. 2013, 32, 1181–1186, (In Chinese with English abstract). [Google Scholar]
- Schultz, N.M.; Griffis, T.J.; Lee, X.; Baker, J.M. Identification and correction of spectral contamination in 2H/1H and 18O/16O measured in leaf, stem, and soil water. Rapid Commun. Mass Sp. 2011, 25, 3360–3368. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual, Version 3.1. Available online: https://cran.r-project.org/web/packages/MixSIAR/readme/README.html (accessed on 14 May 2020).
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plant. Cell Environ. 2010, 15, 1073–1082. [Google Scholar] [CrossRef]
- Tokumoto, I.; Heilman, J.L.; Schwinning, S.; Mcinnes, K.J.; Litvak, M.E.; Morgan, C.; Kamps, R.H. Small-scale variability in water storage and plant available water in shallow, rocky soils. Plant. Soil. 2014, 385, 193–204. [Google Scholar] [CrossRef]
- Fu, T.G.; Chen, H.S.; Fu, Z.Y.; Wang, K.L. Surface soil water content and its controlling factors in a small karst catchment. Environ. Earth Sci. 2016, 75, 1406. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, Y.G.; Fan, D.Y.; Guo, K. Plant drought tolerance assessment for re-vegetation in heterogeneous karst landscapes of southwestern China. Flora 2012, 207, 30–38. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Ding, Y.L.; Zou, Q.Y.; Ma, X.Y.; Wang, K.L. Qualitative identification of hydrologically different water sources used by plants in rock-dominated environments. J. Hydrol. 2019, 573, 386–394. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Jia, G.D.; Yu, X.X. Variation of water uptake in degradation agroforestry shelterbelts on the North China Plain. Agric. Ecosyst. Environ. 2020, 287, 106697. [Google Scholar] [CrossRef]
- Williams, D.G.; Ehleringer, J.R. Intra- and Interspecific Variation for Summer Precipitation Use in Pinyon-Juniper Woodlands. Ecol Monogr. 2000, 70, 517–537. [Google Scholar]
- Li, D.D.; Liu, J.Q.; Anne, V.; Xi, B.Y.; Virginia, H.S. Understanding the relationship between biomass production and water use of Populus tomentosa trees throughout an entire short-rotation. Agric. Water Manag. 2021, 246, 106710. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Dawson, T.E.; Nicolas, E.; Querejeta, J.I. Isotopes reveal contrasting water use strategies among coexisting plant species in a Mediterranean ecosystem. New Phytol. 2012, 196, 489–496. [Google Scholar] [CrossRef]
- Renninger, H.J.; Nicholas, C.; Clark, K.L.; Schfer, K.V.R. Physiological strategies of co-occurring oaks in a water- and nutrient-limited ecosystem. Tree Physiol. 2014, 2, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Gaines, K.P.; Stanley, J.W.; Meinzer, F.C.; Mcculloh, K.A.; Woodruff, D.R.; Weile, C.; Adams, T.S.; Lin, H.; Eissenstat, D.M. Reliance on shallow soil water in a mixed-hardwood forest in central Pennsylvania. Tree Physiol. 2016, 36, 444–458. [Google Scholar] [CrossRef] [Green Version]
- Zhan, L.C.; Chen, J.S.; Li, L.; Xin, P. Plant water use strategies indicated by isotopic signatures of leaf water: Observations in southern and northern China. Agric. For. Meteorol. 2019, 276–277, 107624. [Google Scholar] [CrossRef]
- Sánchez-Costa, E.; Poyatos, R.; Sabaté, S. Contrasting growth and water use strategies in four co-occurring Mediterranean tree species revealed by concurrent measurements of sap flow and stem diameter variations. Agric. For. Meteorol. 2015, 207, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, H.S.; Nie, Y.P.; Wang, K.L. Dynamic variations in profile soil water on karst hillslopes in Southwest China. Catena 2019, 172, 655–663. [Google Scholar] [CrossRef]
- Magh, R.K.; Eiferle, C.; Burzlaff, T.; Dannenmann, M.; Dubbert, M. Competition for water rather than facilitation in mixed beech-fir forests after drying-wetting cycle. J. Hydrol. 2020, 587, 124944. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Jia, G.D.; Yu, X.X. Water uptake and WUE of Apple tree-Corn Agroforestry in the Loess hilly region of China. Agric. Water Manag. 2020, 234, 106138. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Ding, Y.L.; Wang, K.L.; Pugnaire, F. Water source segregation along successional stages in a degraded karst region of subtropical China. J. Veg. Sci. 2018, 29, 933–942. [Google Scholar] [CrossRef]
- Du, H.; Zeng, F.; Song, T.; Liu, K.; Liu, M. Water depletion of climax forests over humid karst terrain: Patterns, controlling factors and implications. Agric. Water Manag. 2021, 244. [Google Scholar] [CrossRef]
- Ellsworth, P.Z.; Sternberg, L.S.L. Seasonal water use by deciduous and evergreen woody species in a scrub community is based on water availability and root distribution. Ecohydrology 2015, 8, 538–551. [Google Scholar] [CrossRef]
- Schwinning, S. The ecohydrology of roots in rocks. Ecohydrology 2010, 3, 238–245. [Google Scholar] [CrossRef]
- Gonzalez, D.; Andres, E.; Camarero, J.J.; Blanco, J.A.; Bosco, I.; Yueh-Hsin, S.-B.; Gabriel, C.; Federico, J. Tree-to-tree competition in mixed European beech-Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J. Ecol. 2018, 106, 59–75. [Google Scholar] [CrossRef] [Green Version]
- Asbjornsen, H. Ecohydrological advances and applications in plant-water relations research: A review. J. Plant. Ecol. 2011, 4, 3–22. [Google Scholar] [CrossRef]
- Hogg, E.H. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar]
- Fan, Z.X.; Thomas, A. Spatiotemporal variability of reference evapotranspiration and its contributing climatic factors in Yunnan Province, SW China, 1961–2004. Clim. Chang. 2013, 116, 309–325. [Google Scholar] [CrossRef]
- Messmer, M.; Simmonds, I. Global analysis of cyclone-induced compound precipitation and wind extreme events. Weather. Clim. Extrem. 2021, 32, 100324. [Google Scholar] [CrossRef]
- Keep, T. To grow or survive: Which are the strategies of a perennial grass to face severe seasonal stress? Funct. Ecol. 2021, 35, 1145–1158. [Google Scholar] [CrossRef]
- Choat, B. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Renninger, H.J.; Carlo, N.J.; Clark, K.L.; Schafer, K.V. Resource use and efficiency, and stomatal responses to environmental drivers of oak and pine species in an Atlantic Coastal Plain forest. Front. Plant. Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Berg, P.; Moseley, C.; Haerter, J.O. Strong increase in convective precipitation in response to higher temperatures. Nat. Geosci. 2013, 6, 181–185. [Google Scholar] [CrossRef]
- Min, S.K.; Zhang, X.; Zwiers, F.W.; Hegerl, G.C. Human contribution to more-intense precipitation extremes. Nature 2011, 470, 378–381. [Google Scholar] [CrossRef]





| Soil Depth (cm) | Edaphic Habitat (g·cm−3) | Rocky Habitat (g·cm−3) |
|---|---|---|
| 0–10 | 0.90 | 0.88 |
| 10–20 | 0.97 | 0.85 |
| 20–30 | 1.09 | 0.91 |
| 30–50 | 1.13 | 1.03 |
| 50–70 | 1.01 | 0.88 |
| 70–90 | 0.91 | - |
| T (℃) | RH (%) | VPD (KPa) | PARmax (umol·m−2·s−1) | |
|---|---|---|---|---|
| May-NP | 26.6 | 63 | 1.29 | 1336.4 |
| June-HP | 30.1 | 74 | 1.11 | 1074.8 |
| August-LP | 31.1 | 66 | 1.54 | 1503.7 |
| October-DP | 20.9 | 68 | 0.79 | 1194.1 |
| Pearson Correlation | Edaphic Habitat (−MPa) | Rocky Habitat (−MPa) | ||
|---|---|---|---|---|
| C. lanceolata | P. massoniana | C. lanceolata | P. massoniana | |
| Shallow layer | −0.41, p > 0.05 | 0.77, p > 0.05 | 0.328, p > 0.05 | −0.825, p > 0.05 |
| Middle layer | 0.152, p > 0.05 | −0.837, p > 0.05 | −0.411, p > 0.05 | −0.78, p > 0.05 |
| Deep layer | 0.441, p > 0.05 | −0.562, p > 0.05 | −0.064, p > 0.05 | −0.886, p > 0.05 |
| Edaphic Habitat (‰) | Rocky Habitat (‰) | |||
|---|---|---|---|---|
| C. lanceolata | P. massoniana | C. lanceolata | P. massoniana | |
| May-NP | −25.78 ± 0.85Aa | −26.67 ± 0.73Aa | −25.66 ± 0.57Aa | −26.93 ± 0.05Aa |
| June-HP | −27.07 ± 0.41Ba | −27.75 ± 0.85Ba | −27.95 ± 1.26Ba | −28.31 ± 0.18Aa |
| August-LP | −27.65 ± 0.69Ba | −28.8 ± 0.42Ba | −27.43 ± 0.18Ba | −29.51 ± 0.27Bb |
| October-DP | −27.22 ± 0.47Ba | −28.23 ± 0.18Bb | −27.86 ± 0.64Ba | −28.86 ± 0.25Ba |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Chen, Y.; Lu, J.; Yan, W. Water Uptake Pattern by Coniferous Forests in Two Habitats Linked to Precipitation Changes in Subtropical Monsoon Climate Region, China. Forests 2022, 13, 708. https://doi.org/10.3390/f13050708
Jia J, Chen Y, Lu J, Yan W. Water Uptake Pattern by Coniferous Forests in Two Habitats Linked to Precipitation Changes in Subtropical Monsoon Climate Region, China. Forests. 2022; 13(5):708. https://doi.org/10.3390/f13050708
Chicago/Turabian StyleJia, Jianbo, Yu Chen, Jia Lu, and Wende Yan. 2022. "Water Uptake Pattern by Coniferous Forests in Two Habitats Linked to Precipitation Changes in Subtropical Monsoon Climate Region, China" Forests 13, no. 5: 708. https://doi.org/10.3390/f13050708






