Quality Analysis and Comprehensive Evaluation of Fruits from Different Cultivars of Pecan (Carya illinoinensis (Wangenheim) K. Koch)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Their Origins
2.2. Sample Determination
2.2.1. Crude Fat Content
2.2.2. Crude Protein Content
2.2.3. Soluble Sugar Content
2.2.4. Tannin Content
2.2.5. Fatty Acid Compositions
2.2.6. Composition of Amino Acids
2.2.7. Metallic Elements
2.3. Data Process
3. Results
3.1. Analysis of the Principal Components of Pecan Kernels
3.1.1. Fatty Acid Composition Analysis
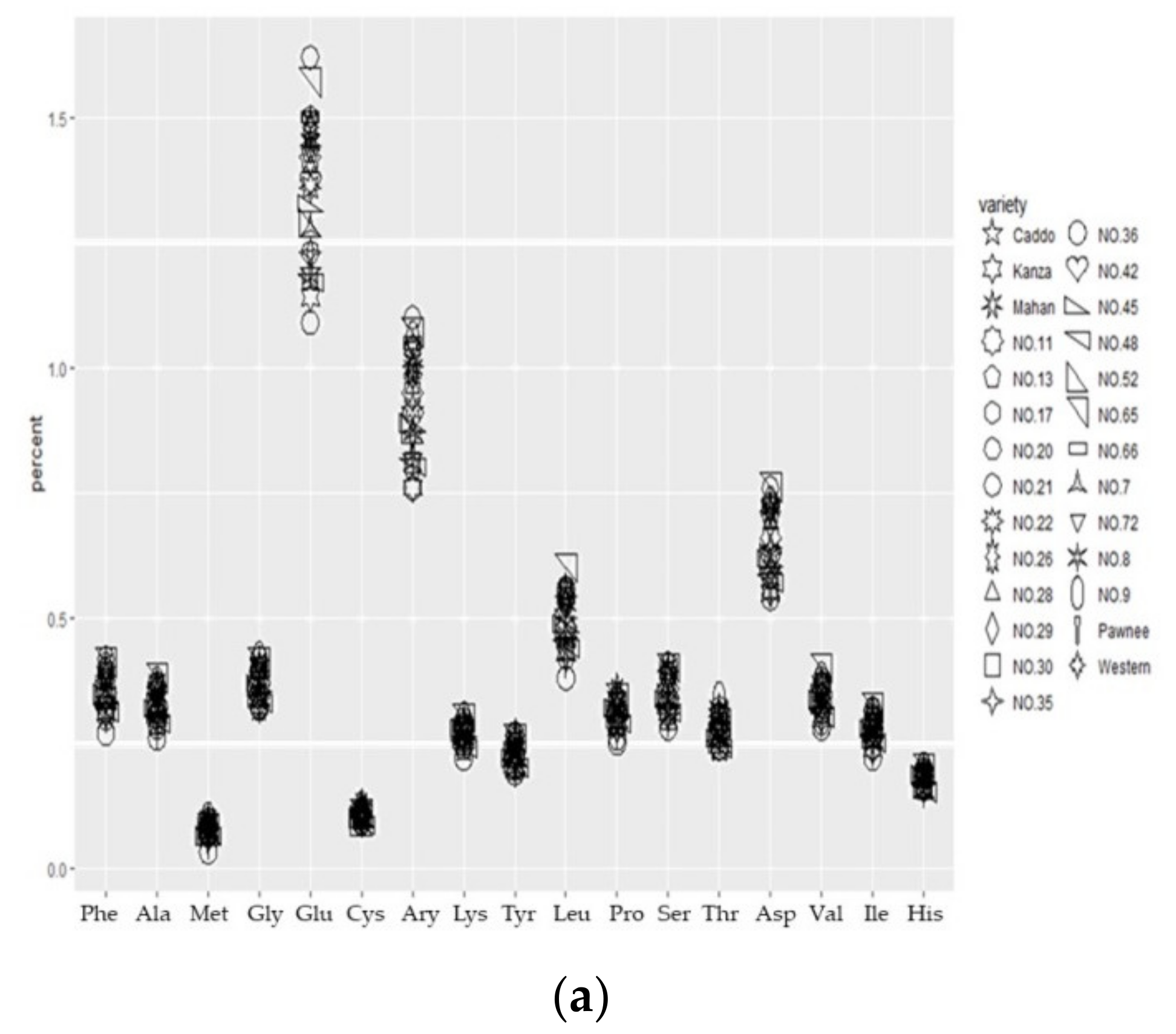
3.1.2. Amino Acid Composition Analysis
3.1.3. Mineral Element Analysis
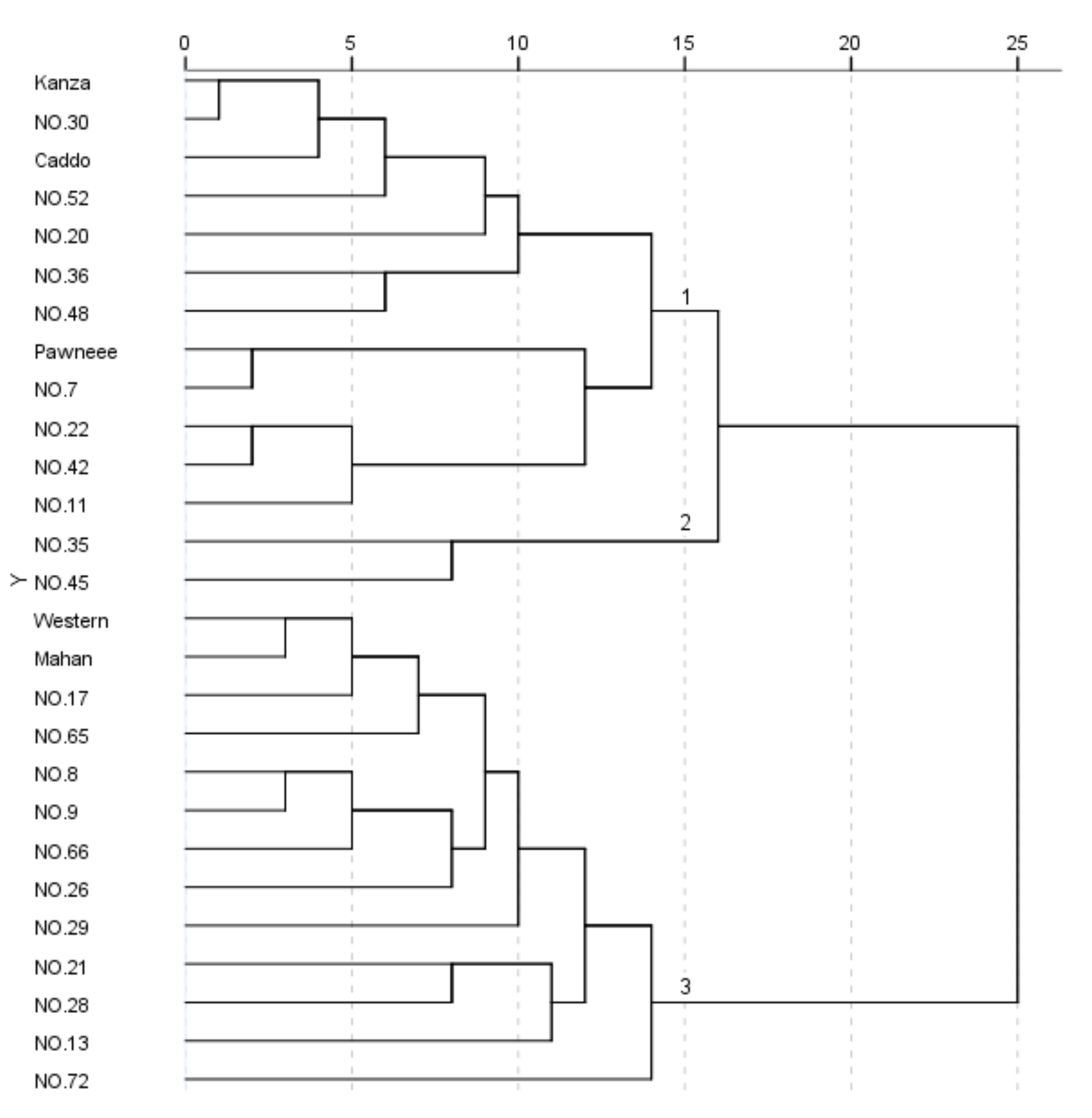
3.2. Cluster Analysis and Differential Characteristic Index Screening of Pecan Fruit
3.2.1. Cluster Analysis of Pecan Fruit
3.2.2. Screening of Differential Trait Indexes in Different Cultivars of Pecan Fruits
3.3. Evaluation of Economic Traits and Model Construction for Pecan Fruits from Different Cultivars
4. Discussion
4.1. Differential Analysis of Nutritional Traits in Different Cultivars of Pecan Fruits
4.2. Comprehensive Evaluation of Fruit Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Yao, X.H.; Wang, K.L.; Gu, X.R. A study on simplifying the indices of the clone fruit characters of Carya illinoinensis. Acta Agric. Univ. Jiangxiensis 2011, 33, 696–700. [Google Scholar]
- Li, C.; Yao, X.H.; Wang, K.L.; Fang, M.Y.; Gu, X.R.; Shao, W.Z. A comparative study on the fruit and nut characters of 12 pecan (Carya illinoemis) Clones and Their Yield. J. Southwest Univ. 2011, 33, 40–44. [Google Scholar]
- Chen, F.; Yao, X.H.; Wang, K.L.; Ren, H.D.; Chang, J. Comparative studies on fruit and nut characters of 33 pecan(Carya illinoemis) clones and their yields. J. Cent. South Univ. For. Technol. 2016, 36, 40–45. [Google Scholar]
- Chen, F.; Yao, X.H.; Teng, J.H.; Ren, H.D.; Wang, K.L.; Chang, J. Comparison of economic characters and quality of Carya illinoinensis clones. J. Chin. Cereals Oils Assoc. 2016, 31, 68–73. [Google Scholar]
- Chang, J.; Ren, H.D.; Yao, X.H.; Yang, S.P.; Wang, K.L. Analysis of dynamic changes of oil and mineral nutrients in pecan at the late stage of fruit development. For. Sci. Res. 2019, 32, 122–129. [Google Scholar]
- Yu, M.; Xu, H.H.; Wang, Z.j.; Si, J.P.; Zhang, A.L. Study on composition and antioxidant activity of Carya cathayensis oil. J. Chin. Cereals Oils Assoc. 2016, 31, 86–90. [Google Scholar]
- Zhou, W.J.; Li, J.; Liu, X. A comparative analysis of the economic characters of 30 pecan varieties in hunan province. Acta Agric. Univ. Jiangxiensis 2021, 43, 807–816. [Google Scholar]
- Yao, X.H.; Chang, J.; Wang, K.L. The Research Proceeding of Pecan in China. Science Press: Zhejiang, China, 2014; pp. 73–158. [Google Scholar]
- Chang, J.; Yao, X.H.; Shao, W.Z.; Chen, H.W.; Wang, K.L.; Chen, F. Effects of different rootstocks of Carya illinoemis on grafting survival rate and growth indexs. J. Cent. South Univ. For. Technol. 2016, 36, 56–60. [Google Scholar]
- Ma, C.G.; Clonal Forestry and Clonal Breeding. National Forest Genetics and breeding academic annual meeting of China forestry society one thousand nine hundred and eighty-six. 1986. Available online: http://www.cnki.com.cn/Article/CJFDTotal-HLKJ198603000.htm (accessed on 26 September 2021).
- Jiang, Y.; Wei, H.L.; Gao, C.H.; Wang, D.G.; Feng, N.K.; Li, R.; Liu, R.R.; Lv, F.D. Observation on flowering phenology and variety combination of Carya illinoinensis in low mountains and hills of Hunan Province. J. Nanjing For. Univ. 2021, 45, 53–62. [Google Scholar]
- Luo, Q.; Xi, X.L.; Zou, W.L.; Fan, Z.Y.; Xv, L.; Ye, C.; Zhang, Q.Q. Breeding of Carya illinoensis ‘barton’ variety. J. West China For. Sci. 2021, 50, 154–162. [Google Scholar]
- Bi, H.H.; Wang, Z.C.; Fu, S.L.; Zhang, Y.B.; Li, J. Effects of different measures on Carya illinois seedlings in South Anhui. Non-Wood For. Res. 2018, 36, 118–122. [Google Scholar]
- Chang, J.; Zhang, X.D.; Yao, X.H.; Yang, S.P.; Wang, K.L.; Ren, H.D. Amino acid composition and nutritional value evaluation of different varieties of Pecan (Carya illinoensis K. Koch). J. Southwest Univ. 2021, 43, 44–52. [Google Scholar]
- Huang, X.Y.; He, P.; Zhang, T.; Song, H.Y.; Zhen, S.F.; Qin, Z.S.; Wang, W.L. Observation and comprehensive evaluation of pecan fruit quality in Guangxi. Southern Agric. 2020, 14, 1–7. [Google Scholar]
- Liang, S.S.; LV, F.D.; Jiang, Y.; Li, J.A.; Wang, S.; Jiang, S.F.; Li, F.S. Principal component analysis and comprehensive evaluation of american pecan nuts. South China Fruits 2015, 44, 123–128. [Google Scholar]
- Guo, K.H. The limited role of PCA in overcoming multiple correlations of variables. Comput. Appl. 2007, 27, 2346–2348. [Google Scholar]
- Tian, T.; Wei, J.J.; Wen, J.H.; Zeng, X.L. Seasonal variability of aroma components of lingyun pekoe green tea. Food Sci. 2020, 41, 252–259. [Google Scholar]
- Zhou, B.B.; Zhang, Q.; Sun, J.; Li, X.L.; Wei, Q.P. Study and application of partial least squares regression on relationship between soil nutrient and fruit quality. Agric. Sci. Technol. 2016, 33, 106–112. [Google Scholar]
- Wu, X.Y.; Xie, Q.S.; Li, Q.Y.; Dong, P.Y.; Hai, Y.D.; Fei, Y.L.; Hui, X.L.; Chun, L. Study on quality evaluation of seabuckthorn(Hippophae rhamnoides L.)seed oil based on determination of 7 kinds of fatty acids content and chemometrics. J. Food Saf. Qual. 2021, 12, 8128–8135. [Google Scholar]
- Zhao, X.Q.; Guo, S.; Lu, Y.Y.; Zhang, F.; Yan, H.; Wang, H.Q.; Duan, J.A. Analysis of water-soluble nutrients in Lycium barbarum leaves and differences between different producing areas. China J. Chin. Mater. Med. 2021, 46, 2084–2093. [Google Scholar]
- Liu, M.J. Studies on the variations and probability gradings of major quantitative characters of Chinese jujube. Acta Hortic. Sin. 1996, 23, 105–109. [Google Scholar]
- Cheng, J.Y.; Li, L.; Zhou, X.H.; Luo, Z.J.; Tu, B.K. Compositions of fatty acids and its correlation among superior trees of Camellia oleifera Abel. For. Sci. Technol. Dev. 2010, 24, 41–43. [Google Scholar]
- Yan, H.Q.; Hou, C.J.; Ma, J.Y.; Deng, Y.; Wang, Q.; Jin, F. Phenotypic character and fatty acid composition and content of olive fruit in different varieties and maturity. China Oils Fats 2019, 44, 105–111. [Google Scholar]
- Venkatachalam, M.; Kshirsagar, H.H.; Seeram, N.P.; Heber, D.; Thompson, T.E.; Roux, K.H.; Sathe, S.K. Biochemical composition and immunological comparison of select pecan Carya illinoinensis (wangenh.) k. koch. cultivarsj. J. Agric. Food Chem. 2007, 55, 9899–9907. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Wang, Z.J.; Xia, G.H.; Huang, J.Q.; Liu, L. Fat content and fatty acid composition of ten Carya illinoensis cultivars. J. Zhejiang A&F Univ. 2013, 30, 714–718. [Google Scholar]
- Wang, W.; Wei, L.P. Analysis of fatty acid compositions in prunus mira kernels from different growing areas in tibet. Food Sci. 2016, 37, 107–111. [Google Scholar]
- Zhang, C.; Zhang, H.; Liu, C.Y.; Xue, W.T.; Liu, R. Research progress of linoleic acid. J. Grain Oil 2010, 5, 18–21. [Google Scholar]
- Wang, J.; Li, Z.G.; Hu, W.; Qiao, H.F.; Mo, W.M.; Hu, B.X. Fatty acid analysis of soybean oil by GC—MS. J. Zhejiang Univ. Sci. Technol. 2003, 15, 16–18. [Google Scholar]
- Wu, M.X.; Wu, G.Y.; Han, Y.; Zhang, P.D. Comparative study on fatty acid composition of Six Edible vegetable oils and their biodiesel. China Grease 2003, 38, 65–67. [Google Scholar]
- Li, H.Y.; Deng, Z.Y.; Li, J.; Fan, Y.W.; Liu, R.; Xiong, H.; Xie, M.Y. Study on oxidative stability of vegetable oil with different fatty acid composition. Food Ind. Sci. Technol. 2010, 31, 173–175. [Google Scholar]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.W. Attention should be paid to the development and utilization of oil rich in linolenic acid. Mach. Cereals Oil Food Process. 2004, 9, 13–14. [Google Scholar]
- Xing, C.H.; Li, J.W.; Wang, X.G.; Jin, Q.Z. Determination of fatty acid composition and vitamin E concentration of Camellia oleifera seed oil by chromatography. J. Food Biotechnol. 2011, 30, 838–842. [Google Scholar]
- Xi, R.C.; Deng, X.M.; Gong, C.; Liu, S.; Gong, C.; Liu, S.; Ao, W.C. Studies on selecting and breeding of high linoleic acid content and high oil yield oiltea camellia clones. For Res. 2006, 19, 158. [Google Scholar]
- Zhu, W.Z.; Fan, J.R.; Peng, J.G.; Yang, H.B.; Yang, B.N.; He, M.B. Analysis of the oil content and its fatty acid composition of fruits for introduced olive cultivars in sichuan province. For. Sci. 2010, 46, 91–100. [Google Scholar]
- Zhang, T.Z. Nutrition, biological characteristics and development and utilization status of Carya cathayensis. Food Ferment. Ind. 2006, 32, 90–93. [Google Scholar]
- Yu, M.; Xu, H.H.; Wang, Z.J.; Si, J.P.; Zhang, A.L. Analysis of morphology and nutritional components of six Carya illinoinensis Varieties. J. Chin. Cereals Oils Assoc. 2013, 28, 74–77. [Google Scholar]
- Bauer, I.; Graessle, S.; Loidl, P.; Hohenstein, K.; Brosch, G. Novel insights into the functional role of three protein arginine methyltransferases in Aspergillus nidulans. Fungal Genet. Biol. 2010, 47, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M. Effects of mineral elements in tea on human health. China Tea 2002, 24, 30–31. [Google Scholar]


| Step | Temperature (℃) | Ramp Time (min) | Hold Time (min) | |
|---|---|---|---|---|
| 1 | 130 | 15 | 10 | |
| 2 | 200 | 10 | 20 |
| Cultivar | Crude Fat (%) | Crude Protein (%) | Soluble Sugar (%) | Tannin (×10−3 mg/kg) |
|---|---|---|---|---|
| Western | 65.50 ± 2.86 | 7.92 ± 0.85 | 3.47 ± 0.71 | 7.60 ± 0.34 |
| Mahan | 61.19 ± 1.72 | 7.52 ± 0.79 | 3.46 ± 0.60 | 3.93 ± 2.12 |
| Pawnee | 63.28 ± 4.32 | 7.24 ± 0.95 | 4.62 ± 0.75 | 5.57 ± 0.7 |
| No. 7 | 61.09 ± 4.00 | 7.07 ± 0.40 | 4.83 ± 0.37 | 5.46 ± 0.54 |
| No. 8 | 66.56 ± 1.18 | 8.13 ± 1.12 | 3.67 ± 1.30 | 7.27 ± 1.68 |
| No. 9 | 65.54 ± 0.68 | 8.34 ± 0.94 | 3.73 ± 0.34 | 5.42 ± 3.54 |
| No. 11 | 62.59 ± 10.43 | 6.55 ± 1.32 | 4.57 ± 0.58 | 7.03 ± 1.21 |
| Kanza | 65.56 ± 1.22 | 7.32 ± 0.59 | 4.29 ± 0.34 | 4.91 ± 0.09 |
| No. 17 | 60.22 ± 0.74 | 8.05 ± 0.59 | 4.16 ± 0.76 | 6.92 ± 0.32 |
| No. 20 | 62.48 ± 5.86 | 7.91 ± 1.69 | 3.80 ± 0.28 | 5.62 ± 0.43 |
| No. 21 | 62.28 ± 7.19 | 7.60 ± 1.17 | 4.11 ± 1.31 | 8.02 ± 1.26 |
| No. 22 | 53.78 ± 2.99 | 7.44 ± 0.80 | 4.59 ± 0.71 | 5.36 ± 0.81 |
| No. 26 | 69.70 ± 1.77 | 7.87 ± 0.24 | 2.63 ± 0.90 | 7.42 ± 1.61 |
| No. 28 | 67.33 ± 4.91 | 7.22 ± 0.74 | 3.81 ± 0.74 | 6.79 ± 1.65 |
| No. 29 | 65.08 ± 1.57 | 7.41 ± 1.28 | 4.42 ± 0.28 | 3.47 ± 1.68 |
| No. 30 | 63.99 ± 0.30 | 7.04 ± 0.36 | 3.93 ± 0.60 | 4.97 ± 0.49 |
| Caddo | 68.78 ± 1.13 | 7.09 ± 0.61 | 2.90 ± 0.17 | 5.88 ± 0.47 |
| No. 35 | 65.12 ± 2.55 | 7.55 ± 0.22 | 2.81 ± 0.34 | 7.24 ± 0.53 |
| No. 36 | 65.80 ± 0.64 | 7.05 ± 0.68 | 4.07 ± 0.54 | 7.68 ± 1.16 |
| No. 42 | 56.08 ± 1.95 | 7.52 ± 0.94 | 3.68 ± 0.13 | 6.35 ± 0.90 |
| No. 45 | 62.47 ± 3.00 | 9.15 ± 1.71 | 4.09 ± 0.80 | 5.45 ± 1.02 |
| No. 48 | 64.23 ± 2.76 | 7.44 ± 1.08 | 4.01 ± 1.48 | 7.39 ± 1.85 |
| No. 52 | 61.75 ± 1.83 | 7.30 ± 0.56 | 4.11 ± 0.79 | 4.09 ± 2.01 |
| No. 65 | 62.80 ± 1.68 | 7.01 ± 1.38 | 3.25 ± 0.68 | 6.90 ± 0.51 |
| No. 66 | 60.92 ± 5.08 | 7.76 ± 0.27 | 3.17 ± 1.05 | 6.98 ± 2.30 |
| No. 72 | 57.99 ± 1.67 | 9.57 ± 1.33 | 3.83 ± 0.12 | 8.38 ± 0.15 |
| No. 13 | 68.42 | 7.09 | 3.65 | 7.59 |
| Average | 63.35 ± 3.69 | 7.60 ± 0.65 | 3.84 ± 0.56 | 6.29 ± 1.32 |
| CV% | 5.72 | 8.40 | 14.38 | 20.59 |
| Cultivar | Palmitic Acid C16:0 | Stearic Acid C18:0 | Oil Oleic Acid C18:1 | Linoleic Acid C18:2 | Linolenic Acid C18:3 | Arachidic Acid C20:0 | Cis-11-20c C20:1 |
|---|---|---|---|---|---|---|---|
| Western | 5.65 ± 0.15 | 2.90 ± 0.10 | 66.55 ± 0.95 | 23.40 ± 1.10 | 1.20 ± 0.00 | 0.10 ± 0.00 | 0.20 ± 0.00 |
| Mahan | 5.70 ± 0.20 | 2.95 ± 0.05 | 66.25 ± 0.65 | 23.40 ± 0.40 | 1.40 ± 0.10 | 0.10 ± 0.00 | 0.25 ± 0.05 |
| Pawnee | 4.03 ± 2.85 | 2.23 ± 0.12 | 65.63 ± 1.67 | 24.27 ± 1.66 | 1.43 ± 0.12 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 7 | 3.73 ± 2.64 | 2.23 ± 0.05 | 68.57 ± 0.90 | 22.00 ± 0.85 | 1.23 ± 0.05 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 8 | 5.17 ± 0.52 | 2.53 ± 0.12 | 73.70 ± 4.52 | 17.17 ± 4.02 | 1.00 ± 0.22 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 9 | 5.20 ± 0.20 | 2.90 ± 0.60 | 72.75 ± 2.35 | 17.75 ± 2.75 | 1.00 ± 0.10 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 11 | 6.00 ± 0.10 | 2.25 ± 0.05 | 62.50 ± 1.00 | 27.25 ± 0.95 | 1.60 ± 0.20 | 0.10 ± 0.00 | 0.25 ± 0.05 |
| Kanza | 5.27 ± 0.21 | 2.03 ± 0.17 | 70.87 ± 1.93 | 20.37 ± 1.46 | 1.10 ± 0.14 | 0.05 ± 0.05 | 0.30 ± 0.00 |
| No. 17 | 5.45 ± 0.05 | 2.15 ± 0.05 | 67.10 ± 1.10 | 23.70 ± 1.00 | 1.20 ± 0.10 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 20 | 5.97 ± 0.25 | 2.17 ± 0.17 | 62.83 ± 1.95 | 27.10 ± 1.56 | 1.57 ± 0.17 | 0.10 ± 0.00 | 0.27 ± 0.05 |
| No. 21 | 5.97 ± 0.25 | 2.10 ± 0.14 | 63.53 ± 2.28 | 26.53 ± 2.15 | 1.57 ± 0.09 | 0.10 ± 0.00 | 0.27 ± 0.05 |
| No. 22 | 5.80 ± 0.29 | 1.93 ± 0.12 | 62.57 ± 1.44 | 27.67 ± 1.07 | 1.60 ± 0.08 | 0.10 ± 0.00 | 0.27 ± 0.05 |
| No. 26 | 5.20 ± 0.20 | 2.65 ± 0.05 | 67.85 ± 2.95 | 22.80 ± 2.60 | 1.10 ± 0.20 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 28 | 6.30 ± 0.60 | 2.10 ± 0.20 | 63.80 ± 5.50 | 25.90 ± 4.20 | 1.55 ± 0.55 | 0.10 ± 0.00 | 0.20 ± 0.00 |
| No. 29 | 5.70 ± 0.22 | 2.40 ± 0.08 | 69.73 ± 1.50 | 20.77 ± 1.25 | 1.13 ± 0.12 | 0.10 ± 0.00 | 0.27 ± 0.05 |
| No. 30 | 5.60 ± 0.20 | 2.25 ± 0.15 | 70.00 ± 1.90 | 20.65 ± 1.85 | 1.05 ± 0.05 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| Caddo | 5.60 ± 0.00 | 2.50 ± 0.00 | 69.80 ± 0.20 | 20.65 ± 0.15 | 1.05 ± 0.05 | 0.10 ± 0.00 | 0.25 ± 0.05 |
| No. 35 | 5.40 ± 0.10 | 2.50 ± 0.10 | 73.10 ± 0.00 | 17.55 ± 0.05 | 1.00 ± 0.00 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 36 | 5.50 ± 0.10 | 2.70 ± 0.10 | 65.70 ± 2.60 | 24.55 ± 2.25 | 1.20 ± 0.10 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 42 | 6.07 ± 0.24 | 2.17 ± 0.17 | 65.13 ± 2.27 | 24.83 ± 2.11 | 1.43 ± 0.05 | 0.10 ± 0.00 | 0.20 ± 0.00 |
| No. 45 | 5.17 ± 0.17 | 2.37 ± 0.05 | 70.90 ± 1.35 | 20.03 ± 1.16 | 1.13 ± 0.12 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 48 | 5.20 ± 0.40 | 2.65 ± 0.25 | 71.45 ± 4.25 | 19.00 ± 4.00 | 1.25 ± 0.15 | 0.10 ± 0.00 | 0.25 ± 0.05 |
| No. 52 | 5.80 ± 0.30 | 2.65 ± 0.25 | 68.50 ± 1.20 | 21.50 ± 1.30 | 1.25 ± 0.05 | 0.05 ± 0.05 | 0.25 ± 0.05 |
| No. 65 | 5.20 ± 0.20 | 2.50 ± 0.10 | 69.05 ± 2.65 | 21.60 ± 2.70 | 1.25 ± 0.05 | 0.10 ± 0.00 | 0.25 ± 0.05 |
| No. 66 | 4.73 ± 0.31 | 2.80 ± 0.00 | 73.97 ± 0.62 | 17.17 ± 0.29 | 0.87 ± 0.05 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 72 | 5.35 ± 0.05 | 2.75 ± 0.05 | 66.55 ± 0.75 | 23.70 ± 0.70 | 1.20 ± 0.10 | 0.10 ± 0.00 | 0.30 ± 0.00 |
| No. 13 | 6.00 | 2.20 | 64.90 | 25.40 | 1.10 | 0.10 | 0.30 |
| Average | 5.44 ± 0.58 | 2.43 ± 0.29 | 67.90 ± 3.50 | 22.47 ± 3.18 | 1.24 ± 0.21 | 0.10 ± 0.02 | 0.27 ± 0.03 |
| CV% | 10.39 | 11.84 | 5.05 | 13.88 | 16.59 | 23.57 | 12.12 |
| Cultivar | K | Mg | Ca | Zn |
|---|---|---|---|---|
| Western | 3886.33 ± 83.74 | 719.30 ± 80.07 | 647.13 ± 33.33 | 38.00 ± 1.41 |
| Mahan | 3757.30 ± 67.07 | 804.40 ± 59.21 | 612.67 ± 53.66 | 37.00 ± 3.74 |
| Pawnee | 3673.63 ± 509.35 | 754.57 ± 55.70 | 777.23 ± 25.20 | 42.67 ± 4.50 |
| No. 7 | 3912.00 ± 246.91 | 822.47 ± 58.68 | 855.93 ± 101.69 | 44.67 ± 5.91 |
| No. 8 | 4280.67 ± 390.58 | 826.17 ± 80.38 | 787.03 ± 258.89 | 44.33 ± 2.49 |
| No. 9 | 4050.60 ± 375.44 | 827.77 ± 14.60 | 702.53 ± 115.31 | 41.00 ± 2.16 |
| No. 11 | 4264.60 ± 146.82 | 789.07 ± 59.30 | 834.37 ± 120.97 | 46.67 ± 9.74 |
| Kanza | 3869.23 ± 162.01 | 774.30 ± 27.79 | 893.47 ± 73.27 | 40.33 ± 2.36 |
| No. 17 | 3693.00 ± 375.10 | 762.00 ± 72.90 | 827.05 ± 45.75 | 37.00 ± 1.00 |
| No. 20 | 4364.73 ± 511.55 | 698.47 ± 68.38 | 830.07 ± 90.35 | 44.00 ± 3.56 |
| No. 21 | 4034.47 ± 372.85 | 711.33 ± 39.32 | 965.33 ± 188.66 | 44.33 ± 8.01 |
| No. 22 | 4218.17 ± 182.40 | 720.13 ± 52.78 | 866.83 ± 99.58 | 43.67 ± 2.62 |
| No. 26 | 4123.20 ± 281.04 | 861.93 ± 75.56 | 1007.70 ± 55.17 | 44.00 ± 0.82 |
| No. 28 | 3899.90 ± 195.03 | 807.70 ± 82.32 | 605.27 ± 34.82 | 45.33 ± 2.87 |
| No. 29 | 4578.70 ± 593.47 | 817.60 ± 84.18 | 713.93 ± 13.58 | 41.33 ± 4.19 |
| No. 30 | 3832.47 ± 148.96 | 811.23 ± 4.38 | 815.93 ± 172.74 | 40.00 ± 2.94 |
| Caddo | 4173.07 ± 111.28 | 742.37 ± 7.58 | 882.03 ± 48.13 | 37.33 ± 4.50 |
| No. 35 | 4229.87 ± 242.66 | 922.60 ± 210.48 | 706.00 ± 19.09 | 42.00 ± 6.48 |
| No. 36 | 4196.40 ± 415.75 | 870.97 ± 57.56 | 673.57 ± 79.92 | 43.00 ± 0.82 |
| No. 42 | 4108.93 ± 321.84 | 712.23 ± 59.74 | 849.80 ± 36.68 | 40.33 ± 4.50 |
| No. 45 | 4181.43 ± 810.44 | 910.3 ± 125.52 | 862.23 ± 50.02 | 46.67 ± 1.70 |
| No. 48 | 4014.87 ± 363.02 | 848.07 ± 90.03 | 728.80 ± 105.94 | 47.33 ± 4.50 |
| No. 52 | 3711.00 ± 118.95 | 793.47 ± 17.07 | 708.73 ± 46.18 | 43.33 ± 3.30 |
| No. 65 | 3899.90 ± 577.27 | 981.43 ± 94.57 | 600.60 ± 82.97 | 39.67 ± 9.39 |
| No. 66 | 4188.20 ± 143.15 | 923.93 ± 92.23 | 702.90 ± 37.03 | 43.00 ± 2.94 |
| No. 72 | 4036.60 ± 292.10 | 857.10 ± 13.50 | 1148.30 ± 7.80 | 43.00 ± 3.00 |
| No. 13 | 4125.97 | 696.23 | 661.50 | 41.67 |
| Average | 4048.34 ± 220.85 | 806.19 ± 75.59 | 787.66 ± 130.20 | 42.28 ± 2.90 |
| CV% | 5.35 | 9.20 | 16.22 | 6.74 |
| Cultivar | Cu | Mn | Fe | Na |
| Western | 6.67 ± 2.35 | 65.60 ± 17.55 | 65.60 ± 17.55 | 1.00 ± 0.28 |
| Mahan | 5.33 ± 1.74 | 58.13 ± 7.08 | 58.13 ± 7.08 | 0.93 ± 0.45 |
| Pawnee | 9.00 ± 2.12 | 42.77 ± 11.48 | 42.77 ± 11.48 | 1.20 ± 0.45 |
| No. 7 | 10.40 ± 2.99 | 53.93 ± 15.72 | 53.93 ± 15.72 | 1.30 ± 0.22 |
| No. 8 | 7.90 ± 3.10 | 55.00 ± 11.63 | 55.00 ± 11.63 | 1.30 ± 0.37 |
| No. 9 | 5.20 ± 1.56 | 50.37 ± 5.99 | 50.37 ± 5.99 | 1.13 ± 0.39 |
| No. 11 | 6.83 ± 3.81 | 37.03 ± 7.11 | 37.03 ± 7.11 | 1.23 ± 0.46 |
| Kanza | 7.23 ± 2.15 | 45.27 ± 15.45 | 45.27 ± 15.45 | 1.23 ± 0.24 |
| No. 17 | 6.95 ± 0.65 | 40.55 ± 5.75 | 40.55 ± 5.75 | 1.15 ± 0.15 |
| No. 20 | 5.70 ± 0.73 | 41.43 ± 14.88 | 41.43 ± 14.88 | 0.90 ± 0.24 |
| No. 21 | 6.63 ± 0.48 | 43.30 ± 4.42 | 43.30 ± 4.42 | 1.10 ± 0.14 |
| No. 22 | 5.97 ± 0.63 | 43.97 ± 4.44 | 43.97 ± 4.44 | 1.17 ± 0.05 |
| No. 26 | 6.20 ± 2.33 | 50.90 ± 8.61 | 50.90 ± 8.61 | 0.87 ± 0.25 |
| No. 28 | 6.67 ± 0.61 | 51.53 ± 6.18 | 51.53 ± 6.18 | 1.40 ± 0.33 |
| No. 29 | 6.63 ± 0.19 | 52.60 ± 11.15 | 52.60 ± 11.15 | 0.93 ± 0.17 |
| No. 30 | 6.23 ± 2.30 | 53.47 ± 7.87 | 53.47 ± 7.87 | 0.93 ± 0.31 |
| Caddo | 4.97 ± 1.57 | 56.17 ± 5.60 | 56.17 ± 5.60 | 0.87 ± 0.37 |
| No. 35 | 6.57 ± 3.19 | 89.63 ± 12.36 | 89.63 ± 12.36 | 1.03 ± 0.58 |
| No. 36 | 6.13 ± 2.15 | 30.57 ± 0.83 | 30.57 ± 0.83 | 1.20 ± 0.36 |
| No. 42 | 6.03 ± 1.23 | 32.87 ± 8.56 | 32.87 ± 8.56 | 1.13 ± 0.49 |
| No. 45 | 9.00 ± 2.06 | 66.57 ± 25.98 | 66.57 ± 25.98 | 1.20 ± 0.28 |
| No. 48 | 8.23 ± 0.76 | 42.80 ± 11.04 | 42.80 ± 11.04 | 1.20 ± 0.43 |
| No. 52 | 5.83 ± 1.97 | 42.10 ± 4.58 | 42.10 ± 4.58 | 1.00 ± 0.37 |
| No. 65 | 6.70 ± 3.24 | 63.97 ± 17.90 | 63.97 ± 17.90 | 0.93 ± 0.48 |
| No. 66 | 8.30 ± 1.99 | 75.53 ± 18.48 | 75.53 ± 18.48 | 1.30 ± 0.29 |
| No. 72 | 8.65 ± 2.75 | 49.70 ± 0.90 | 49.70 ± 0.90 | 1.05 ± 0.25 |
| No. 13 | 4.50 | 40.03 | 7.63 | 0.80 |
| Average | 6.83 ± 1.38 | 50.95 ± 13.08 | 10.65 ± 1.64 | 1.09 ± 0.16 |
| CV% | 19.88 | 25.19 | 15.10 | 14.42 |
| Index | Component | |||
|---|---|---|---|---|
| RC1 | RC2 | RC3 | RC4 | |
| Oleic acid P3 | −0.010 | 0.983 | −0.038 | 0.014 |
| Linoleic acid P4 | 0.007 | −0.977 | 0.048 | −0.025 |
| Linolenic acid P5 | −0.037 | −0.891 | 0.192 | −0.086 |
| Magnesium P9 | 0.059 | 0.661 | 0.317 | 0.372 |
| Manganese P11 | 0.198 | 0.619 | 0.104 | 0.503 |
| Crude fat P14 | −0.018 | 0.320 | −0.829 | 0.193 |
| Crude protein P15 | 0.312 | 0.294 | 0.513 | 0.023 |
| Soluble sugar P16 | −0.177 | −0.309 | 0.259 | −0.828 |
| Aspartic acid P18 | 0.968 | 0.027 | 0.037 | 0.199 |
| Threonine P19 | 0.880 | 0.101 | −0.036 | −0.203 |
| Serine P20 | 0.983 | 0.010 | 0.013 | 0.132 |
| Glutamic acid P21 | 0.959 | −0.118 | 0.143 | 0.119 |
| Glycine P22 | 0.977 | 0.021 | 0.033 | −0.017 |
| Alanine P23 | 0.971 | 0.076 | 0.006 | 0.090 |
| Valine P24 | 0.944 | 0.190 | 0.146 | 0.131 |
| Methionine P26 | 0.835 | −0.151 | −0.324 | −0.111 |
| Isoleucine P27 | 0.948 | 0.165 | 0.075 | 0.135 |
| Leucine P28 | 0.948 | 0.125 | 0.049 | 0.239 |
| Tyrosine P29 | 0.971 | −0.026 | 0.053 | 0.112 |
| Phenylalanine P30 | 0.989 | 0.046 | 0.035 | 0.057 |
| Lysine P31 | 0.873 | 0.110 | 0.223 | −0.084 |
| Histidine P32 | 0.928 | −0.009 | 0.227 | −0.023 |
| Arginine P33 | 0.973 | −0.009 | 0.090 | 0.114 |
| Proline P34 | 0.849 | 0.321 | 0.047 | 0.197 |
| Contribution rate (E)/% | 60.88 | 17.82 | 5.84 | 3.79 |
| Cultivars | Component | Comprehensive Component RC* | |||
|---|---|---|---|---|---|
| RC1 | RC2 | RC3 | RC4 | ||
| No. 9 | 1.329/2 | 1.397/4 | −0.478/20 | −1.378/25 | 0.978/1 |
| No. 65 | 1.092/3 | 0.217/12 | 0.639/6 | 1.755/2 | 0.808/2 |
| No. 66 | 0.572/12 | 1.631/1 | 0.927/5 | 0.942/5 | 0.729/3 |
| No. 72 | 0.894/5 | 0.038/15 | 2.340/1 | 0.254/11 | 0.697/4 |
| No. 8 | 0.827/7 | 1.487/3 | −0.688/22 | −0.895/21 | 0.694/5 |
| No. 21 | 1.579/1 | −1.407/24 | −0.114/15 | −0.315/16 | 0.692/6 |
| No. 17 | 1.070/4 | −0.245/16 | 0.286/8 | −1.091/23 | 0.583/7 |
| No. 26 | 0.781/9 | 0.042/14 | −1.135/25 | 1.737/3 | 0.483/8 |
| No. 29 | 0.627/11 | 0.642/8 | −0.183/18 | −0.952/22 | 0.449/9 |
| Mahan | 0.838/6 | −0.570/20 | 0.020/13 | 0.715/7 | 0.437/10 |
| Western | 0.746/10 | −0.371/18 | −0.572/21 | 0.688/8 | 0.381/11 |
| No. 13 | 0.802/8 | −0.718/21 | −2.210/27 | −0.331/17 | 0.219/12 |
| No. 28 | 0.519/13 | −1.054/22 | −0.906/24 | 0.745/6 | 0.103/13 |
| No. 45 | −0.377/16 | 1.175/5 | 1.904/2 | 0.071/13 | 0.094/14 |
| No. 35 | −0.668/20 | 1.575/2 | 0.073/10 | 1.889/1 | −0.05/15 |
| No. 7 | −0.276/15 | 0.360/10 | 0.320/7 | −1.574/27 | −0.145/16 |
| No. 11 | 0.182/14 | −1.498/25 | −0.147/16 | −0.559/20 | −0.186/17 |
| No. 52 | −0.407/17 | 0.079/13 | 0.091/9 | −0.476/19 | −0.247/18 |
| No. 42 | −0.464/19 | −1.065/23 | 1.312/4 | 0.127/12 | −0.391/19 |
| No. 22 | −0.423/18 | −1.699/27 | 1.431/3 | −0.434/18 | −0.493/20 |
| Pawnee | −0.815/21 | −0.447/19 | 0.048/11 | −1.249/24 | −0.620/21 |
| No. 30 | −1.185/23 | 0.607/9 | −0.371/19 | −0.172/14 | −0.641/22 |
| Kanza | −1.149/22 | 0.753/6 | −0.818/23 | −1.386/26 | −0.666/23 |
| No. 48 | −1.378/26 | 0.672/7 | −0.172/17 | −0.268/15 | −0.739/24 |
| Caddo | −1.238/24 | 0.318/11 | −1.654/26 | 1.232/4 | −0.747/25 |
| No. 20 | −1.362/25 | −1.590/26 | 0.047/12 | 0.668/9 | −1.084/26 |
| No. 36 | −2.118/27 | −0.330/17 | 0.009/14 | 0.260/10 | −1.338/27 |
| Comprehensive Evaluation | Comprehensive Component Score | Cultivar |
|---|---|---|
| Level 5 | >0.857 | No. 9 |
| Level 4 | 0.857~0.681 | No. 65, No. 66, No. 72, No. 8, No. 21 |
| Level 3 | 0.681~0.435 | No. 17, No. 26, No. 29, Mahan |
| Level 2 | 0.435~0.258 | Western |
| Level 1 | <0.258 | No. 13, No. 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Yao, X.; Wang, K.; Yang, S.; Ren, H.; Huang, M.; Chang, J. Quality Analysis and Comprehensive Evaluation of Fruits from Different Cultivars of Pecan (Carya illinoinensis (Wangenheim) K. Koch). Forests 2022, 13, 746. https://doi.org/10.3390/f13050746
Wu S, Yao X, Wang K, Yang S, Ren H, Huang M, Chang J. Quality Analysis and Comprehensive Evaluation of Fruits from Different Cultivars of Pecan (Carya illinoinensis (Wangenheim) K. Koch). Forests. 2022; 13(5):746. https://doi.org/10.3390/f13050746
Chicago/Turabian StyleWu, Shuang, Xiaohua Yao, Kailiang Wang, Shuiping Yang, Huadong Ren, Mei Huang, and Jun Chang. 2022. "Quality Analysis and Comprehensive Evaluation of Fruits from Different Cultivars of Pecan (Carya illinoinensis (Wangenheim) K. Koch)" Forests 13, no. 5: 746. https://doi.org/10.3390/f13050746
APA StyleWu, S., Yao, X., Wang, K., Yang, S., Ren, H., Huang, M., & Chang, J. (2022). Quality Analysis and Comprehensive Evaluation of Fruits from Different Cultivars of Pecan (Carya illinoinensis (Wangenheim) K. Koch). Forests, 13(5), 746. https://doi.org/10.3390/f13050746






