Carbon, Nitrogen and Phosphorus Stoichiometry in Natural and Plantation Forests in China
Abstract
:1. Introduction
2. Materials and Methods
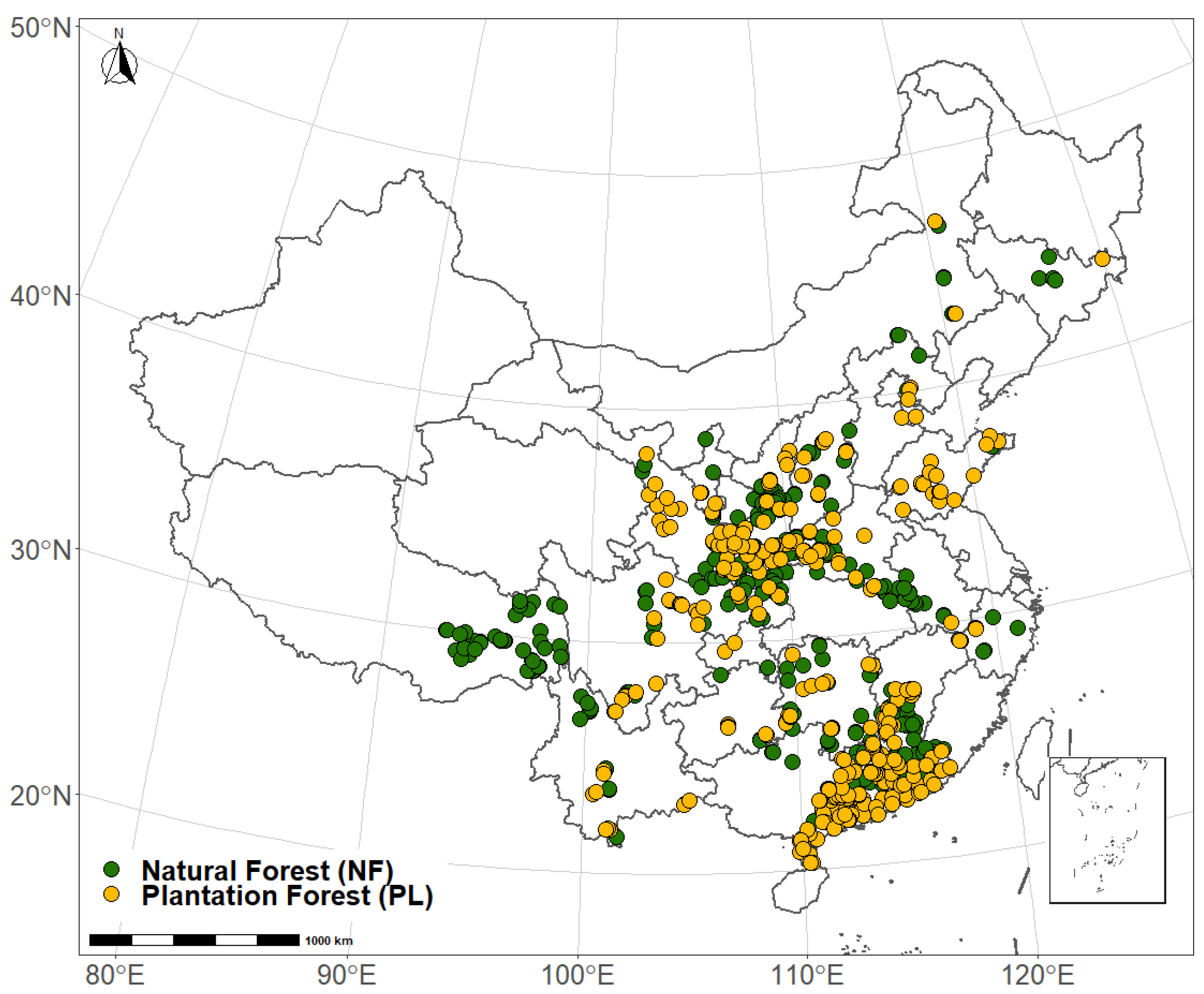
2.1. Samples and Measurement
2.2. Climate Data
2.3. Statistical Analyses
3. Results
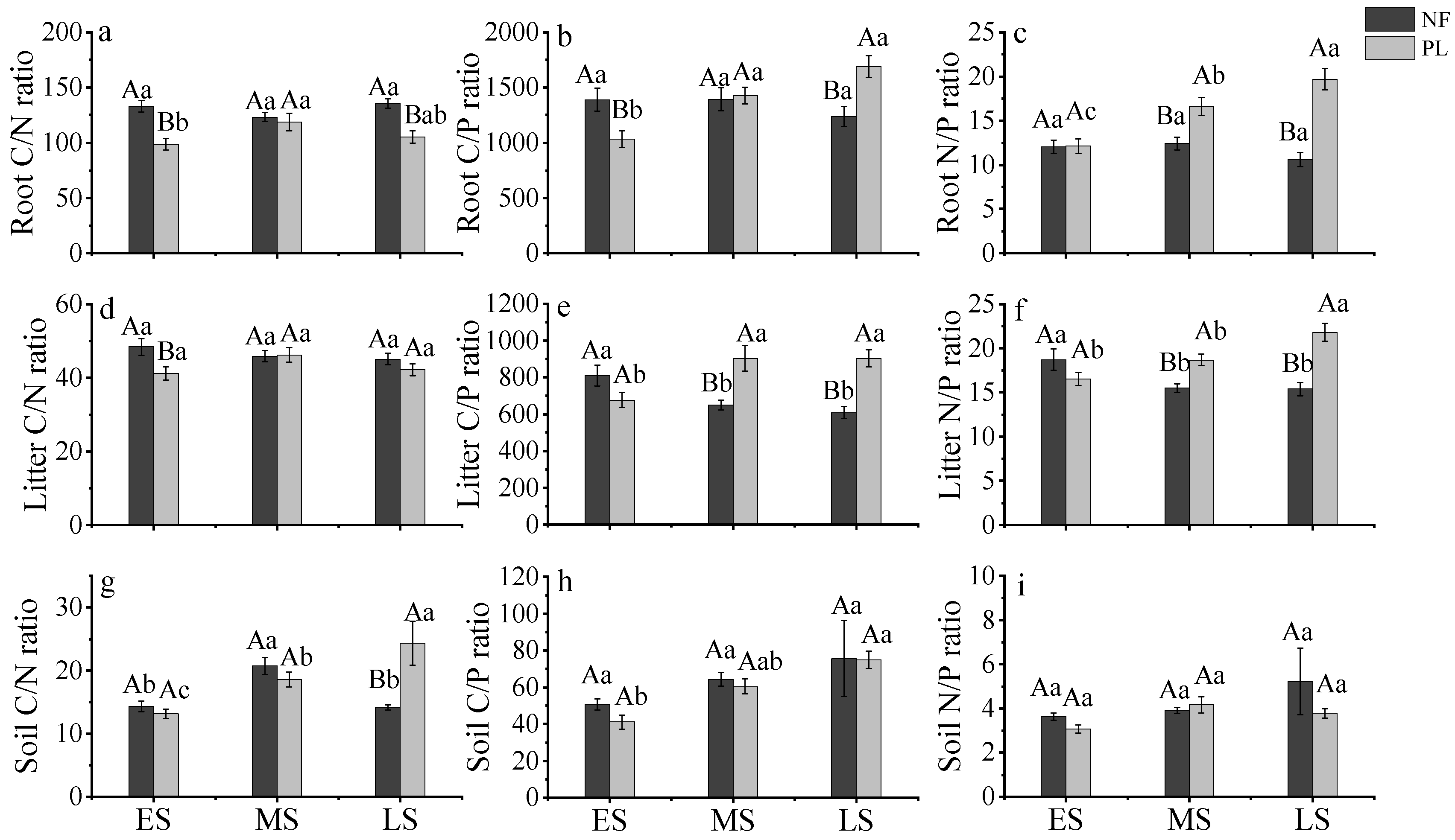
3.1. C, N and P Concentrations in Roots, Litter and Soil in Forests of Different Origins in China
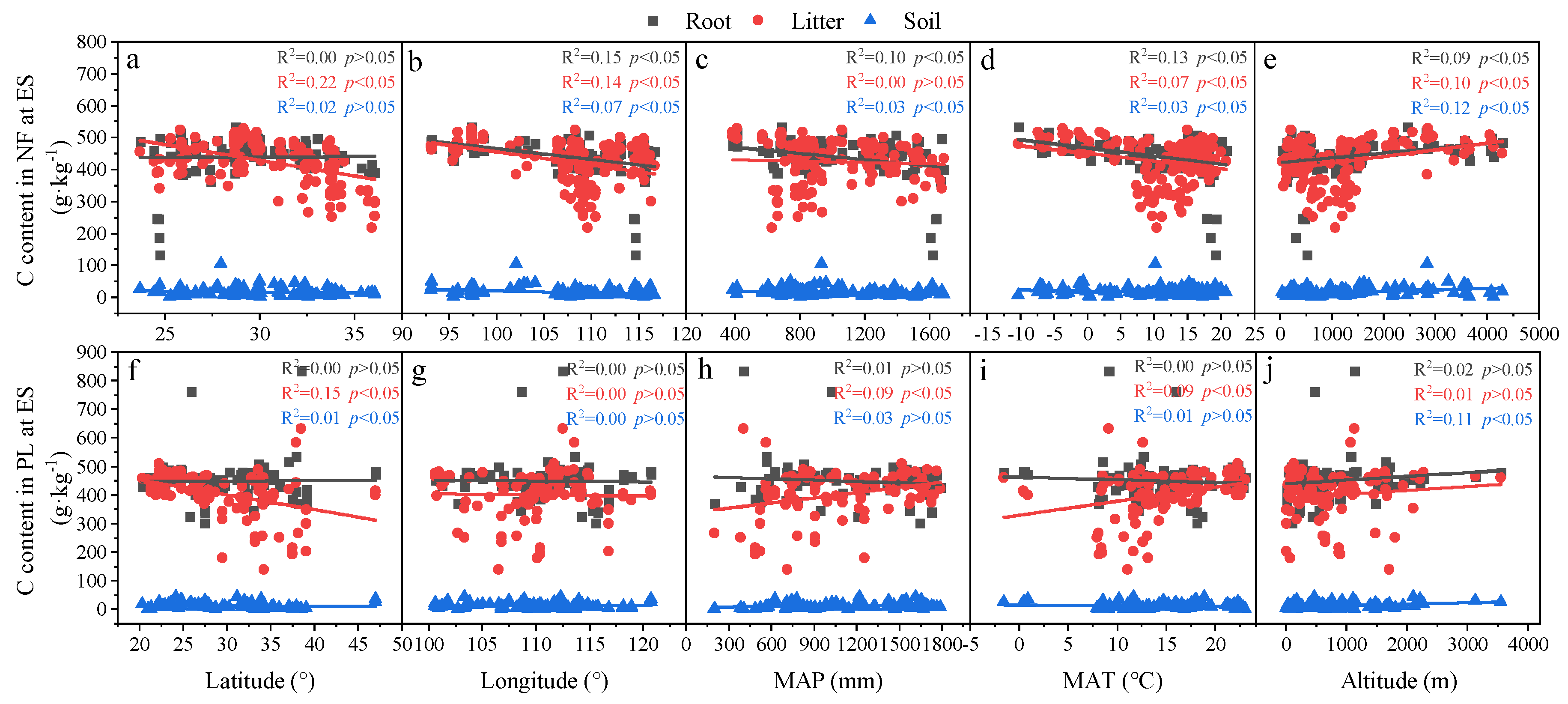
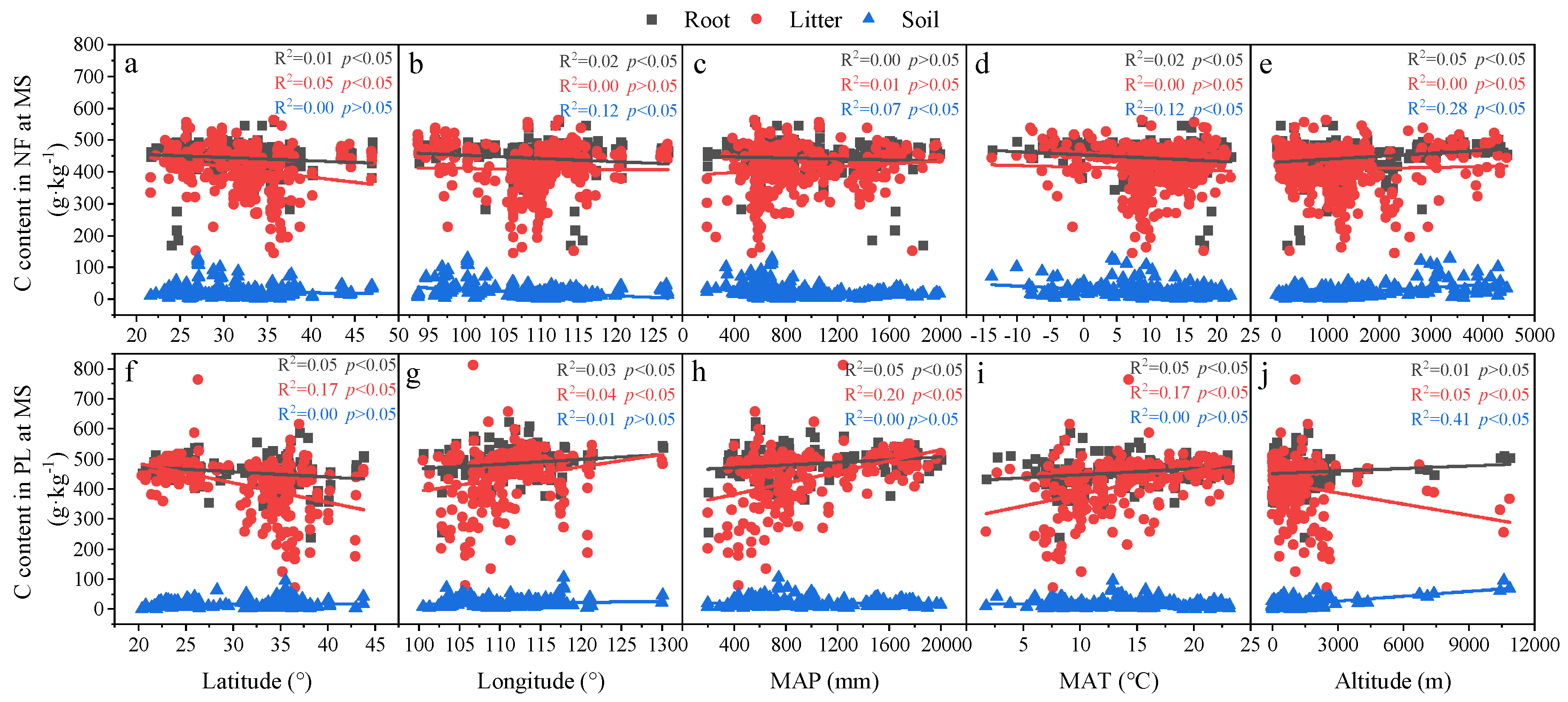
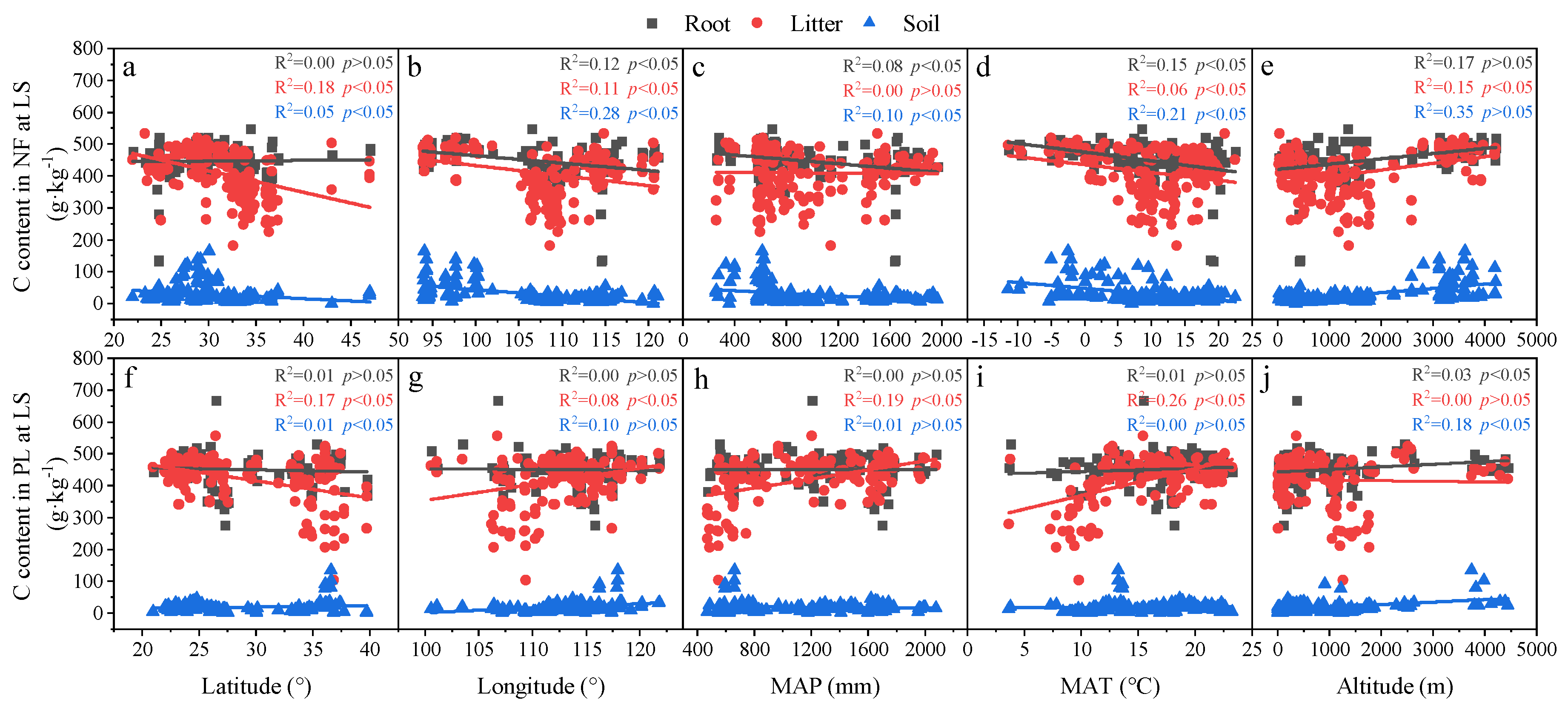
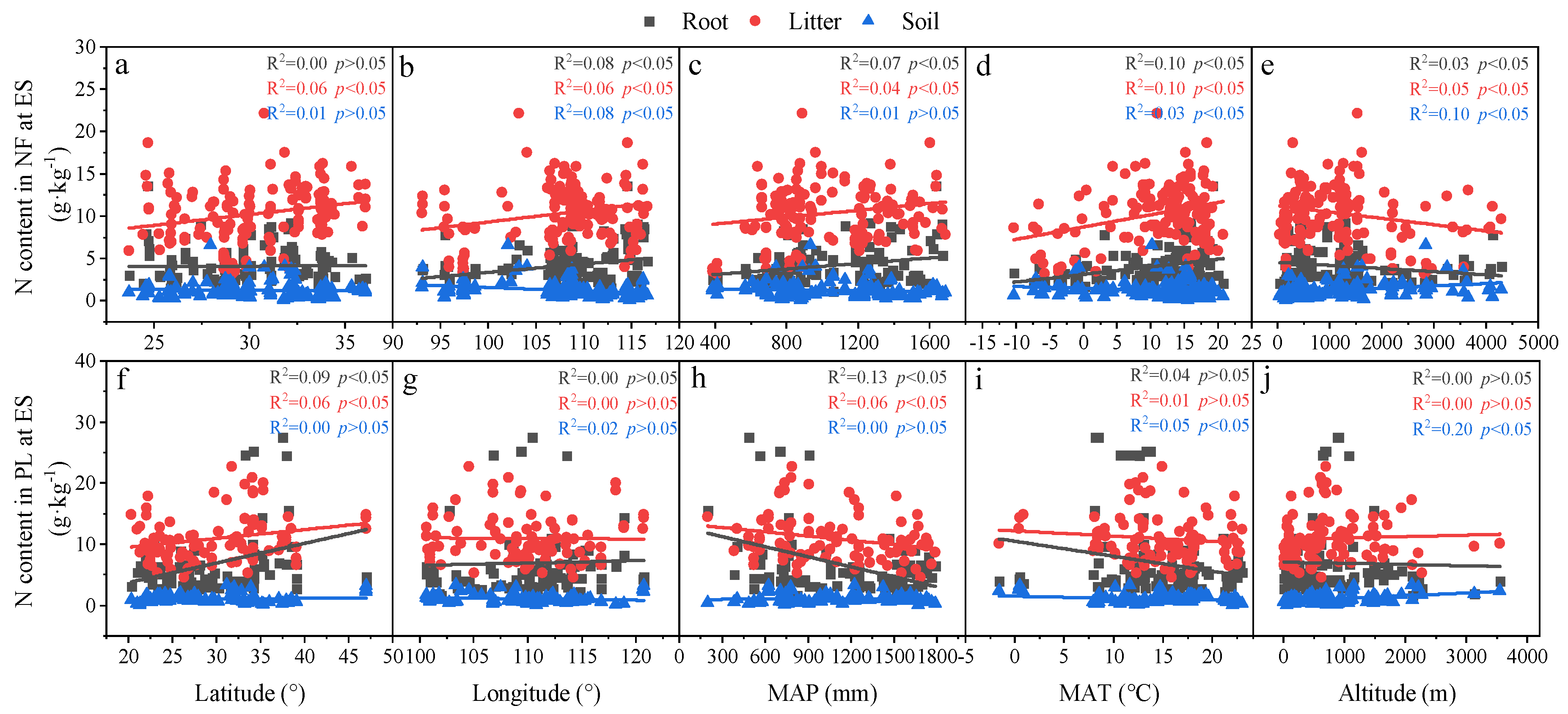
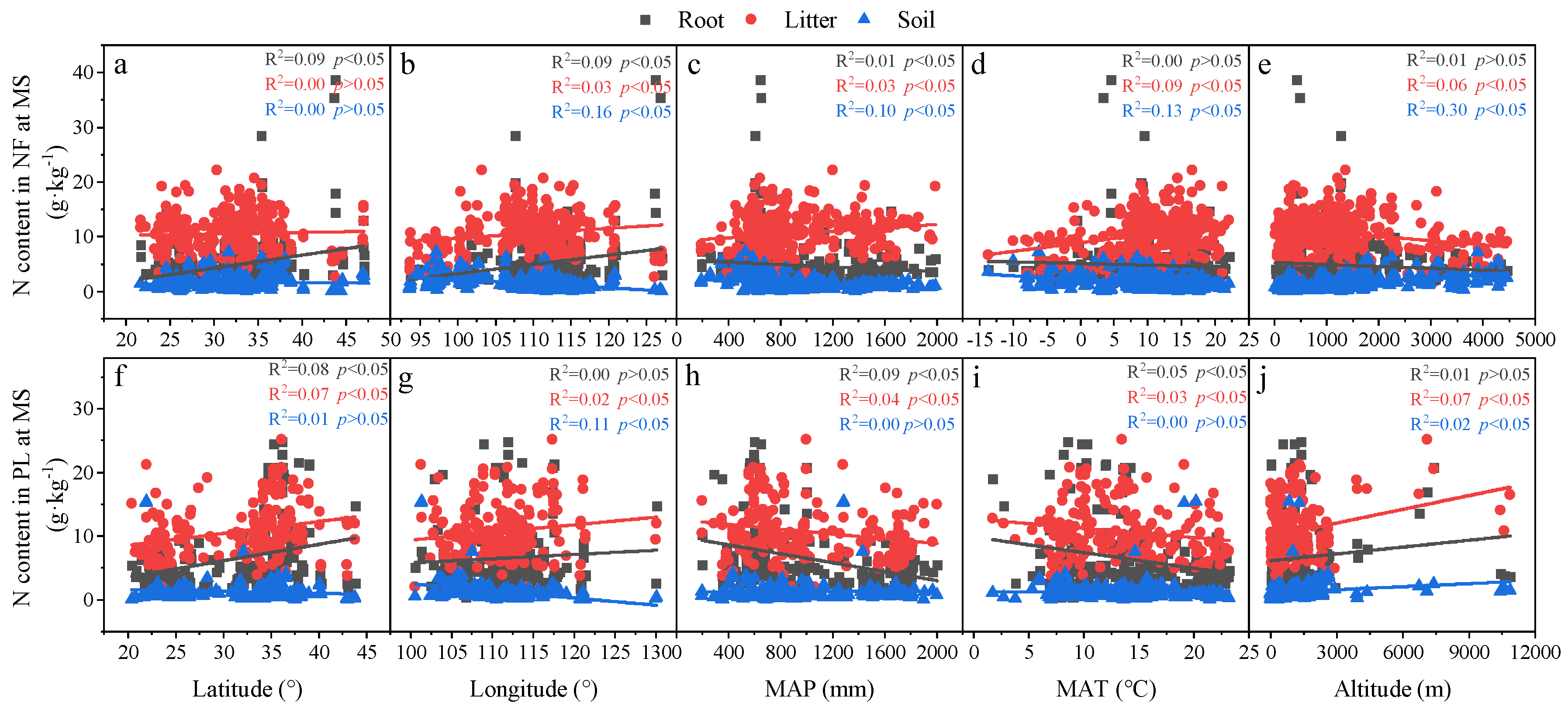
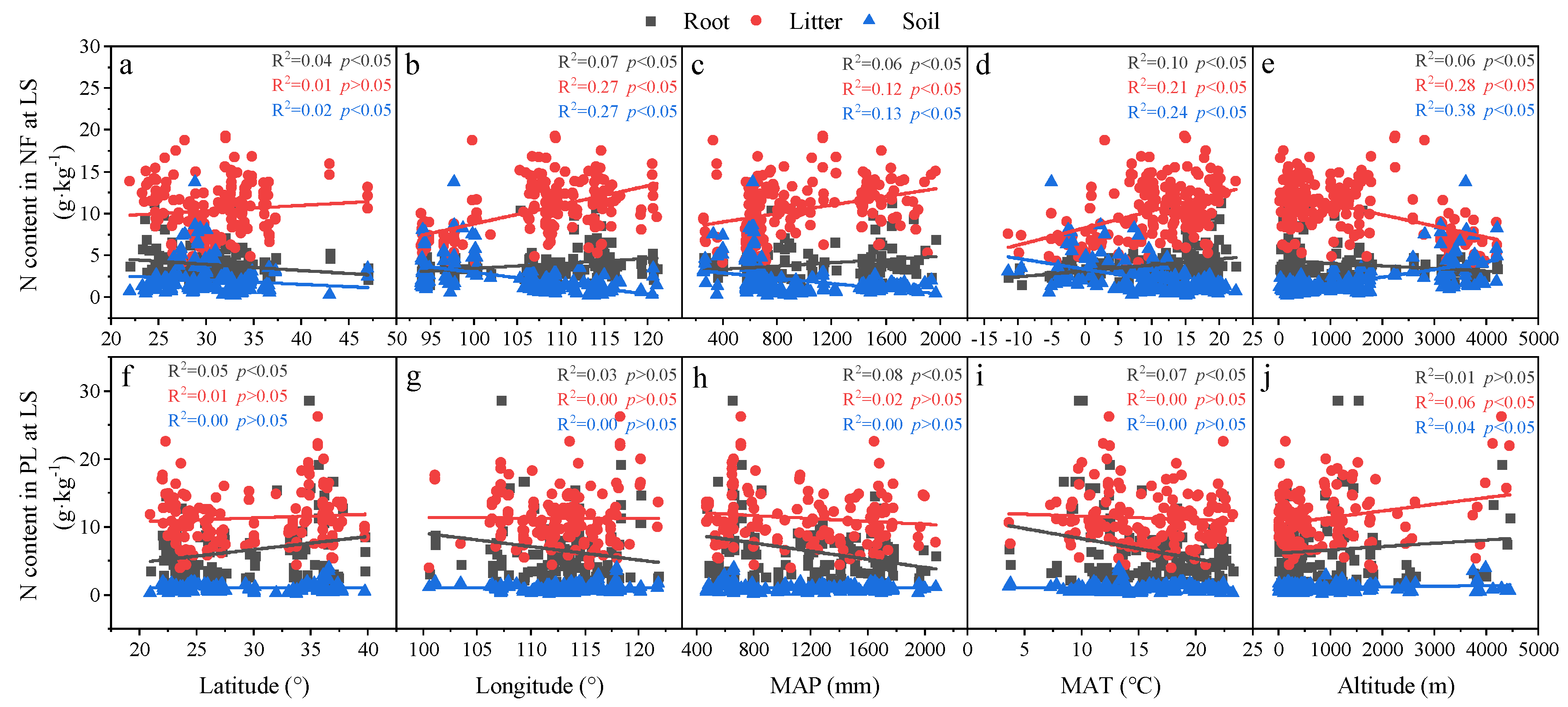
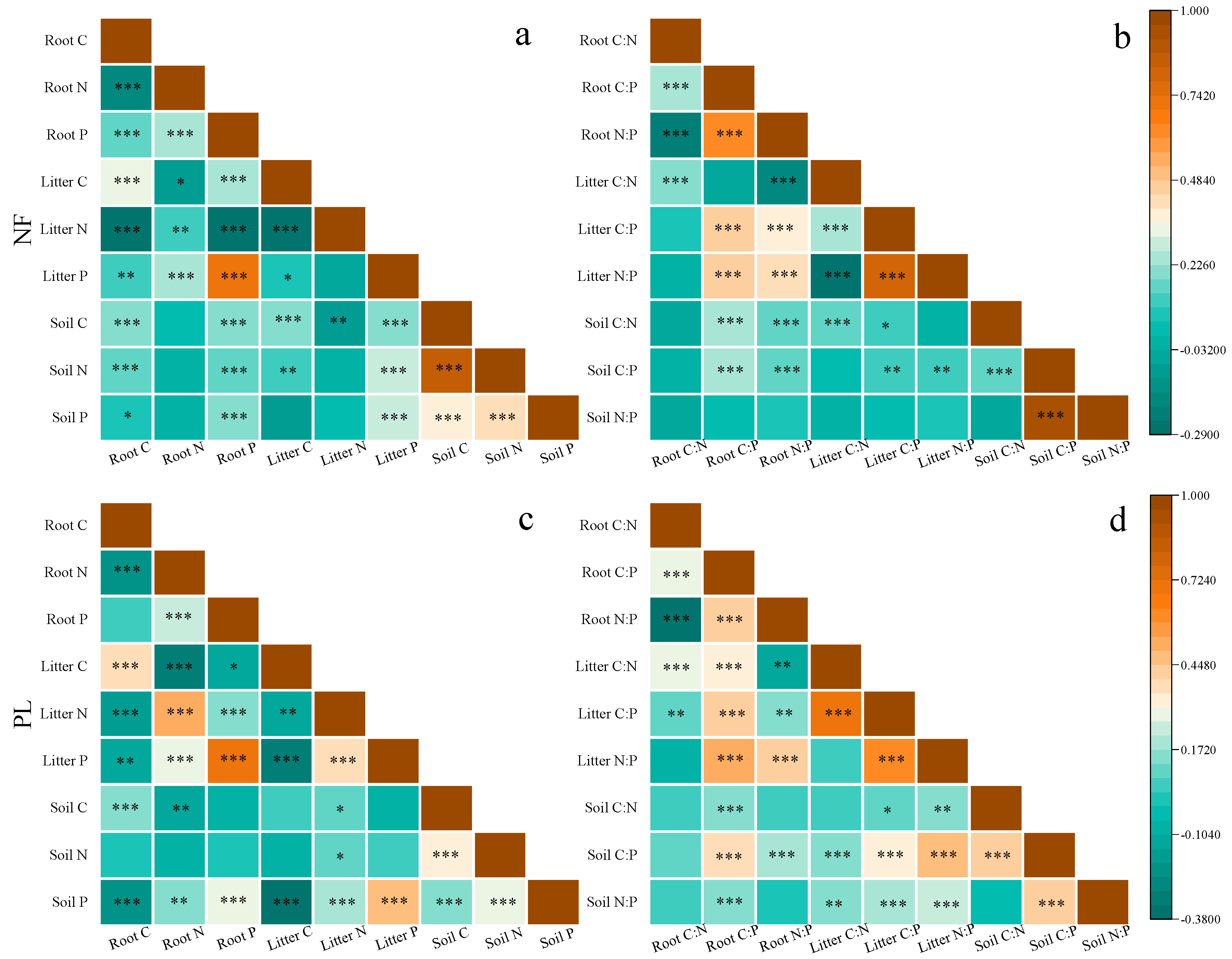
3.2. Relationships in C, N and P Concentrations and Ecological Stoichiometry in Roots, Litter and Soil in Different Forests
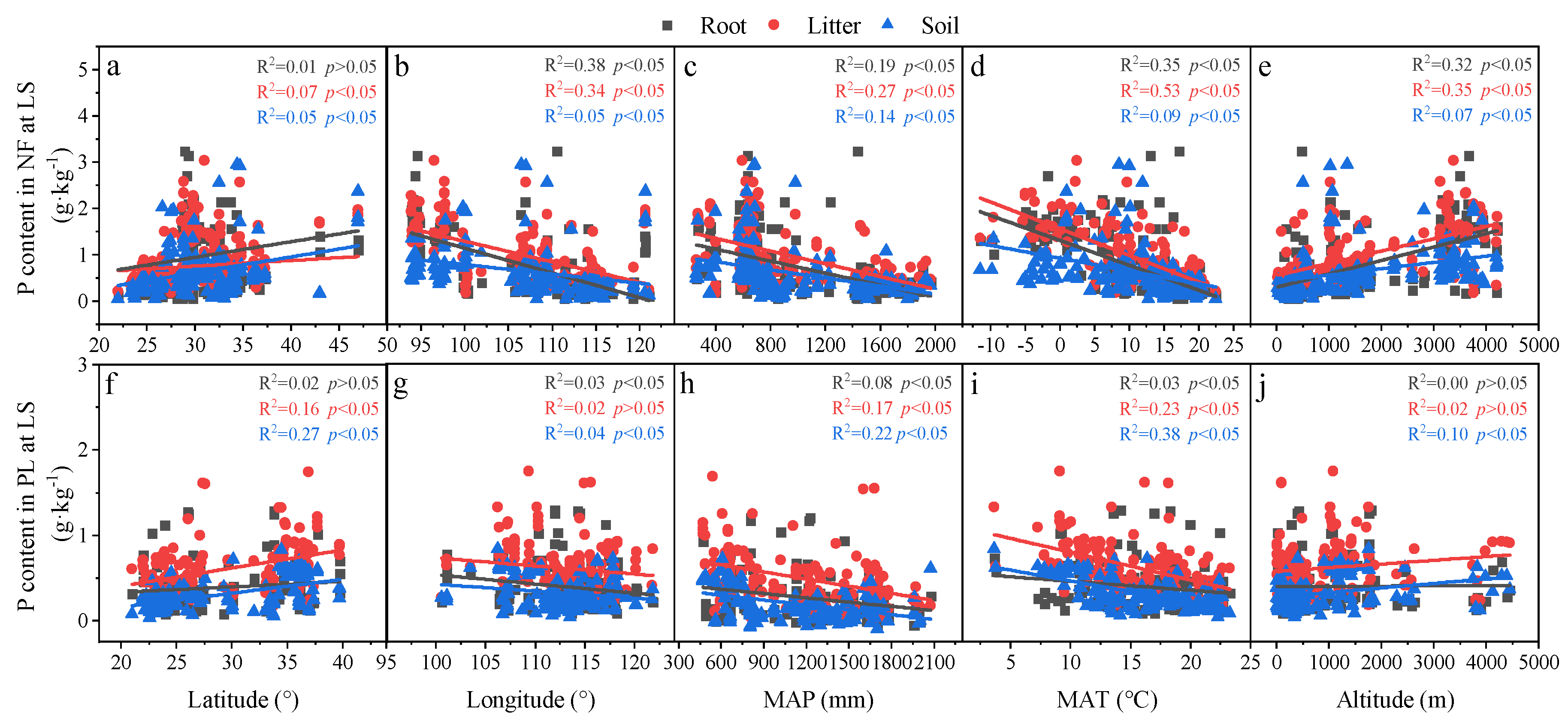
3.3. Relationships in Determinants and C, N and P Concentrations and Ecological Stoichiometry in Roots, Litter and Soil between NF and PL
4. Discussion
4.1. C, N and P Concentrations and Stoichiometry in Roots, Litter and Soil in Different Forests
4.2. Factors Affecting C, N and P Concentrations and Their Stoichiometry in Different Succession Stages in NF and PL
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Carbon |
| N | Nitrogen |
| P | Phosphorus |
| NF | Natural forests |
| PL | Plantation forests |
| ES | Early stage |
| MS | Middle stage |
| LS | Late stage |
| MAT | Mean annual temperature |
| MAP | Mean annual precipitation |
Appendix A
| Forest Origin | n | Growth Stages | N | Dominant Species |
|---|---|---|---|---|
| NF | 667 | ES | 149 | Quercus wutaishansea Mary, Populus simonii Carr, Betula platyphylla Suk., Betula albosinensis Burk., Quercus variabilis Bl., Quercus acutissima Carruth., Pinus tabuliformis Carr., Pinus massoniana Lamb., Quercus glandulifera var. brevipetiolata Nakai, Abies fabri (Mast.) Craib, Quercus semicarpifolia Smith, Pinus yunnanensis Franch., Cyclobalanopsis glauca (Thunb.) Oerst., Lithocarpus glaber (Thunb.) Nakai, Pinus densata Mast., Picea spinulosa (Griff.) A. Henry, Cunninghamia lanceolata (Lamb.) Hook., Abies georgei Orr var. smithii (Viguie et Gaussen) Cheng et L. |
| MS | 344 | Fraxinus rhynchophylla Hance, Quercus mongolica Fisch. ex Ledeb, Juglans mandshurica Maxim., Populus davidiana Dode, Betula dahurica Pall., Quercus aliena Bl. var. acuteserrata Maxim. ex Wenz., Robinia pseudoacacia L., Betula platyphylla Suk., Quercus wutaishansea Mary, Pinus armandii Franch., Pinus tabuliformis Carr., Picea crassifolia Kom., Sabina przewalskii Kom., Larix gmelinii (Rupr.) Kuzen., Quercus acutissima Carruth., Acer ginnala Maxim., Quercus variabilis Bl., Juniperus formosana Hayata, Quercus aliena Bl. var. acuteserrata Maxim. ex Wenz., Vernicia fordii (Hemsl.) Airy Shaw, Populus tomentosa Carr, Pinus tabuliformis var. henryi (Masters) C. T. Kuan, Cunninghamia lanceolata (Lamb.) Hook., Pinus massoniana Lamb., Bothrocaryum controversum, Abies fabri (Mast.) Craib, Pinus yunnanensis Franch., Quercus semicarpifolia Smith, Abies georgei Orr var. smithii (Viguie et Gaussen) Cheng et L., Picea spinulosa (Griff.) A. Henry | ||
| LS | 174 | Ulmus pumila L., Larix gmelinii (Rupr.) Kuzen., Quercus variabilis Bl., Cyclobalanopsis glauca (Thunb.) Oerst., Quercus aliena Bl. var. acuteserrata Maxim. ex Wenz., Pinus tabuliformis Carr., Picea crassifolia Kom., Pinus armandii Franch., Platycladus orientalis (L.) Franco, Quercus wutaishansea Mary, Betula albosinensis Burk., Quercus acutissima Carruth., Quercus aliena Bl. var. acuteserrata Maxim. ex Wenz., Pinus massoniana Lamb., Liquidambar formosana Hance, Quercus glandulifera var. brevipetiolata Nakai, Pinus yunnanensis Franch., Schima superba Gardn. et Champ., Loropetalum chinense (R. Br.) Oliver, Castanopsis fargesii Franch., Bothrocaryum controversum, Castanopsis sclerophylla (Lindl.) Schott., Cinnamomum porrectum (Roxb.) Kosterm., Castanopsis hystrix J. D. Hooker et Thomson ex A. De Candolle, Castanopsis carlesii (Hemsl.) Hayata., Cunninghamia lanceolata (Lamb.) Hook., Abies georgei Orr, Abies delavayi Franch., Abies georgei Orr var. smithii (Viguie et Gaussen) Cheng et L., Pinus densata Mast., Quercus semicarpifolia Smith, Picea spinulosa (Griff.) A. Henry | ||
| PL | 445 | ES | 102 | Larix gmelinii (Rupr.) Kuzen., Populus gansuensis C. Wang et H. L. Yang, Robinia pseudoacacia L., Platycladus orientalis (L.) Franco, Pinus tabuliformis Carr., Populus simonii Carr, Betula albosinensis Burk., Populus euramericana cv.‘I-214’, Pinus massoniana Lamb., Abies fabri (Mast.) Craib, Quercus semicarpifolia Smith, Cinnamomum camphora (L.) Presl., Pinus yunnanensis Franch., pinus elliottii, Eucalyptus robusta Smith, Cunninghamia lanceolata (Lamb.) Hook., Acacia mangium Willd., Schima superba Gardn. et Champ., E. urophylla × E. grandis |
| MS | 199 | Larix gmelinii (Rupr.) Kuzen., Pinus sylvestris var. mongolica Litv., Picea asperata Mast., Pinus tabuliformis Carr., Larix gmelinii var. principis-rupprechtii (Mayr) Pilger, Robinia pseudoacacia L., Larix kaempferi (Lamb.) Carr., Pinus thunbergii Parlatore, Quercus acutissima Carruth., Styphnolobium japonicum (L.) Schott, Populus tomentosa Carr, Quercus aliena Bl. var. acuteserrata Maxim. ex Wenz., Populus tomentosa Carr, Quercus variabilis Bl., Cunninghamia lanceolata (Lamb.) Hook., Cupressus funebris Endl., Eucalyptus robusta Smith, Acacia mangium Willd. | ||
| LS | 144 | Pinus tabuliformis Carr., Larix gmelinii (Rupr.) Kuzen., Populus davidiana Dode, Pinus densiflora Sieb. et Zucc., Platycladus orientalis (L.) Franco, Pinus thunbergii Parlatore, Populus simonii Carr, Populus cathayana Rehd., Populus tomentosa Carr, Larix kaempferi (Lamb.) Carr., Cunninghamia lanceolata (Lamb.) Hook., Pinus massoniana Lamb., pinus elliottii, Cinnamomum camphora (L.) Presl., Quercus variabilis Bl., Loropetalum chinense (R. Br.) Oliver, Acacia mangium Willd., Schima superba Gardn. et Champ., Liquidambar formosana Hance |
References
- Reich, P.B.; Tjoelker, M.; Machado, J.L.; Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006, 439, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; p. 439. [Google Scholar]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elser, J.J.; Dobberfuhl, D.R.; Mackay, N.A.; Schampel, J.H. Organism size, life history, and N:P stoichiometry. Bioscience 1996, 46, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 369–397. [Google Scholar]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.X.; Wang, K.L.; Zeng, F.P.; Song, X.L.; Song, X.J. Litter decomposition and nutrient release in typical secondary and primary forests in karst region, Northwest of Guangxi. Acta. Ecol. Sin. 2012, 32, 2720–2728. [Google Scholar] [CrossRef] [Green Version]
- Ngaba, M.J.Y.; Hu, Y.L.; Bol, R.; Ma, X.Q.; Jin, S.F.; Mgelwa, A.S. Effects of land use change from natural forest to plantation on C, N and natural abundance of 13C and 15N along a climate gradient in eastern China. Sci. Rep. 2019, 9, 16516–16518. [Google Scholar] [CrossRef]
- Sheng, W.T. On the Maintenance of Long-term Productivity of Plantation in China. Forest. Res. 2018, 31, 1–14. [Google Scholar]
- Yu, Z.; Zhao, H.; Liu, S.; Zhou, G.; Fang, J.; Yu, G.; Tang, X.; Wang, W.; Yan, J.; Wang, G.; et al. Mapping forest type and age in China’s plantations. Sci. Total Environ. 2020, 744, 140790. [Google Scholar] [CrossRef]
- Ostertag, R.; DiManno, N.M. Detecting terrestrial nutrient limitation: A global meta-analysis of foliar nutrient concentrations after fertilization. Front. Earth. Sci. 2016, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New. Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Xu, S.; Sardans, J.; Zhang, J.L.; Penuelas, J. Variations in foliar carbon:nitrogen and nitrogen:phosphorus ratios under global change: A meta-analysis of experimental field studies. Sci. Rep. 2020, 10, 12156. [Google Scholar] [CrossRef] [PubMed]
- Sophie, Z.B.; Maria, K.K.; Maria, M.; Josep, P.; Andreas, R.; Sardans, J.; Wolfgang, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2016, 85, 133–155. [Google Scholar]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Chen, H.Y.H. Global trends in senesced-leaf nitrogen and phosphorus. Glob. Ecol. Biogeogr. 2009, 18, 532–542. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat. Clim. Change 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Townsend, A.R.; Cleveland, C.C.; Asner, G.P.; Bustamante, M.M.C. Controls over foliar N:P ratios in tropical rain forests. Ecology 2007, 88, 107–118. [Google Scholar] [CrossRef]
- Bai, X.J.; Wang, B.R.; An, S.S.; Zeng, Q.C.; Zhang, H.X. Response of forest species to C:N:P in the plant-litter-soil system and stoichiometric homeostasis of plant tissues during afforestation on the Loess Plateau, China. Catena 2019, 183, 104186. [Google Scholar] [CrossRef]
- Tang, X.L.; Zhao, X.; Bai, Y.F.; Tang, Z.Y.; Wang, W.T.; Zhao, Y.C.; Wan, H.W.; Xie, Z.Q.; Shi, X.Z.; Wu, B.F.; et al. Carbon pools in China’s terrestrial ecosystems: New estimates based on an intensive field survey. Proc. Natl. Acad. Sci. USA 2018, 115, 4021–4026. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; pp. 14, 31, 43, 47. [Google Scholar]
- Yu, Z.; Zhou, G.Y.; Liu, L.; Manzoni, S.; Ciais, P.; Goll, D.; Peñuelas, J.; Sardans, J.; Wang, W.T.; Zhu, J.; et al. Natural forests promote phosphorus retention in soil. Glob. Change Biol. 2021, 28, 1678–1689. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 28, 457–467. [Google Scholar]
- Tang, Z.Y.; Xu, W.T.; Zhou, G.Y.; Bai, Y.F.; Li, J.X.; Tang, X.L.; Chen, D.M.; Liu, Q.; Ma, W.H.; Xiong, G.M.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcclaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover and nutrient dynamics of above- and below-ground detritus of world forests. Adv. Ecol. Res. 1986, 15, 3030–3377. [Google Scholar]
- Yuan, Z.Y.; Chen, H.Y.H.; Reich, P.B. Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nat. Commun. 2011, 2, 344. [Google Scholar] [CrossRef] [Green Version]
- Beyer, F.; Hertel, D.; Leuschner, C. Fine root morphological and functional traits in Fagus sylvatica and Fraxinus excelsior saplings as dependent on species, root order and competition. Plant. Soil. 2013, 373, 143–156. [Google Scholar] [CrossRef]
- Guo, L.B.; Halliday, M.J.; Gifford, R.M. Fine root decomposition under grass and pine seedlings in controlled environmental conditions. Appl. Soil Ecol. 2006, 33, 22–29. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Liu, J.X.; Fang, X.; Tang, X.L.; Wang, W.T.; Zhou, G.Y.; Xu, S.; Huang, W.J.; Wang, G.X.; Yan, J.H.; Ma, K.P.; et al. Patterns and controlling factors of plant nitrogen and phosphorus stoichiometry across China’s forests. Biogeochemistry 2019, 143, 191–205. [Google Scholar] [CrossRef]
- Zhou, G.Y. Old-growth forests can accumulate carbon in soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Zhang, S.J.; Liu, Z.D.; Li, L.D.; Chen, J.L.; Wang, L.F.; Fang, X. Effects of vegetation restoration on soil organic carbon concentration and density in the mid-subtropical region of China. Acta Ecol. Sin. 2018, 42, 595–608. [Google Scholar]
- Liu, S.R.; Wang, H.; Luan, J.W. A review of research progress and future prospective of forest soil carbon stock and soil carbon process in China. Acta Ecol. Sin. 2011, 31, 5437–5448. [Google Scholar]
- Lambers, H.; Bishop, J.G.; Hopper, S.D.; Laliberté, E.; Zúñiga-Feest, A. Phosphorus-mobilization ecosystem engineering: The roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann. Bot. 2012, 110, 329–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Gao, G.L.; Ding, G.D.; Zhang, Y.; Guo, M.S.; Cao, H.Y.; Su, M. Stoichiometric characteristics of nitrogen and phosphorus in leaf-litter-soil system of Pinus sylvestris var. mongolica plantations. Chin. J. Appl. Ecol. 2019, 30, 36–43. [Google Scholar]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Glob. Ecol. Biogeogr. 2013, 22, 14. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W.; Verhoeven, J. Biomass N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Chen, W.X. Soil and Environmental Microbiology; Beijing Agricultural University Press: Beijing, China, 1990. [Google Scholar]
- Herbert, D.A.; Williams, M.; Rastetter, E.B. A model analysis of N and P limitation on carbon accumulation in Amazonian secondary forest after alternate land-use abandonment. Biogeochemistry 2003, 65, 121–150. [Google Scholar] [CrossRef]
- Mckane, R.B.; Rastetter, E.B.; Melillo, J.M.; Shaver, G.R.; Hopkinson, C.S.; Fernandes, D.N.; Skole, D.L.; Chomentowski, W.H. Effects of global change on carbon storage in tropical forests of South America. Glob. Biogeochem. Cycles 1995, 9, 329–350. [Google Scholar] [CrossRef]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.B.; Zhang, J.L.; Slik, J.F.; Cao, K.F. Leaf element concentrations of terrestrial plants across China are influenced by taxonomy and the environment. Glob. Ecol. Biogeogr. 2012, 21, 809–818. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Bodegom, P.; Witte, J.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2010, 18, 137–149. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zheng, J.Y.; Shao, M.A. Comparison of the Attributes of Natural Forests and Plantations in Ziwuling Mountain. Acta Biol. Boreali-Occident. Sin. 2005, 25, 2447–2456. [Google Scholar]
- Sardans, J.; Penuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.Z.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New. Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Weih, M.; Karlsson, P.S. Growth response of Mountain birch to air and soil temperature: Is increasing leaf-nitrogen content an acclimation to lower air temperature? New Phytol. 2001, 150, 147–155. [Google Scholar] [CrossRef]
- Luo, W.; Elser, J.J.; Lü, X.T.; Wang, Z.; Bai, E.; Yan, C.; Wang, C.; Li, M.-H.; Zimmermann, N.E.; Han, X.; et al. Plant nutrients do not covary with soil nutrients under changing climatic conditions. Glob. Biogeochem. Cycles 2015, 29, 1298–1308. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.P.; Wang, M.H.; Huang, Z.Q.; Lin, T.C.; Vadeboncoeur, M.A.; Searle, E.B.; Chen, H.Y.H. Temporal changes in soil C-N-P stoichiometry over the past 60 years across subtropical China. Glob. Change Biol. 2017, 24, 1308–1320. [Google Scholar] [CrossRef]



| Parameter | Forest Origin | C Concentration (g·kg−1) | N Concentration (g·kg−1) | P Concentration (g·kg−1) |
|---|---|---|---|---|
| Root | NF | 443.30 ± 1.87 B | 4.4 ± 0.12 B | 0.73 ± 0.03 A |
| PL | 451.80 ± 2.41 A | 6.64 ± 0.26 A | 0.56 ± 0.03 B | |
| Litter | NF | 412.71 ± 2.69 A | 10.48 ± 0. 13 B | 0.93 ± 0.03 A |
| PL | 410.40 ± 4.05 A | 10.96 ± 0.19 A | 0.71 ± 0.02 B | |
| Soil | NF | 22.00 ± 0.83 A | 1.56 ± 0.05 A | 0.48 ± 0.02 A |
| PL | 16.83 ± 0.69 B | 1.14 ± 0.06 B | 0.39 ± 0.01 B |
| Parameter | Forest Origin | C:N Ratio | C:P Ratio | N:P Ratio |
|---|---|---|---|---|
| Root | NF | 128.71 ± 2.70 A | 1352.19 ± 62.18 A | 11.86 ± 0.45 B |
| PL | 109.79 ± 4.16 B | 1421.91 ± 50.82 A | 16.56 ± 0.65 A | |
| Litter | NF | 46.19 ± 1.02 A | 674.38 ± 20.95 B | 16.19 ± 0.42 B |
| PL | 43.74 ± 1.11 A | 853.67 ± 36.52 A | 19.22 ± 0.48 A | |
| Soil | NF | 17.58 ± 0.75 A | 64.18 ± 5.76 A | 4.20 ± 0.40 A |
| PL | 19.21 ± 1.27 A | 60.64 ± 2.62 A | 3.79 ± 0.18 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, L.; Yu, Z.; Peñuelas, J.; Sardans, J.; Chen, Q.; Xu, J.; Zhou, G. Carbon, Nitrogen and Phosphorus Stoichiometry in Natural and Plantation Forests in China. Forests 2022, 13, 755. https://doi.org/10.3390/f13050755
Li L, Liu L, Yu Z, Peñuelas J, Sardans J, Chen Q, Xu J, Zhou G. Carbon, Nitrogen and Phosphorus Stoichiometry in Natural and Plantation Forests in China. Forests. 2022; 13(5):755. https://doi.org/10.3390/f13050755
Chicago/Turabian StyleLi, Lin, Lei Liu, Zhen Yu, Josep Peñuelas, Jordi Sardans, Qifei Chen, Jiangbing Xu, and Guoyi Zhou. 2022. "Carbon, Nitrogen and Phosphorus Stoichiometry in Natural and Plantation Forests in China" Forests 13, no. 5: 755. https://doi.org/10.3390/f13050755






