Preparation and Characterization of Heat-Treated Douglas Fir Wood with Core–Shell Structure
Abstract
1. Introduction
2. Materials and Methods
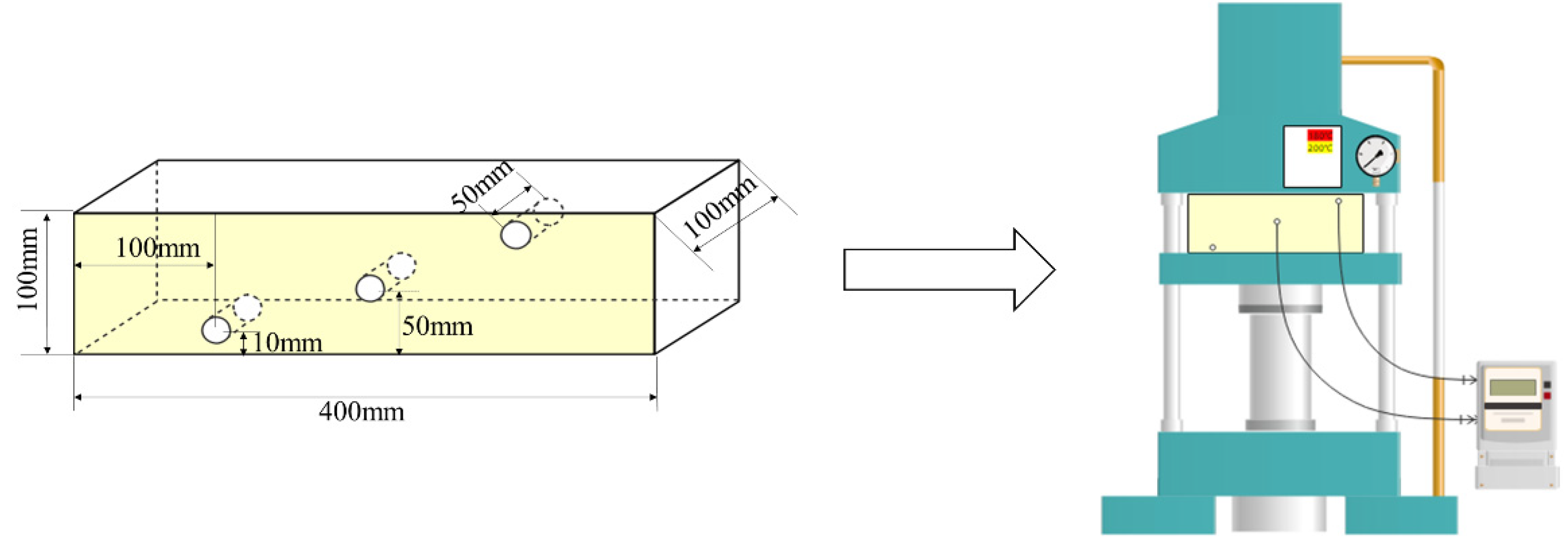
2.1. Wood Heat Treatment
2.2. Wood Colour Measurement
2.3. Dynamic Vapor Sorption (DVS)
2.4. Dynamic Mechanical Analysis (DMA)
2.5. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Sample Temperature Evolution during the Treatments
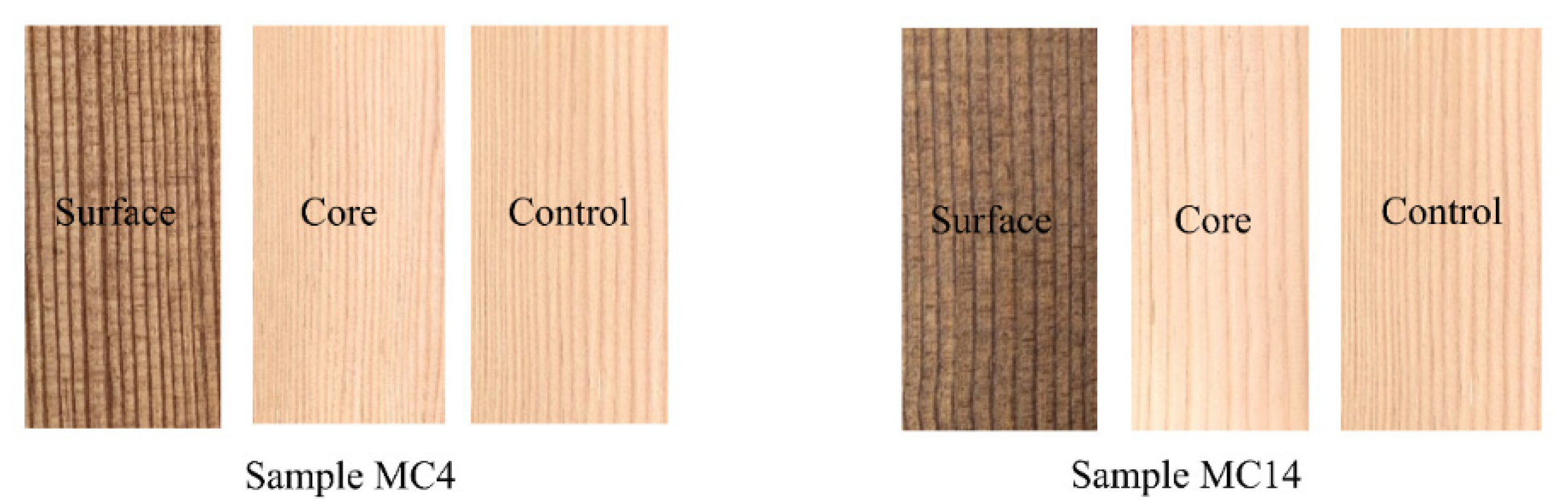
3.2. Colour and Appearance
3.3. Hygroscopicity
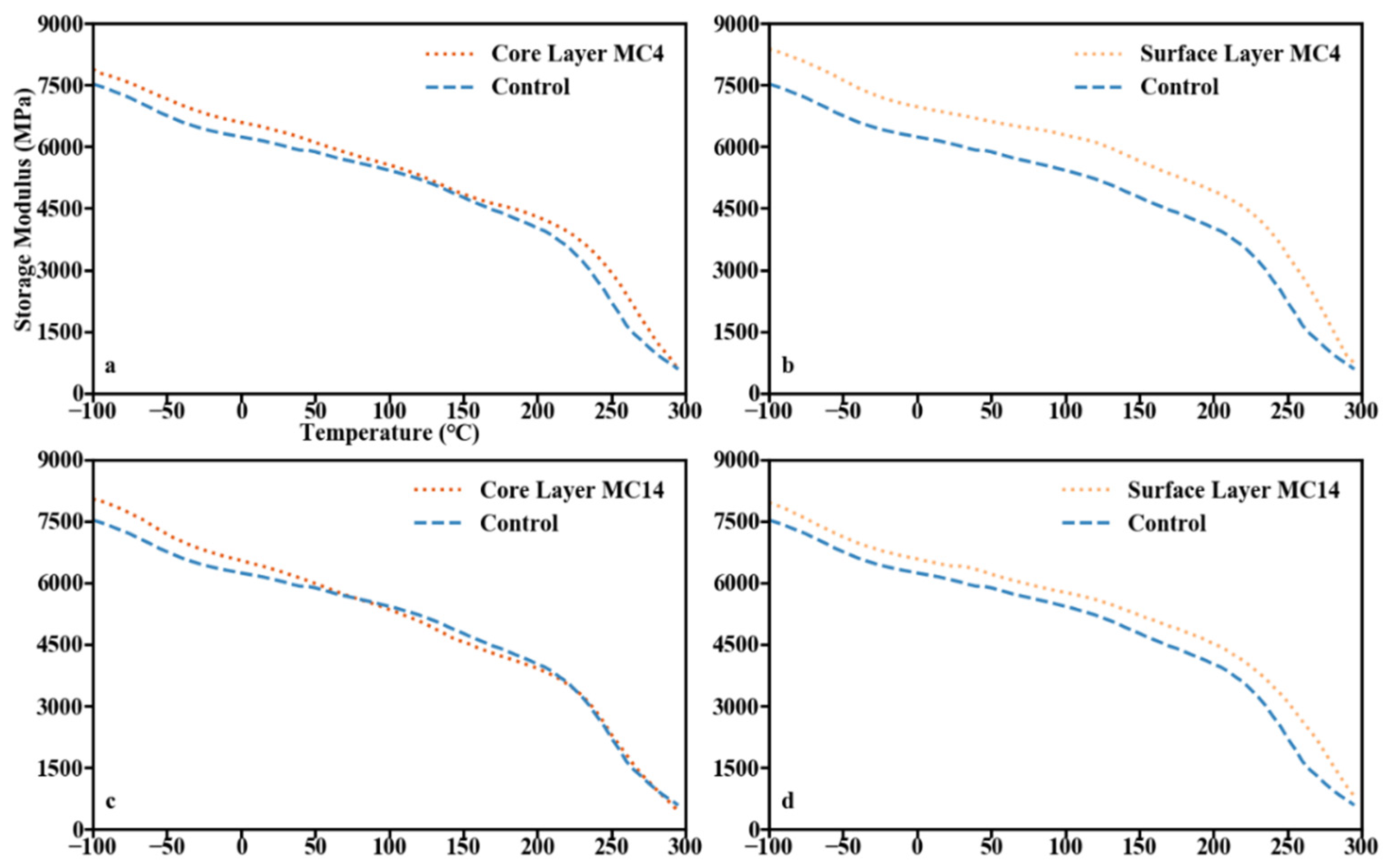
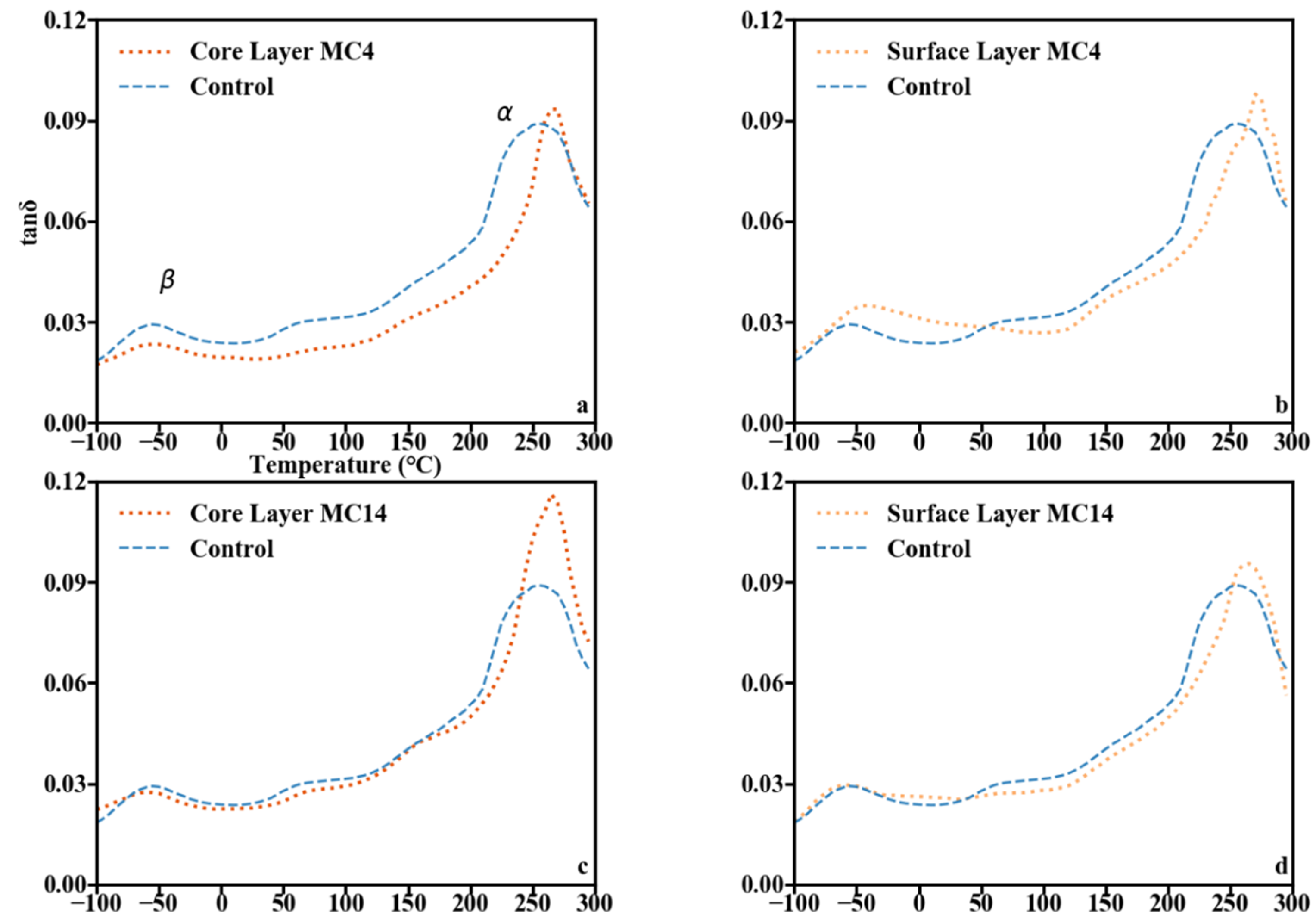
3.4. Dynamic Mechanical Analysis
3.5. XPS Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.; Sandberg, D. A Review of Wood Modification Globally-Updated Findings from COST FP1407. Interdiscip. Perspect. Built Environ. 2020, 1, 1–31. [Google Scholar] [CrossRef]
- Popescu, C.M.; Zeniya, N.; Endo, K.; Genkawa, T.; Obataya, E. Modifications in spruce wood structure following hydro-thermal treatment evaluated by NIR spectroscopy. Pro Ligno 2019, 15, 48–54. [Google Scholar]
- Barboutis, I.; Kamperidou, V. Impact of Heat Treatment on the Quality of Tree-of-Heaven Wood. Drv. Ind. 2019, 70, 351–358. [Google Scholar] [CrossRef]
- He, Z.; Qu, L.; Wang, Z.; Qian, J.; Yi, S. Evaluation of the hygroscopicity and dimensional stability of silicone oil treated wood. Holzforschung 2020, 74, 811–815. [Google Scholar] [CrossRef]
- Cao, Y.J.; Lu, J.X.; Huang, R.F.; Jiang, J.L. Increased dimensional stability of Chinese fir through steam-heat treatment. Eur. J. Wood Wood Prod. 2012, 70, 441–444. [Google Scholar] [CrossRef]
- CEN/TS 15083-2:2005; Durability of Wood and Wood-Based Products—Determination of the Natural Durability of Solid Wood against Wood Destroying Fungi, Test Methods—Part 2: Soft Rotting Micro-Fungi. European Committee for Standardization (CEN): Brussels, Belgium, 2005.
- Candelier, K.; Hannouz, S.; Thévenon, M.-F.; Guibal, D.; Gérardin, P.; Pétrissans, M.; Collet, R. Resistance of thermally modified ash (Fraxinus excelsior L.) wood under steam pressure against rot fungi, soil-inhabiting micro-organisms and termites. Eur. J. Wood Wood Prod. 2017, 75, 249–262. [Google Scholar] [CrossRef]
- Boonstra, M.; Tjeerdsma, B. Chemical analysis of heat treated softwoods. Holz Roh Werkst. 2006, 64, 204–211. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Ben Mlouka, S.; Belt, T.; Merk, V.; Liljeström, V.; Hänninen, T.; Uimonen, T.; Kostiainen, M.; Rautkari, L. Chemical, water vapour sorption and ultrastructural analysis of Scots pine wood thermally modified in high-pressure reactor under saturated steam. J. Mater. Sci. 2018, 53, 3027–3037. [Google Scholar] [CrossRef]
- Čabalová, I.; Výbohová, E.; Igaz, R.; Kristak, L.; Kačík, F.; Antov, P.; Papadopoulos, A.N. Effect of oxidizing thermal modification on the chemical properties and thermal conductivity of Norway spruce (Picea abies L.) wood. Wood Mater. Sci. Eng. 2021, 1–10. [Google Scholar] [CrossRef]
- Torniainen, P.; Popescu, C.M.; Jones, D.; Scharf, A.; Sandberg, D. Correlation of studies between colour, structure and mechanical properties of commercially produced ThermoWood® treated Norway spruce and Scots pine. Forests 2021, 12, 1165. [Google Scholar] [CrossRef]
- Guo, F.; Huang, R.; Lu, J.; Chen, Z.J.; Cao, Y.J. Evaluating the effect of heat treating temperature and duration on selected wood properties using comprehensive cluster analysis. J. Wood Sci. 2014, 60, 255–262. [Google Scholar] [CrossRef]
- Poncsak, S.; Kocaefe, D.; Bouzazra, M.; Pichette, A. Effect of high temperature treatment on the mechanical properties of birch (Betula papyrifera). Wood Sci. Technol. 2006, 40, 647–663. [Google Scholar] [CrossRef]
- Bal, B. A comparative study of some of the mechanical properties of pine wood heat treated in vacuum, nitrogen, and air atmospheres. BioResources 2018, 13, 5504–5511. [Google Scholar]
- Kariz, M.; Kuzman, M.K.; Sernek, M.; Hughes, M.; Rautkari, L.; Kamke, F.A.; Kutnar, A. Influence of temperature of thermal treatment on surface densification of spruce. Eur. J. Wood Wood Prod. 2017, 75, 113–123. [Google Scholar] [CrossRef]
- Sözbir, G.D.; Bektas, I.; Ak, A.K. Influence of Combined Heat Treatment and Densification on Mechanical Properties of Poplar Wood. Maderas. Cienc. Tecnol. 2019, 21, 481–492. [Google Scholar] [CrossRef]
- Awoyemi, L.; Westermark, U. Effects of borate impregnation on the response of wood strength to heat treatment. Wood Sci. Technol. 2005, 39, 484–491. [Google Scholar] [CrossRef]
- Kartal, S.N.; Hwang, W.J.; Imamura, Y. Combined effect of boron compounds and heat treatments on wood properties: Chemical and strength properties of wood. J. Mater. Process. Technol. 2008, 198, 234–240. [Google Scholar] [CrossRef]
- Perçin, O.; Sofuoglu, S.D.; Uzun, O. Effects of boron impregnation and heat treatment on some mechanical properties of oak (Quercus petraea Liebl.) wood. BioResources 2015, 10, 3963–3978. [Google Scholar] [CrossRef][Green Version]
- Sun, B.L.; Wang, X.H.; Liu, J.L. Changes in dimensional stability and mechanical properties of Eucalyptus pellita by melamine–urea–formaldehyde resin impregnation and heat treatment. Eur. J. Wood Wood Prod. 2013, 71, 557–562. [Google Scholar] [CrossRef]
- Wang, W.; Ran, Y.Y.; Wang, J.M. Improved performance of thermally modified wood via impregnation with carnauba wax/organoclay emulsion. Constr. Build. Mater. 2020, 247, 118586. [Google Scholar] [CrossRef]
- Kamperidou, V. The Biological durability of thermally- and chemically-modified black pine and poplar wood against basidiomycetes and mold action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef]
- Čermák, P.; Dömény, J.; Brabec, M.; Milch, J.; Baar, J.; Horáček, P.; Rademacher, P. Unevenly distributed thermal treatment of wood: Preliminary study—Density profiles. Eur. J. Wood Wood Prod. 2016, 74, 629–631. [Google Scholar] [CrossRef]
- Čermák, P.; Dejmal, A.; Paschová, Z.; Kymäläinen, M.; Dömény, J.; Brabec, M.; Hess, D.; Rautkari, L. One-sided surface charring of beech wood. J. Mater. Sci. 2019, 54, 9497–9506. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Hautamäki, S.; Lillqvist, K.; Segerholm, K.; Rautkari, L. Surface modification of solid wood by charring. J. Mater. Sci. 2017, 52, 6111–6119. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Turunen, H.; Čermák, P.; Hautamäki, S.; Rautkari, L. Sorption-related characteristics of surface charred spruce wood. Materials 2018, 11, 2083. [Google Scholar] [CrossRef]
- De Jesus, M.S.; Carneiro, A.D.O.; Martinez, C.L.M.; Vital, B.R.; Vital, B.R.; de Assis, M.R. Thermal decomposition fundamentals in large-diameter wooden logs during slow pyrolysis. Wood Sci. Technol. 2019, 53, 1353–1372. [Google Scholar] [CrossRef]
- Poncsak, S.; Kocaefe, D.; Younsi, R.; Kocaefe, Y.; Gastonguay, L. Thermal treatment of electrical poles. Wood Sci. Technol. 2019, 43, 471–486. [Google Scholar] [CrossRef]
- Fortino, S.; Genoese, A.; Genoese, A.; Rautkari, L. FEM simulation of the hygro-thermal behaviour of wood under surface densification at high temperature. J. Mater. Sci. 2013, 48, 7603–7612. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Hale, M.D. Colour in thermally modified wood of beech, Norway spruce and Scots pine. Part 1: Colour evolution and colour changes. Holzforschung 2009, 63, 385–393. [Google Scholar] [CrossRef]
- Sundqvist, B.; Karlsson, O.; Westermark, U. Determination of formic-acid and acetic acid concentrations formed during hydrothermal treatment of birch wood and its relation to colour, strength and hardness. Wood Sci. Technol. 2006, 40, 549–561. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Hale, M.D. Colour in thermally modified wood of beech, Norway spruce and Scots pine. Part 2: Property predictions from colour changes. Holzforschung 2009, 63, 394–401. [Google Scholar] [CrossRef]
- Jalaludin, Z.; Hill, C.; Xie, Y.J.; Samsi, H.W.; Husain, H.; Awang, K.; Curling, S.F. Analysis of the water vapour sorption isotherms of thermally modified acacia and sesendok. Wood Mater. Sci. Eng. 2010, 5, 194–203. [Google Scholar]
- Olek, W.; Majka, J.; Czajkowski, L. Sorption isotherms of thermally modified wood. Holzforschung 2013, 67, 183–191. [Google Scholar] [CrossRef]
- CEN/TS 15679:2007; Thermal Modified Timber—Definitions and Characteristics. European Committee for Standardization (CEN): Brussels, Belgium, 2007.
- Yildiza, S.; Gezerb, E.; Yildiza, U. Mechanical and chemical behavior of spruce wood modified by heat. Build. Environ. 2006, 41, 1762–1766. [Google Scholar] [CrossRef]
- Blanchet, P.; Kaboorani, A.; Bustos, C. Understanding effects of drying methods on wood mechanical properties at ultra and cellular levels. Wood Fiber Sci. 2016, 48, 1–12. [Google Scholar]
- Kelley, S.S.; Rials, T.G.; Glasser, W.G. Relaxation behaviour of the amorphous components of wood. J. Mater. Sci. 1987, 22, 617–624. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Z.J. Dynamic viscoelastic properties of heat-treated glycerol-impregnated poplar wood. Eur. J. Wood Wood Prod. 2018, 76, 611–616. [Google Scholar] [CrossRef]
- Sun, N.; Das, S.; Frazier, C.E. Dynamic mechanical analysis of dry wood: Linear viscoelastic response region and effects of minor moisture changes. Holzforschung 2007, 61, 28–33. [Google Scholar] [CrossRef]
- Kocaefe, D.; Ponscak, S.; Boluk, Y. Effect of thermal treatment on the chemical composition and mechanical properties of birch and aspen. BioResources 2008, 3, 517–537. [Google Scholar]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Havimo, M. A literature-based study on the loss tangent of wood in connection with mechanical pulping. Wood Sci. Technol. 2009, 43, 627–642. [Google Scholar] [CrossRef]
- Wikberg, H.; Maunu, S.L. Characterisation of thermally modified hard- and softwoods by 13C CPMAS NMR. Carbohydr. Polym. 2004, 58, 461–466. [Google Scholar] [CrossRef]
- Sinn, G.; Reiterer, A.; Stanzl-Tschegg, S.E. Surface analysis of different wood species using X-ray photoelectron spectroscopy (XPS). J. Mater. Sci. 2001, 36, 4673–4680. [Google Scholar] [CrossRef]
- Wei, Y.A.; Huang, Y.X.; Yu, Y.L.; Gao, R.Q.; Yu, W.J. The surface chemical constituent analysis of poplar fibrosis veneers during heat treatment. J. Wood Sci. 2018, 64, 485–500. [Google Scholar] [CrossRef]
- Rowell, R.M. Cell wall chemistry. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Pettersen, R., Tshabalala, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Chapter 3. [Google Scholar]





| Relative Humidity/% | Step Length/% |
|---|---|
| 0~10 | 5 |
| 10~90 | 10 |
| 90~95 | 5 |
| 95~98 | 3 |
| Relative Humidity (%) | Sample MC4 (%) | Sample MC14 (%) | ||
|---|---|---|---|---|
| Adsorption | Desorption | Adsorption | Desorption | |
| 10 | 99 | 93 | 95 | 73 |
| 30 | 100 | 98 | 91 | 82 |
| 60 | 99 | 93 | 88 | 85 |
| 90 | 90 | 89 | 83 | 83 |
| Carbon Components | Binding Energy (eV) | Binding Type |
|---|---|---|
| C1 | 284.8 | C-H, C-C |
| C2 | 286.5 | C-OH, C-O-C |
| C3 | 288.0 | C=O, O-C-O |
| C4 | 289.5 | O-C=O |
| Sample | Percentage (%) | ||||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | ||
| Control | 55.6 | 28.8 | 11.5 | 4.1 | |
| MC4 | surface layer | 67.1 | 22.4 | 7.3 | 3.2 |
| core layer | 57.6 | 26.2 | 11.9 | 4.3 | |
| MC14 | surface layer | 68.8 | 22.4 | 5.9 | 2.9 |
| core layer | 53.8 | 30.2 | 11.8 | 4.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, T.; Cheng, Y.; Jiang, T. Preparation and Characterization of Heat-Treated Douglas Fir Wood with Core–Shell Structure. Forests 2022, 13, 825. https://doi.org/10.3390/f13060825
Ding T, Cheng Y, Jiang T. Preparation and Characterization of Heat-Treated Douglas Fir Wood with Core–Shell Structure. Forests. 2022; 13(6):825. https://doi.org/10.3390/f13060825
Chicago/Turabian StyleDing, Tao, Yafei Cheng, and Tianle Jiang. 2022. "Preparation and Characterization of Heat-Treated Douglas Fir Wood with Core–Shell Structure" Forests 13, no. 6: 825. https://doi.org/10.3390/f13060825
APA StyleDing, T., Cheng, Y., & Jiang, T. (2022). Preparation and Characterization of Heat-Treated Douglas Fir Wood with Core–Shell Structure. Forests, 13(6), 825. https://doi.org/10.3390/f13060825






