Defoliation-Induced Growth Reduction of Pinus sylvestris L. after a Prolonged Outbreak of Diprion pini L.—A Case Study from Eastern Finland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Data Sets
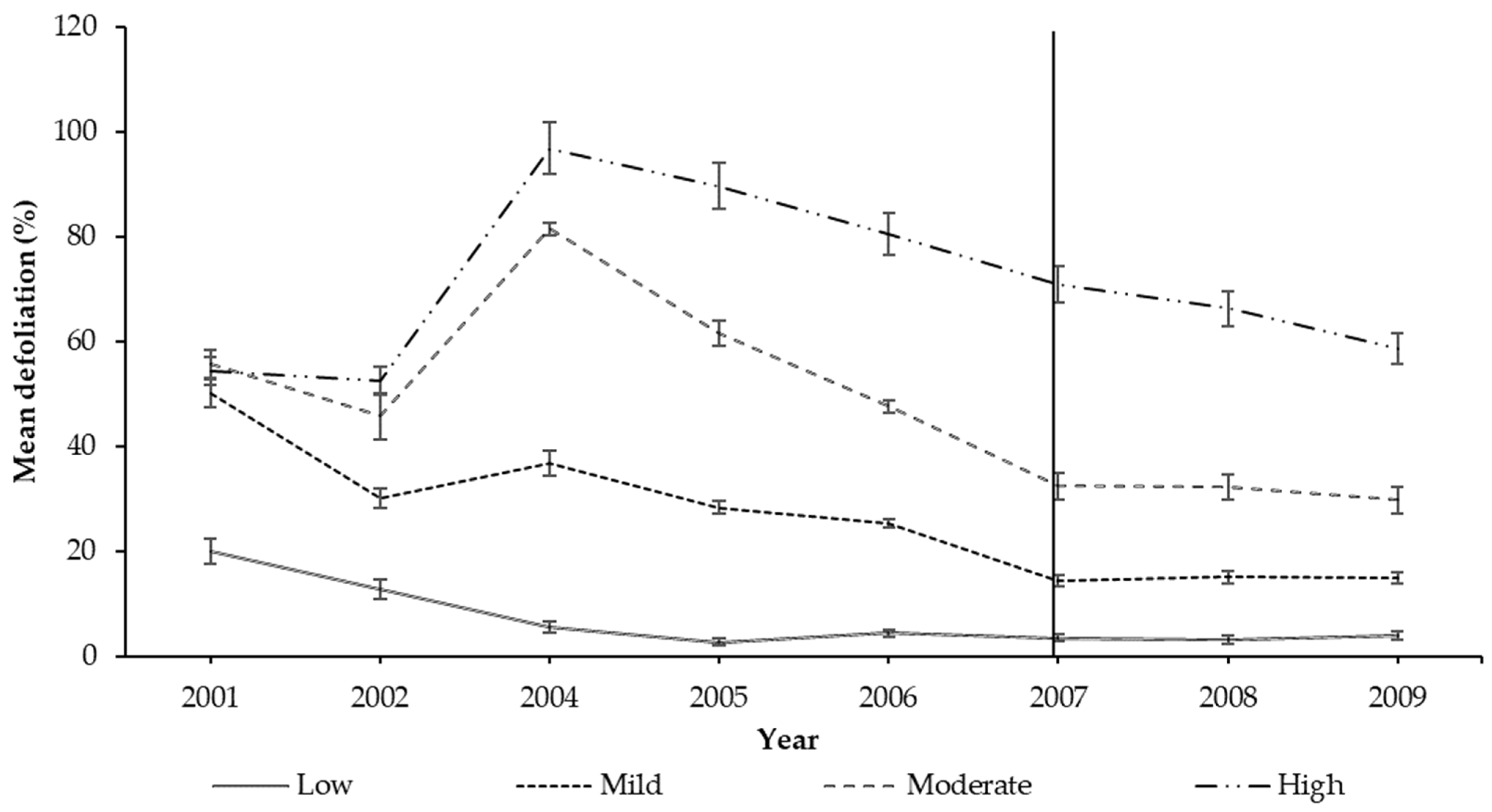
2.2. Defoliation Assessment
2.3. Radial Tree Growth Measurement
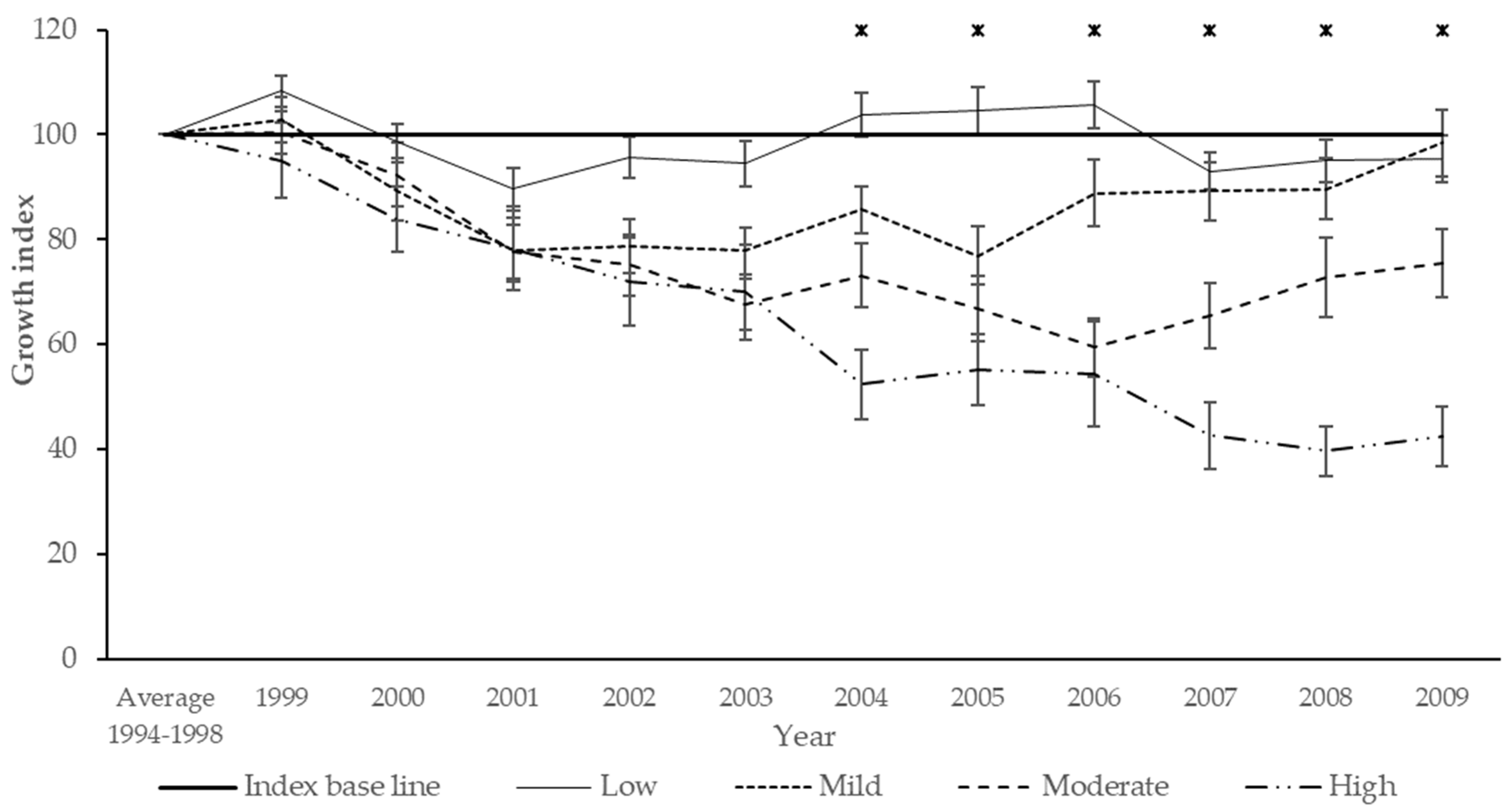
2.4. Growth Indices
2.5. Estimation of Tree Volume and Economic Impact
3. Results
3.1. Radial Growth and Growth Indices
3.2. Volume Growth and Economic Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dajoz, R. Insects and Forests: The Role and Diversity of Insects in the Forest Environment; Intercept Limited: Paris, France, 2000; ISBN 1898298688. [Google Scholar]
- Twery, M.J. Effects of defoliation by Gypsy moth. In US Department of Agriculture Interagency Gypsy Moth Research Review; Gottschalk, K.W., Twery, M.J., Smith, S.I., Eds.; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA; East Windsor, CT, USA, 1990; pp. 27–39. [Google Scholar]
- Niquidet, K.; Tang, J.; Peter, B. Economic analysis of forest insect pests in Canada. Can. Entomol. 2016, 148, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Austarå, Ø.; Orlund, A.; Svendsrud, A.; Veidahl, A. Growth loss and economic consequences following two years defoliation of Pinus sylvestris by the pine sawfly Neodiprion sertifer in West-Norway. Scand. J. For. Res. 1987, 2, 111–119. [Google Scholar] [CrossRef]
- Lyytikäinen-Saarenmaa, P.; Niemela, P.; Annila, E. Growth Responses and Mortality of Scots Pine (Pinus sylvestris L.) after a Pine Sawfly Outbreak. In IUFRO Kanazawa 2003 “Forest Insect Population Dynamics and Host Influences”; Kanazawa University: Kanazawa, Japan, 2006; pp. 81–85. [Google Scholar]
- De Somviele, B.; Lyytikäinen-Saarenmaa, P.; Niemelä, P. Sawfly (Hym., Diprionidae) outbreaks on Scots pine: Effect of stand structure, site quality and relative tree position on defoliation intensity. For. Ecol. Manag. 2004, 194, 305–317. [Google Scholar] [CrossRef]
- Lyytikäinen-Saarenmaa, P.; Tomppo, E. Impact of sawfly defoliation on growth of Scots pine Pinus sylvestris (Pinaceae) and associated economic losses. Bull. Entomol. Res. 2002, 92, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, J.S.; Orazio, C.; Jactel, H. Defoliation by processionary moth significantly reduces tree growth: A quantitative review. Ann. For. Sci. 2012, 69, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Lyytikäinen-Saarenmaa, P. Growth responses of scots pine. Ecol. Appl. 1999, 9, 469–474. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Krause, S.C.; Vanderklein, D.W.; Raffs, K.F.; Tabone, T. Growth, nutrition and gas exchange of Pinus resinosa following artificial defoliation. Trees 1993, 7, 67–77. [Google Scholar] [CrossRef]
- Lyytikäinen-Saarenmaa, P. The responses of Scots pine, Pinus sylvestris, to natural and artificial defoliation stress. Ecol. Appl. 1999, 9, 469–474. [Google Scholar]
- Krause, C.; Gionest, F.; Morin, H.; MacLean, D.A. Temporal relations between defoliation caused by spruce budworm (Choristoneura fumiferana Clem.) and growth of balsam fir (Abies balsamea (L.) Mill.). Dendrochronologia 2003, 21, 23–31. [Google Scholar] [CrossRef]
- Wainhouse, D. Ecological Methods in Forest Pest Management; Oxford University Press: Oxford, UK, 2005; ISBN 9780198505648. [Google Scholar]
- Krause, S.C.; Raffa, K.F. Differential growth and recovery rates following defoliation in related deciduous and evergreen trees. Trees 1996, 10, 308. [Google Scholar] [CrossRef]
- Jacquet, J.S.; Bosc, A.; O’Grady, A.; Jactel, H. Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Tree Physiol. 2014, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, B.Y.A.; Larsson, S.; Tenow, O. Effects of Early and Late Season Defoliation on Growth and Carbohydrate Dynamics in Scots Pine. J. Appl. Ecol. 1980, 17, 747–769. [Google Scholar] [CrossRef]
- Nordkvist, M.; Klapwijk, M.J.; Edenius, L.R.; Björkman, C. Interacting effects of insect and ungulate herbivory on Scots pine growth. Sci. Rep. 2020, 10, 22341. [Google Scholar] [CrossRef]
- Tiihonen, P. Ruskean Mäntypistiäisen (N. sertifer Geoffr.) Tuhojen Vaikutuksesta Männiköiden Kasvuun Etelä-Pohjanmaalla, Pohjois-Pohjanmaalle ja Länsi-Uudellamaalla Vuosina 1960–1967 (English Abstact Available); Communicationes Instituti Forestalis Fenniae: Helsinki, Finland, 1970. [Google Scholar]
- Talvitie, M.; Kantola, T.; Holopainen, M.; Lyytikäinen-Saarenmaa, P. Adaptive Cluster Sampling in Inventorying Forest Damage by the Common Pine Sawfly. J. For. Plan. 2011, 16, 141–147. [Google Scholar]
- Geri, C. The pine sawfly in central France. In Dynamics of Forest Insect Populations: Patterns, Causes and Implications; Berryman, A., Ed.; Plenum Press: New York, NY, USA, 1988; pp. 377–405. ISBN 978-1-4899-0789-9. [Google Scholar]
- Viitasaari, M.; Varama, M. Sawflies 4. Conifer Sawflies (Diprionidae). English Summary in Sahapistiäiset 4. Havupistiäiset (Diprinidae), 10th ed.; Maatalous- ja Metsäeläintieteen Laitos; Helsingin Yliopisto: Helsinki, Finland, 1987; ISBN 951-45-4179-0. [Google Scholar]
- De Somviele, B.; Lyytikäinen-Saarenmaa, P.; Niemelä, P. Stand edge effects on distribution and condition of Diprionid sawflies. Agric. For. Entomol. 2007, 9, 17–30. [Google Scholar] [CrossRef]
- Aalto, J.; Pirinen, P.; Kauppi, P.E.; Rantanen, M.; Lussana, C.; Lyytikäinen-Saarenmaa, P.; Gregow, H. High-resolution analysis of observed thermal growing season variability over northern Europe. Clim. Dyn. 2022, 58, 1477–1493. [Google Scholar] [CrossRef]
- Långström, B.; Annila, E.; Hellqvist, C.; Varama, M.; Niemelä, P. Tree mortality, needle biomass recovery and growth losses in Scots pine following defoliation by Diprion pini (L.) and subsequent attack by Tomicus piniperda (L.). Scand. J. For. Res. 2001, 16, 342–353. [Google Scholar] [CrossRef]
- Annila, E.; Långström, B.; Varama, M.; Hiukka, R.; Niemelä, P. Susceptibility of defoliated Scots pine to spontaneous and induced attack by Tomicus piniperda and Tomicus minor. Silva Fenn. 1999, 33, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, J.S.; Bosc, A.; O’Grady, A.P.; Jactel, H. Pine growth response to processionary moth defoliation across a 40-year chronosequence. For. Ecol. Manag. 2013, 293, 29–38. [Google Scholar] [CrossRef]
- Blomqvist, M.; Lyytikäinen-Saarenmaa, P.; Kantola, T.; Kosunen, M.; Talvitie, M.; Holopainen, M. Impacts of natural enemies and stand characteristics on cocoon mortality of the pine sawfly Diprion pini in a Fennoscandian boreal forest. Silva Fenn. 2016, 50, 20. [Google Scholar] [CrossRef] [Green Version]
- Mikola, P. Application of vegetation science to forestry in Finland. In Handbook of Vegetation Science, Part 12; Jahn, G., Ed.; Dr. Junk Publishers: The Hague, The Netherlands, 1982; pp. 199–224. [Google Scholar]
- Eichhorn, J. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests. In Visual Assessment of Crown Condition and Submanual on Visual Assessment of Crown Condition on Intensive Monitoring Plots; United Nations Economic Commission for Europe Convention on LongRange Transboundary Air Pollution; United Nations Economic Commission for Europe: Hamburg, Germany, 1998; p. 69. [Google Scholar]
- Sokal, R.; Rohlf, F. Biometry: The Principles and Practice of Statistics in Biological Research; W. H. Freeman and Company: New York, NY, USA, 1995; ISBN 978-0716724117. [Google Scholar]
- Näslund, M. Skogsförsöksanstaltens gallringsförsök i tallskog. In Meddelanden Från Statens Skogsförsöksanstalt; Meddelanden Fran Statens Skogsforsoksanstalt: Stockholm, Sweden, 1936; Volume 29, pp. 1–169. [Google Scholar]
- Laasasenaho, J. Taper Curve and Volume Functions for Pine, Spruce and Birch; Metsäntutkimuslaitos: Helsinki, Finland, 1982; Volume 108, ISBN 951-40-0589-9. [Google Scholar]
- Rantala, S. (Ed.) Tapion Taskukirja, 25th ed.; Metsäkustannus Oy: Helsinki, Finland, 2008; ISBN 9789525694260. [Google Scholar]
- Lynch, A.M. What Tree-Ring Reconstruction Tells Us about Conifer Defoliator Outbreaks. Insect Outbreaks Revisit. 2012, 126–154. [Google Scholar] [CrossRef]
- Magnussen, S.; Alfaro, R.I. Linking aerial survey data of forest insect defoliation and tree ring data to estimate forest level growth losses. Dendrochronologia 2012, 30, 287–294. [Google Scholar] [CrossRef]
- Perot, T.; Vallet, P.; Archaux, F. Growth compensation in an oak-pine mixed forest following an outbreak of pine sawfly (Diprion pini). For. Ecol. Manag. 2013, 295, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Kosunen, M.; Kantola, T.; Starr, M.; Blomqvist, M.; Talvitie, M.; Lyytikäinen-Saarenmaa, P. Influence of soil and topography on defoliation intensity during an extended outbreak of the common pine sawfly (Diprion pini L.). Iforest Biogeosci. For. 2017, 10, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Le Mellec, A.; Michalzik, B. Impact of a pine lappet (Dendrolimus pini) mass outbreak on C and N fluxes to the forest floor and soil microbial properties in a Scots pine forest in Germany. Can. J. For. Res. 2008, 38, 1829–1841. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Trumble, J.T.; Kolodny-Hirsch, D.M.; Ting, I.P. Plant Compensation for arthropod herbivory. Ann. For. Sci. 1993, 38, 93–119. [Google Scholar] [CrossRef]
- Palacio, S.; Hernández, R.; Maestro-Martínez, M.; Camarero, J.J. Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees Struct. Funct. 2012, 26, 1627–1640. [Google Scholar] [CrossRef] [Green Version]
- Salemaa, M.; Vanha-Majamaa, I.; Derome, J. Understorey vegetation along a heavy-metal pollution gradient in SW Finland. Environ. Pollut. 2001, 112, 339–350. [Google Scholar] [CrossRef]
- Ericsson, A.; Hellqvist, C.; Langstrom, B.; Larsson, S.; Tenow, O. Effects on Growth of Simulated and Induced Shoot Pruning by Tomicus piniperda as Related to Carbohydrate and Nitrogen Dynamics in Scots Pine. J. Appl. Ecol. 1985, 22, 105. [Google Scholar] [CrossRef]
- Czokajlo, D.; Wink, R.A.; Warren, J.C.; Teale, S.A. Growth reduction of Scots pine, Pinus sylvestris, caused by the larger pine shoot beetle, Tomicus piniperda (Coleoptera, Scolytidae), in New York State. Can. J. For. Res. 1997, 27, 1394–1397. [Google Scholar] [CrossRef]
- Hu, M.; Lehtonen, A.; Minunno, F.; Mäkelä, A. Age effect on tree structure and biomass allocation in Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies [L.] Karst.). Ann. For. Sci. 2020, 77, 90. [Google Scholar] [CrossRef]
| Defoliation Classes | Low | Mild | Moderate | High |
|---|---|---|---|---|
| N of trees | 83 | 43 | 32 | 26 |
| Age | 76.8 (±12.8) | 74.4 (±15.8) | 75.9 (±10.9) | 74.2 (±12.8) |
| Height | 18.8 (±1.7) | 18.7 (±1.9) | 18.8 (±1.7) | 18.5 (±2.2) |
| Dbh | 21.3 (±3.8) | 21.3 (±3.7) | 22.9 (±3.8) | 22.7 (±4.2) |
| Model | Coefficients | Estimate | Std. Error | t-Value | p-Value | DF | F | Significance of F | R² | Adjusted R² |
|---|---|---|---|---|---|---|---|---|---|---|
| Growth 2003 ~DEF 2002 | Intercept | 0.864 | 0.092 | 9.401 | <0.001 * | |||||
| DEF 2002 | −0.003 | 0.002 | −1.505 | 0.137 | 64 | 2.266 | 0.137 | 0.034 | 0.019 | |
| Growth 2005 ~DEF 2004 | Intercept | 0.865 | 0.081 | 10.715 | <0.001 * | |||||
| DEF 2004 | −0.006 | 0.002 | −2.408 | 0.02 * | 64 | 5.797 | 0.019 * | 0.083 | 0.069 | |
| Growth 2006 ~DEF 2005 | Intercept | 0.829 | 0.059 | 14.002 | <0.001 * | |||||
| DEF 2005 | −0.004 | 0.001 | −3.088 | 0.003 * | 64 | 9.536 | 0.003 * | 0.130 | 0.116 | |
| Growth 2007 ~DEF 2006 | Intercept | 0.695 | 0.050 | 13.779 | <0.001 * | |||||
| DEF 2006 | −0.003 | 0.001 | −2.817 | 0.006 * | 64 | 7.933 | 0.006 * | 0.110 | 0.096 | |
| Growth 2008 ~DEF 2007 | Intercept | 0.956 | 0.041 | 23.182 | <0.001 * | |||||
| DEF 2007 | −0.006 | 0.001 | −5.791 | <0.001 * | 190 | 33.54 | <0.000* | 0.150 | 0.146 | |
| Growth 2009 ~DEF 2008 | Intercept | 1.006 | 0.045 | 22.261 | <0.001 * | |||||
| DEF 2008 | −0.009 | 0.001 | −5.797 | <0.001 * | 190 | 33.6 | <0.000 * | 0.150 | 0.146 | |
| Growth 2010 ~DEF 2009 | Intercept | 1.283 | 0.060 | 21.228 | <0.001 | |||||
| DEF 2009 | −0.012 | 0.002 | −5.768 | <0.001 | 124 | 33.27 | <0.000 * | 0.212 | 0.205 |
| Defoliation Class | Annual Volume Growth (m3/ha) | Growth (%) | Cumulative Economic Loss (€/ha) |
|---|---|---|---|
| Low | 0.40 | 16.5 | +146 |
| Mild | −0.11 | −4.2 | −51 |
| Moderate | −0.57 | −20.8 | −272 |
| High | −1.44 | −40.4 | −734 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blomqvist, M.; Lyytikäinen-Saarenmaa, P.; Kosunen, M.; Kantola, T.; Holopainen, M. Defoliation-Induced Growth Reduction of Pinus sylvestris L. after a Prolonged Outbreak of Diprion pini L.—A Case Study from Eastern Finland. Forests 2022, 13, 839. https://doi.org/10.3390/f13060839
Blomqvist M, Lyytikäinen-Saarenmaa P, Kosunen M, Kantola T, Holopainen M. Defoliation-Induced Growth Reduction of Pinus sylvestris L. after a Prolonged Outbreak of Diprion pini L.—A Case Study from Eastern Finland. Forests. 2022; 13(6):839. https://doi.org/10.3390/f13060839
Chicago/Turabian StyleBlomqvist, Minna, Päivi Lyytikäinen-Saarenmaa, Maiju Kosunen, Tuula Kantola, and Markus Holopainen. 2022. "Defoliation-Induced Growth Reduction of Pinus sylvestris L. after a Prolonged Outbreak of Diprion pini L.—A Case Study from Eastern Finland" Forests 13, no. 6: 839. https://doi.org/10.3390/f13060839






